Spider Silk-CBD-Cellulose Nanocrystal Composites: Mechanism of Assembly
Abstract
:1. Introduction
2. Results and Discussion
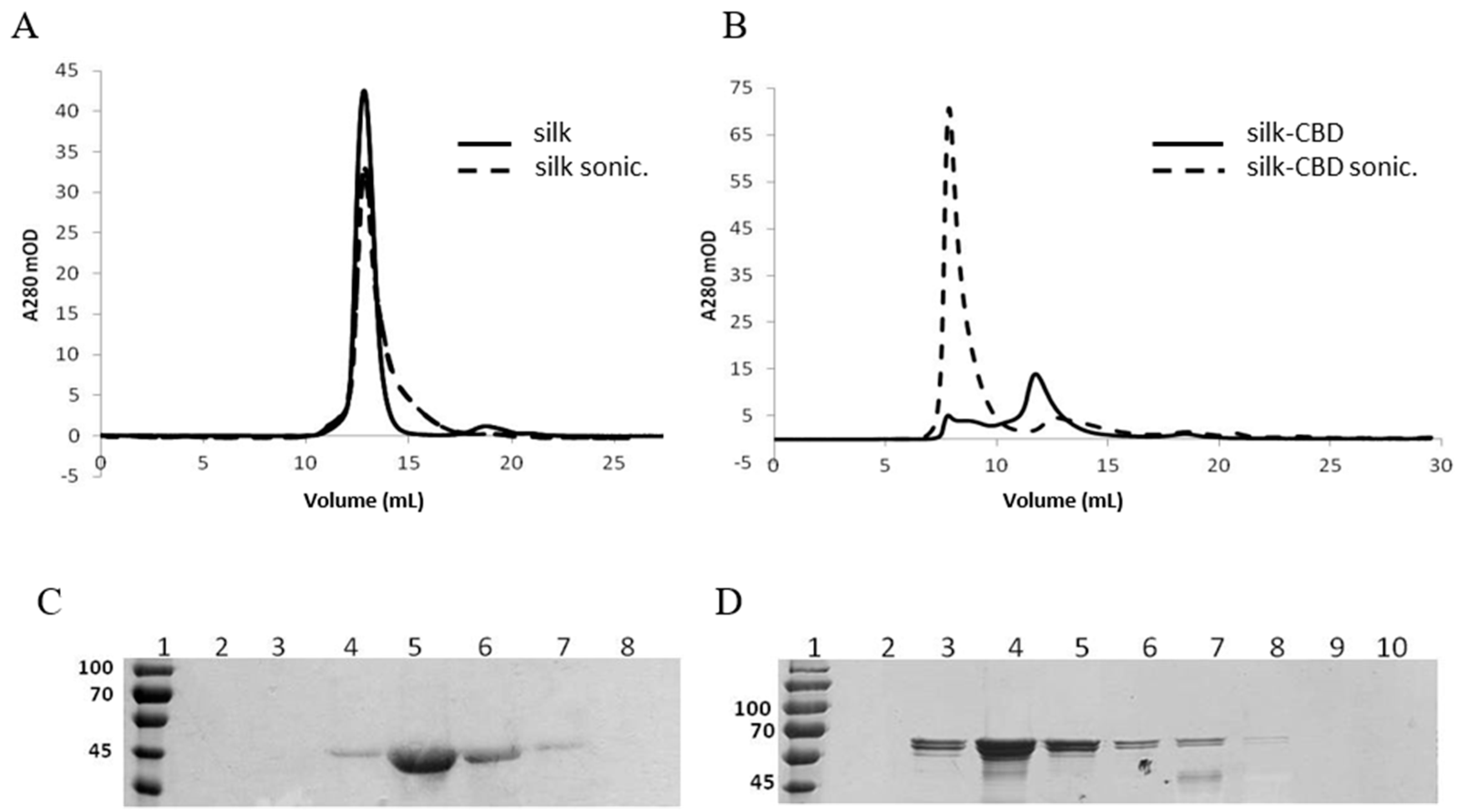
2.1. Protein Expression and Purification
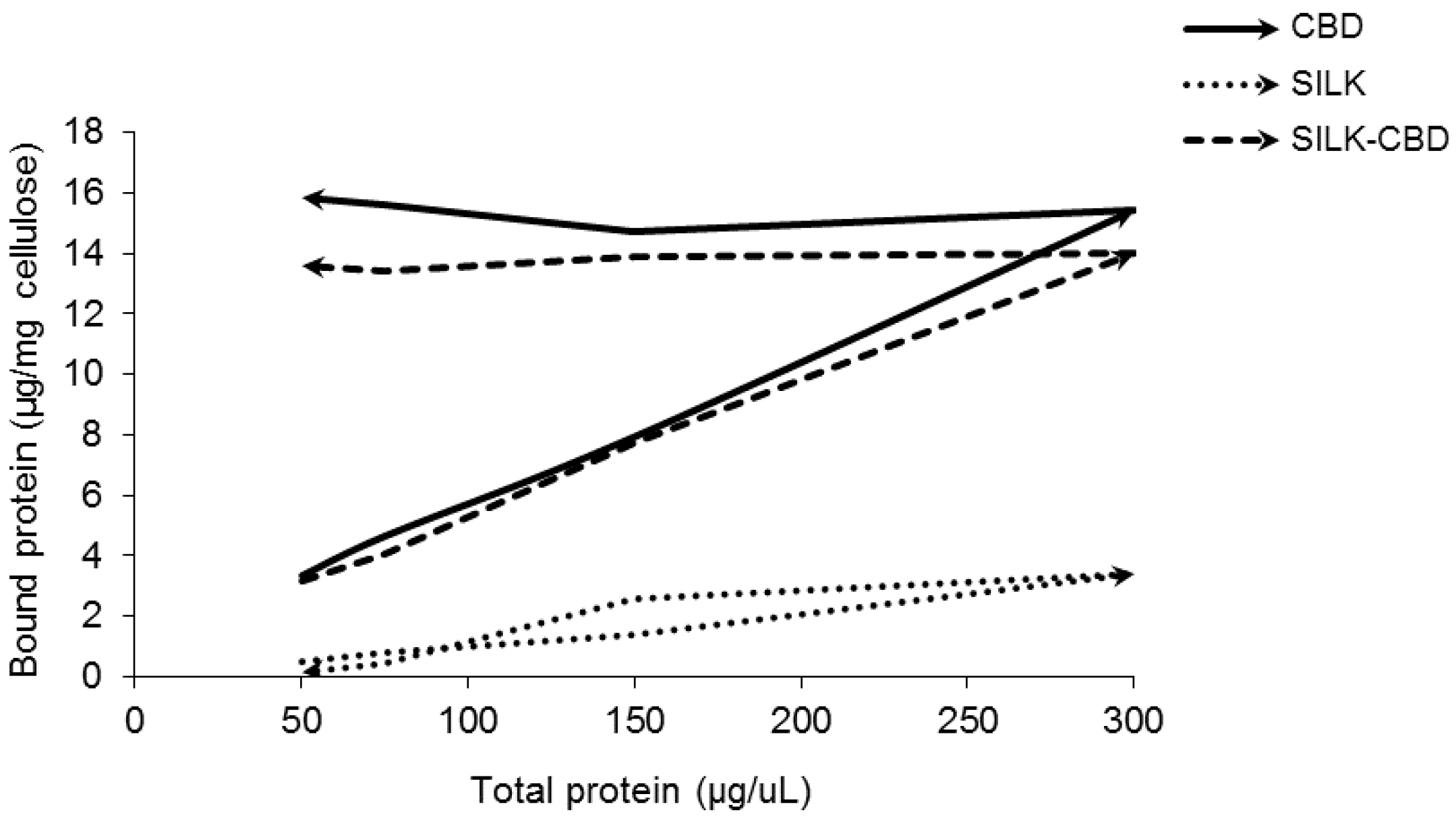
2.2. Quantitative Cellulose Binding Assay
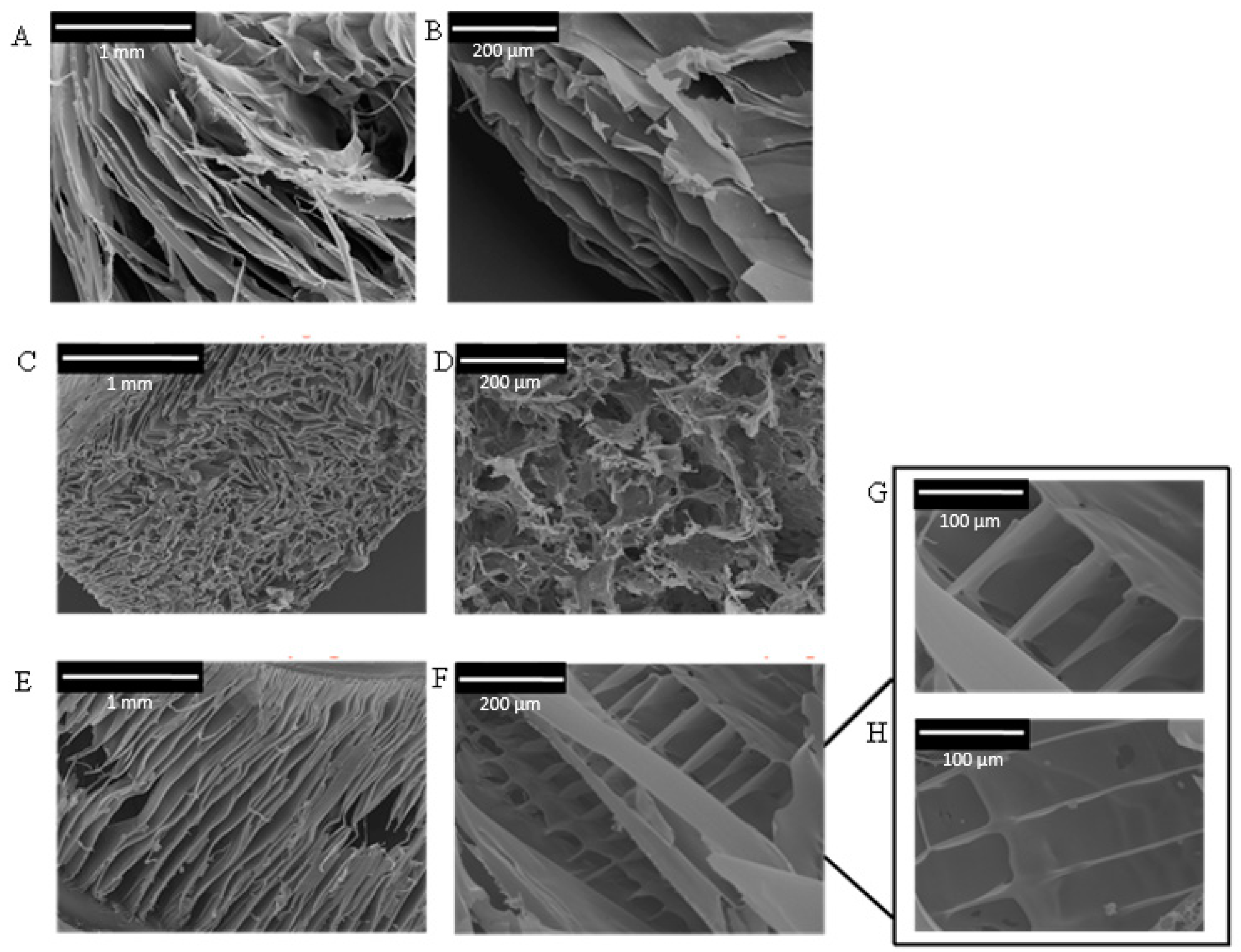
2.3. Composite CNC/Spider Silk Sponges
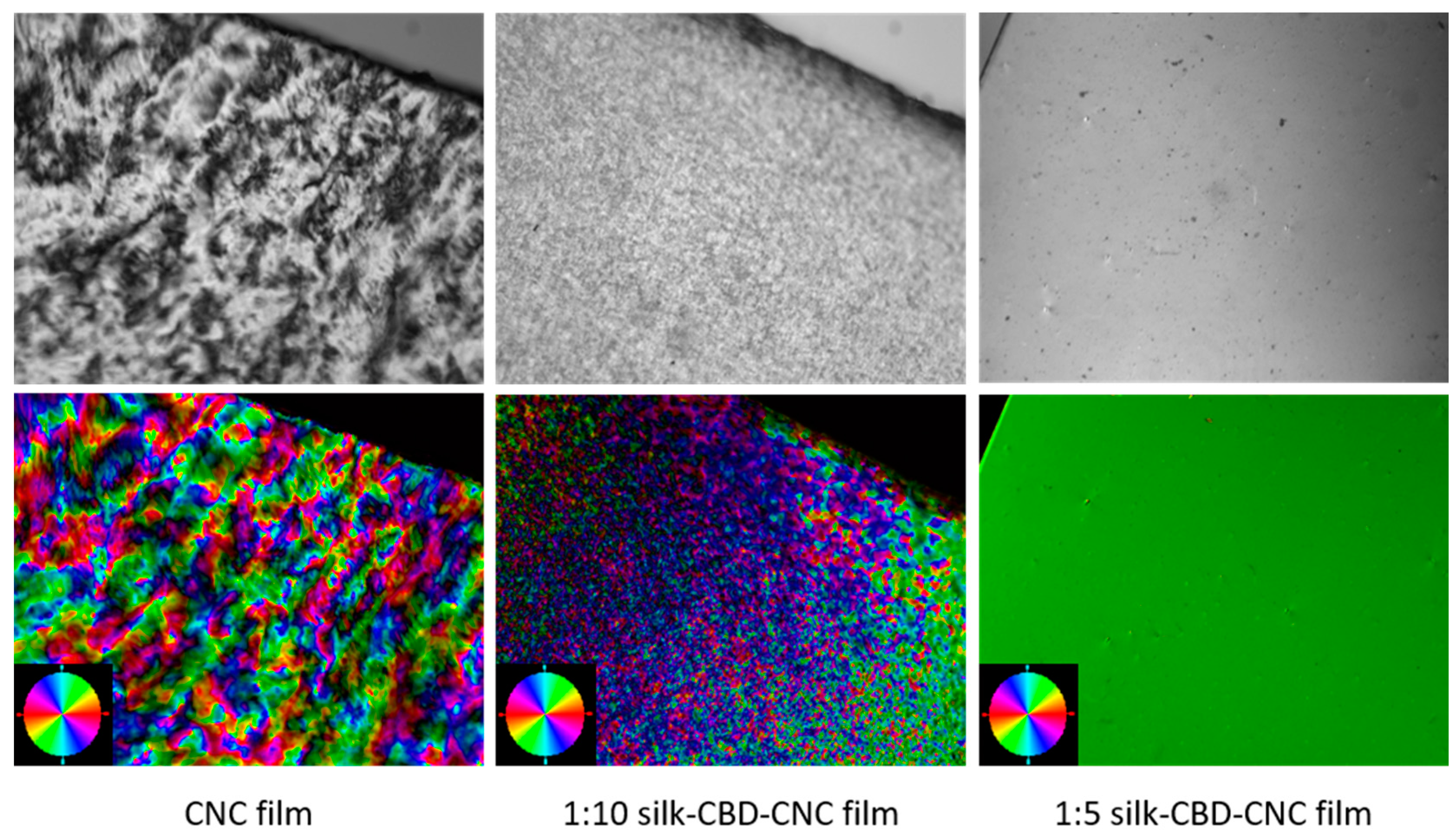
2.4. Composite CNC/Spider Silk Films
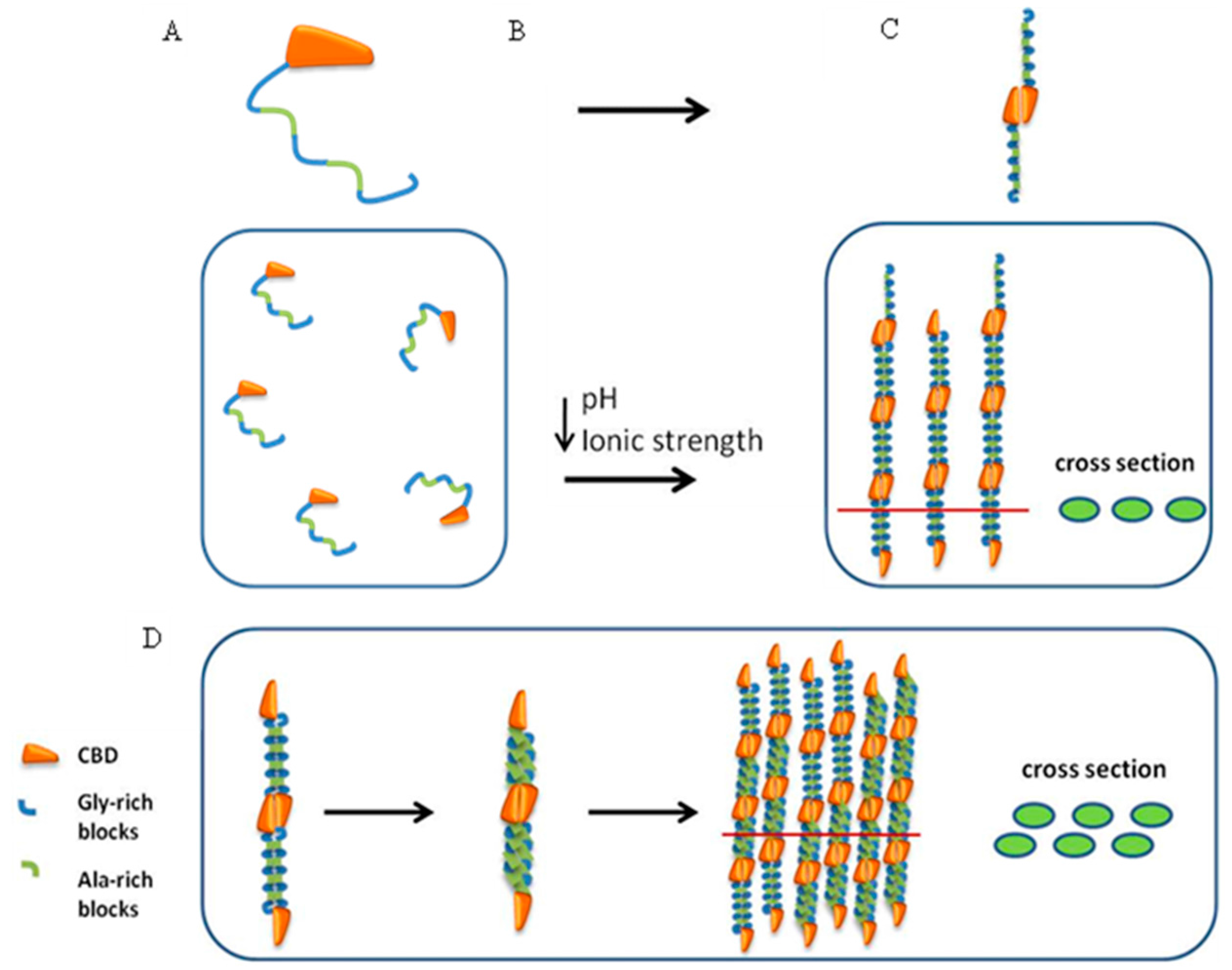
2.5. Spider Silk-CBD Fusion Protein Assembly
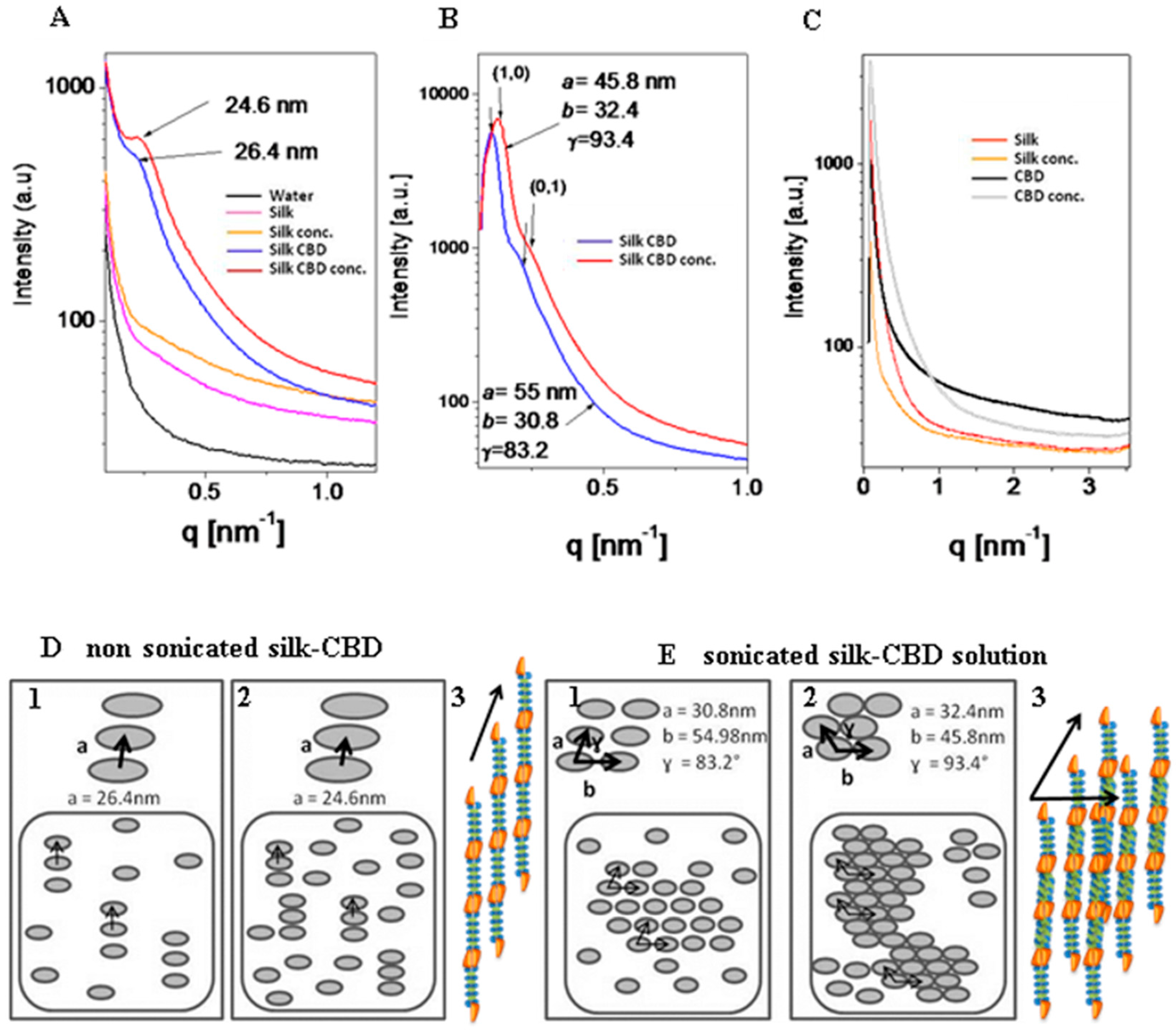
2.6. Small Angle X-ray Scattering (SAXS) of Solutions of Silk and Silk-CBD
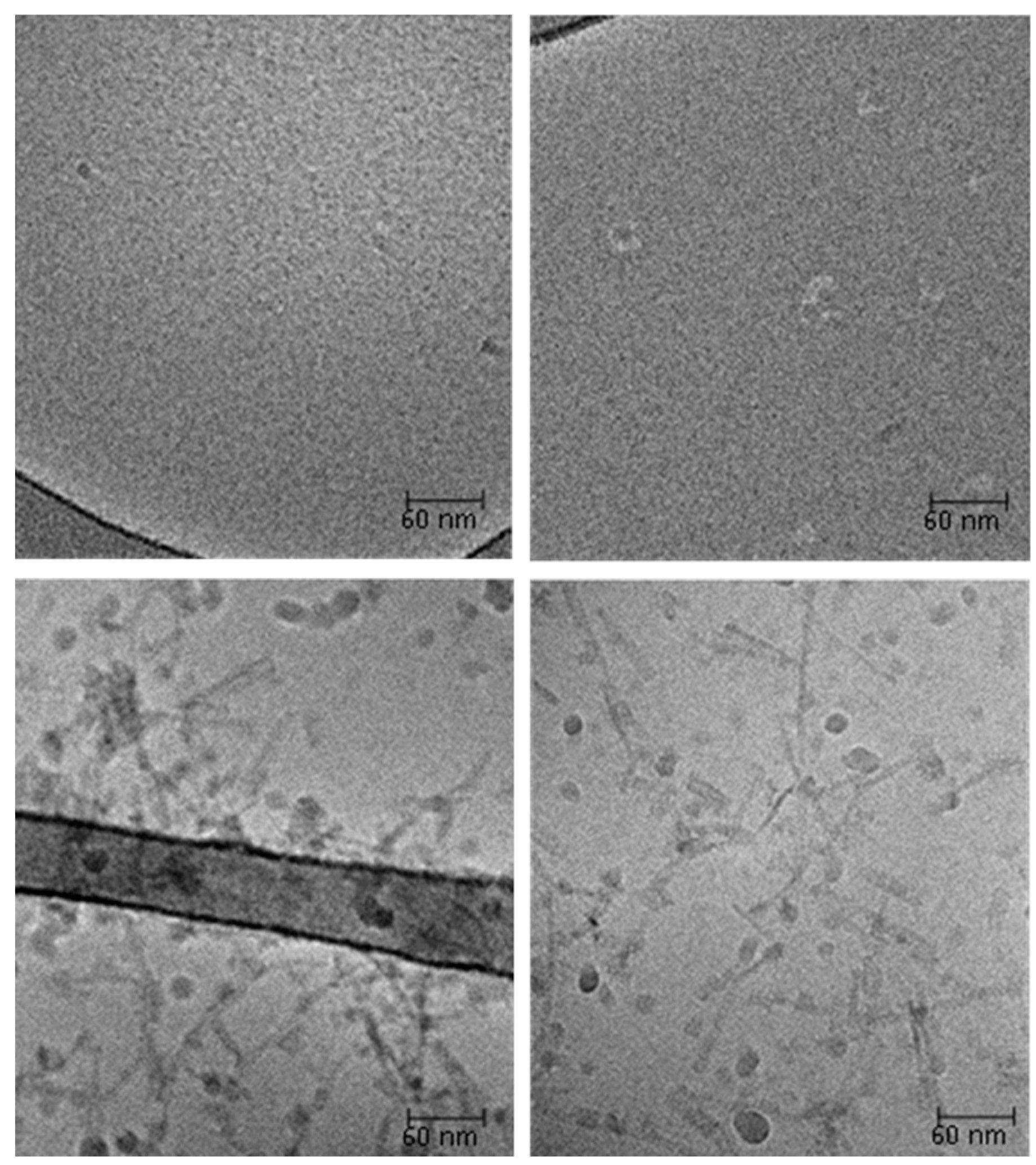
2.7. Cryo-Transmission Electron Microscopy (Cryo-TEM)
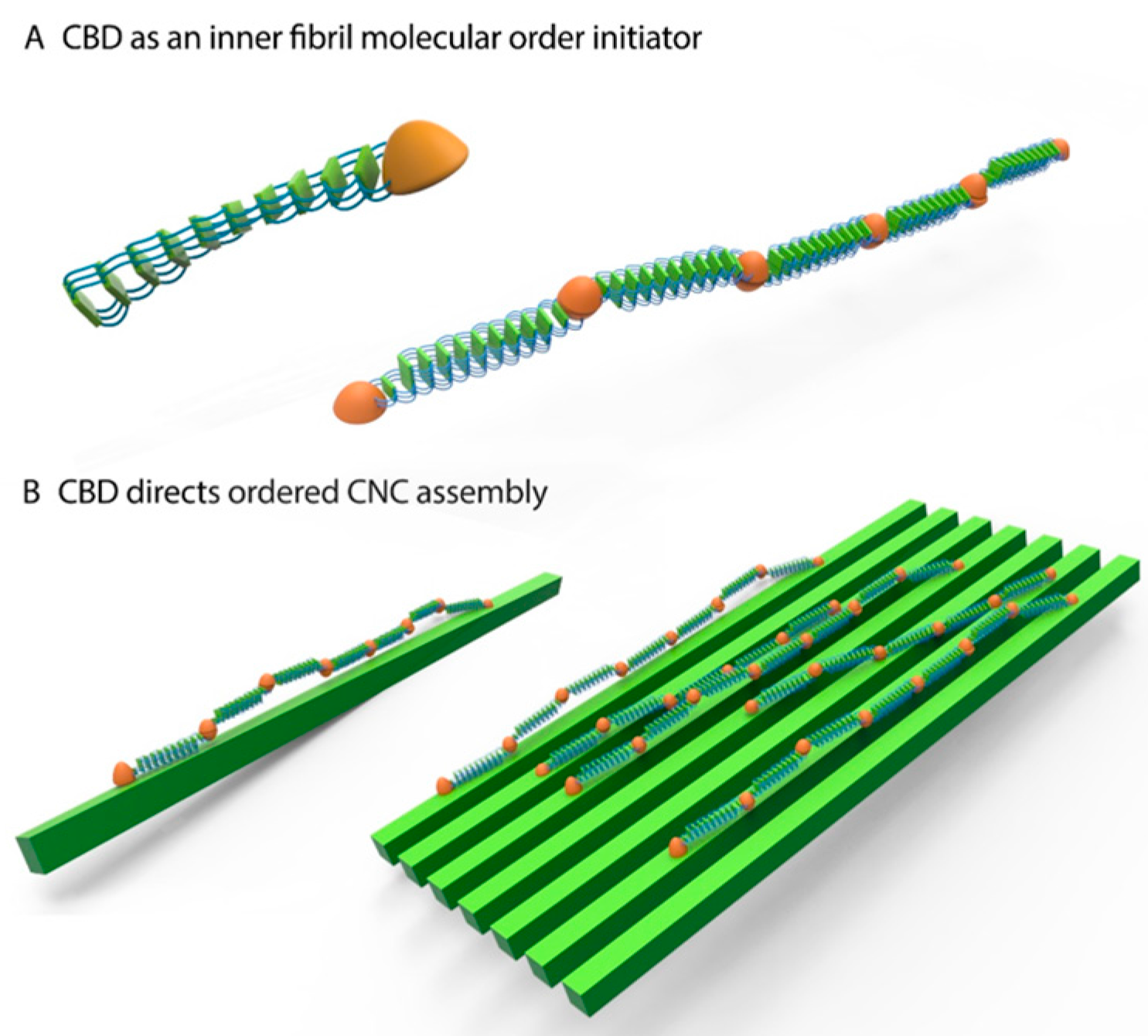
2.8. Silk-CBD Assembly Model
3. Material and Methods
3.1. Design and Expression of the Recombinant Spider Silk Proteins
3.2. Purification of 6H-Silk and 6H-Silk-CBD
3.3. Quantitative Cellulose Binding Reversibility Assay for CBD, Silk and Silk-CBD
3.4. Production of Composite Silk/Silk-CBD and CNC Sponges
3.5. CNC and Silk-CBD-CNC Films Preparation
3.6. Scanning Electron Microscopy (SEM)
3.7. Polarized Light Microscopy (POM)
3.8. Differential Scanning Calorimeter (DSC) Analysis
3.9. Gel Filtration
3.10. Dynamic Light Scattering (DLS)
3.11. Small Angle X-ray Scattering (SAXS)
3.12. Cryo-Transmision Electrom Microscopy (TEM)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vollrath, F. Strength and structure of spiders’ silks. Rev. Mol. Biotechnol. 2000, 74, 67–83. [Google Scholar] [CrossRef]
- Rising, A.; Hjälm, G.; Engström, W.; Johansson, J. N-terminal nonrepetitive domain common to dragline, flagelliform, and cylindriform spider silk proteins. Biomacromolecules 2006, 7, 3120–3124. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, N.A.; Garb, J.E.; Tinghitella, R.M.; Collin, M.A.; Hayashi, C.Y. Blueprint for a high-performance biomaterial: Full-length spider dragline silk genes. PLoS ONE 2007, 2, e514. [Google Scholar] [CrossRef] [PubMed]
- Simmons, A.; Ray, E.; Jelinski, L. Solid-state 13C NMR of Nephila clavipes dragline silk establishes structure and identity of crystalline regions. Macromolecules 1994, 27, 5235–5237. [Google Scholar] [CrossRef]
- Hinman, M.B.; Jones, J.A.; Lewis, R.V. Synthetic spider silk: A modular fiber. Trends Biotechnol. 2000, 18, 374–379. [Google Scholar] [CrossRef]
- Parkhe, A.D.; Seeley, S.K.; Gardner, K.; Thompson, L.; Lewis, R.V. Structural studies of spider silk proteins in the fiber. J. Mol. Recognit. 1997, 10, 1–6. [Google Scholar] [CrossRef]
- Hayashi, C.Y.; Shipley, N.H.; Lewis, R.V. Hypotheses that correlate the sequence, structure, and mechanical properties of spider silk proteins. Int. J. Biol. Macromol. 1999, 24, 271–275. [Google Scholar] [CrossRef]
- Rising, A.; Nimmervoll, H.; Grip, S.; Fernandez-Arias, A.; Storckenfeldt, E.; Knight, D.P.; Vollrath, F.; Engström, W. Spider silk proteins—Mechanical property and gene sequence. Zool. Sci. 2005, 22, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.K.; Jovicic, J. Modeling of mechanical properties and structural design of spider web. Biomacromolecules 2004, 5, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Kluge, J.A.; Rabotyagova, O.; Leisk, G.G.; Kaplan, D.L. Spider silks and their applications. Trends Biotechnol. 2008, 26, 244–521. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R. Unraveling the weave of spider silk. Bioscience 1996, 46, 636–638. [Google Scholar] [CrossRef]
- Fahnestock, S.R.; Bedzyk, L.A. Production of synthetic spider dragline silk protein in Pichia pastoris. Appl. Microbiol. Biotechnol. 1997, 47, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Fahnestock, S.R.; Irwin, S.L. Synthetic spider dragline silk proteins and their production in Escherichia coli. Appl. Microbiol. Biotechnol. 1997, 47, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Zhang, Y.; Nakagaki, K.; Zhao, T.; Zhao, A.; Meng, Y.; Nakagaki, M.; Park, E.Y.; Maenaka, K. Expression of spider flagelliform silk protein in Bombyx mori cell line by a novel Bac-to-Bac/BmNPV baculovirus expression system. Appl. Microbiol. Biotechnol. 2006, 71, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Barr, L.A.; Fahnestock, S.R.; Yang, J. Production and purification of recombinant DP1B silk-like protein in plants. Mol. Breed. 2004, 13, 345–356. [Google Scholar] [CrossRef]
- Xia, X.X.; Qian, Z.G.; Ki, C.S.; Park, Y.H.; Kaplan, D.L.; Lee, S.Y. Native-sized recombinant spider silk protein produced in metabolically engineered Escherichia coli results in a strong fiber. Proc. Natl. Acad. Sci. USA 2010, 107, 14059–14063. [Google Scholar] [CrossRef] [PubMed]
- Stark, M.; Grip, S.; Rising, A.; Hedhammar, M.; Engström, W.; Hjälm, G.; Johansson, J. Macroscopic fibers self-assembled from recombinant miniature spider silk proteins. Biomacromolecules 2007, 8, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Kaplan, D.L. Mechanism of silk processing in insects and spiders. Nature 2003, 424, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Vollrath, F.; Knight, D.P. Liquid crystalline spinning of spider silk. Nature 2001, 410, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Rivkin, A.; Meirovitch, S.; Shoseyov, O.; Kaplan, D.L. Bio-inspired elastic composites of nano crestalline celulose and cellulose binding resilin. In Proceedings of the 34th Risø International Symposium on Materials Science: Processing of Fibre Composites—Challenges for Maximum Materials Performance, Roskilde, Denmark, 2–5 September 2013.
- Šturcová, A.; Davies, G.R.; Eichhorn, S.J. Elastic modulus and stress-transfer properties of tunicate cellulose whiskers. Biomacromolecules 2005, 6, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Noishiki, Y.; Nishiyama, Y.; Wada, M.; Kuga, S.; Magoshi, J. Mechanical properties of silk fibroin–microcrystalline cellulose composite films. J. Appl. Polym. Sci. 2002, 86, 3425–3429. [Google Scholar] [CrossRef]
- Shoseyov, O.; Doi, R.H. Essential 170-kDa subunit for degradation of crystalline cellulose by Clostridium cellulovorans cellulase. Proc. Natl. Acad. Sci. USA 1990, 87, 2192–2195. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, M.A.; Takagi, M.; Hashida, S.; Shoseyov, O.; Doi, R.H.; Segel, I.H. Characterization of the cellulose-binding domain of the Clostridium cellulovorans cellulose-binding protein A. J. Bacteriol. 1993, 175, 5762–5768. [Google Scholar] [PubMed]
- Tomme, P.; Warren, R.A.J.; Gilkes, N.R. Cellulose hydrolysis by bacteria and fungi. Adv. Microb. Physiol. 1995, 37, 1–81. [Google Scholar] [PubMed]
- Bolam, D.N.; Ciruela, A.; McQueen-Mason, S.; Simpson, P.; Williamson, M.P.; Rixon, J.E.; Boraston, A.; Hazlewood, G.P.; Gilbert, H.J. Pseudomonas cellulose-binding domains mediate their effects by increasing enzyme substrate proximity. Biochem. J. 1998, 331, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Haynes, C.A.; Norde, W. Globular proteins at solid/liquid interfaces. Colloids Surf. B Biointerfaces 1994, 2, 517–566. [Google Scholar] [CrossRef]
- Li, M.; Lu, S.; Wu, Z.; Yan, H.; Mo, J.; Wang, L. Study on porous silk fibroin materials. I. Fine structure of freeze dried silk fibroin. J. Appl. Polym. Sci. 2001, 79, 2185–2191. [Google Scholar] [CrossRef]
- Li, M.; Wu, Z.; Zhang, C.; Lu, S.; Yan, H.; Huang, D.; Ye, H. Study on porous silk fibroin materials. II. Preparation and characteristics of spongy porous silk fibroin materials. J. Appl. Polym. Sci. 2001, 79, 2192–2199. [Google Scholar] [CrossRef]
- Nazarov, R.; Hyoung-Joon, J.; Kaplan, D.L. Porous 3D scaffolds from regenerated Antheraea pernyi silk fibroin. Biomacromolecules 2004, 5, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Svagan, A.J.; Samir, M.S.; Berglund, L.A. Biomimetic foams of high mechanical performance based on nanostructured cell walls reinforced by native cellulose nanofibrils. Adv. Mater. 2008, 20, 1263–1269. [Google Scholar]
- Wang, X.; Kluge, J.A.; Leisk, G.G.; Kaplan, D.L. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials 2008, 29, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Shmelev, K.; Sun, L.; Gil, E.S.; Park, S.H.; Cebe, P.; Kaplan, D.L. Regulation of silk material structure by temperature-controlled water vapor annealing. Biomacromolecules 2011, 12, 1686–1696. [Google Scholar] [CrossRef] [PubMed]
- Cunniff, P.; Fossey, S. Mechanical and thermal properties of dragline silk from the spider Nephila clavipes. Polym. Adv. Technol. 1994, 5, 401–410. [Google Scholar] [CrossRef]
- Guess, K.B.; Viney, C. Thermal analysis of major ampullate (drag line) spider silk: The effect of spinning rate on tensile modulus. Thermochim. Acta 1998, 315, 61–66. [Google Scholar] [CrossRef]
- Magoshi, J.; Nakamura, S. Studies on physical properties and structure of silk. Glass transition and crystallization of silk fibroin. J. Appl. Polym. Sci. 1975, 19, 1013–1015. [Google Scholar] [CrossRef]
- Xu, Y.; Foong, F.C. Characterization of a cellulose binding domain from Clostridium cellulovorans endoglucanase-xylanase D and its use as a fusion partner for soluble protein expression in Escherichia coli. J. Biotechnol. 2008, 135, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Craig, S.J.; Shu, A.; Xu, Y.; Foong, F.C.; Nordon, R. Chimeric protein for selective cell attachment onto cellulosic substrates. Protein Eng. Des. Sel. 2007, 20, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.; O’Brien, J. Molecular weight distribution of Nephila clavipes dragline silk. Macromolecules 1995, 28, 5975–5977. [Google Scholar] [CrossRef]
- Sponner, A.; Schlott, B.; Vollrath, F.; Unger, E.; Grosse, F.; Weisshart, K. Characterization of the protein components of Nephila clavipes dragline silk. Biochemistry 2005, 44, 4727–4736. [Google Scholar] [CrossRef] [PubMed]
- Hedhammar, M.; Rising, A.; Grip, S.; Martinez, A.S.; Nordling, K.; Casals, C.; Stark, M.; Johansson, J. Structural properties of recombinant nonrepetitive and repetitive parts of major ampullate spidroin 1 from Euprosthenops australis: Implications for fiber formation. Biochemistry 2008, 47, 3407–3417. [Google Scholar] [CrossRef] [PubMed]
- Askarieh, G.; Hedhammar, M.; Nordling, K.; Saenz, A.; Casala, C.; Rising, A.; Johansson, J.; Knight, D.S. Self-assembly of spider silk proteins is controlled by a pH-sensitive relay. Nature 2010, 465, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Hagn, F.; Eisoldt, L.; Hardy, J.G.; Vendrely, C.; Coles, M.; Scheibel, T.; Kessler, H. A conserved spider silk domain acts as a molecular switch that controls fibre assembly. Nature 2010, 465, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Bini, E.; Foo, C.W.; Huang, J.; Karageorgiou, V.; Kitchel, B.; Kaplan, D.L. RGD-Functionalized Bioengineered Spider Dragline Silk Biomaterial. Biomacromolecules 2006, 7, 3139–3145. [Google Scholar] [CrossRef] [PubMed]
- Nadler, M.; Steiner, A.; Dvir, T.; Szekely, O.; Szekely, P.; Ginsburg, A.; Asor, R.; Resh, R.; Tamburu, C.; Peres, M.; et al. Following the structural changes during zinc-induced crystallization of charged membranes using time-resolved solution X-ray scattering. Soft Matter 2011, 7, 1512–1523. [Google Scholar] [CrossRef]
- Székely, P.; Ginsburg, A.; Ben-Nun, T.; Raviv, U. Solution X-ray scattering form factors of supramolecular self-assembled structures. Langmuir 2010, 26, 13110–13129. [Google Scholar] [CrossRef] [PubMed]

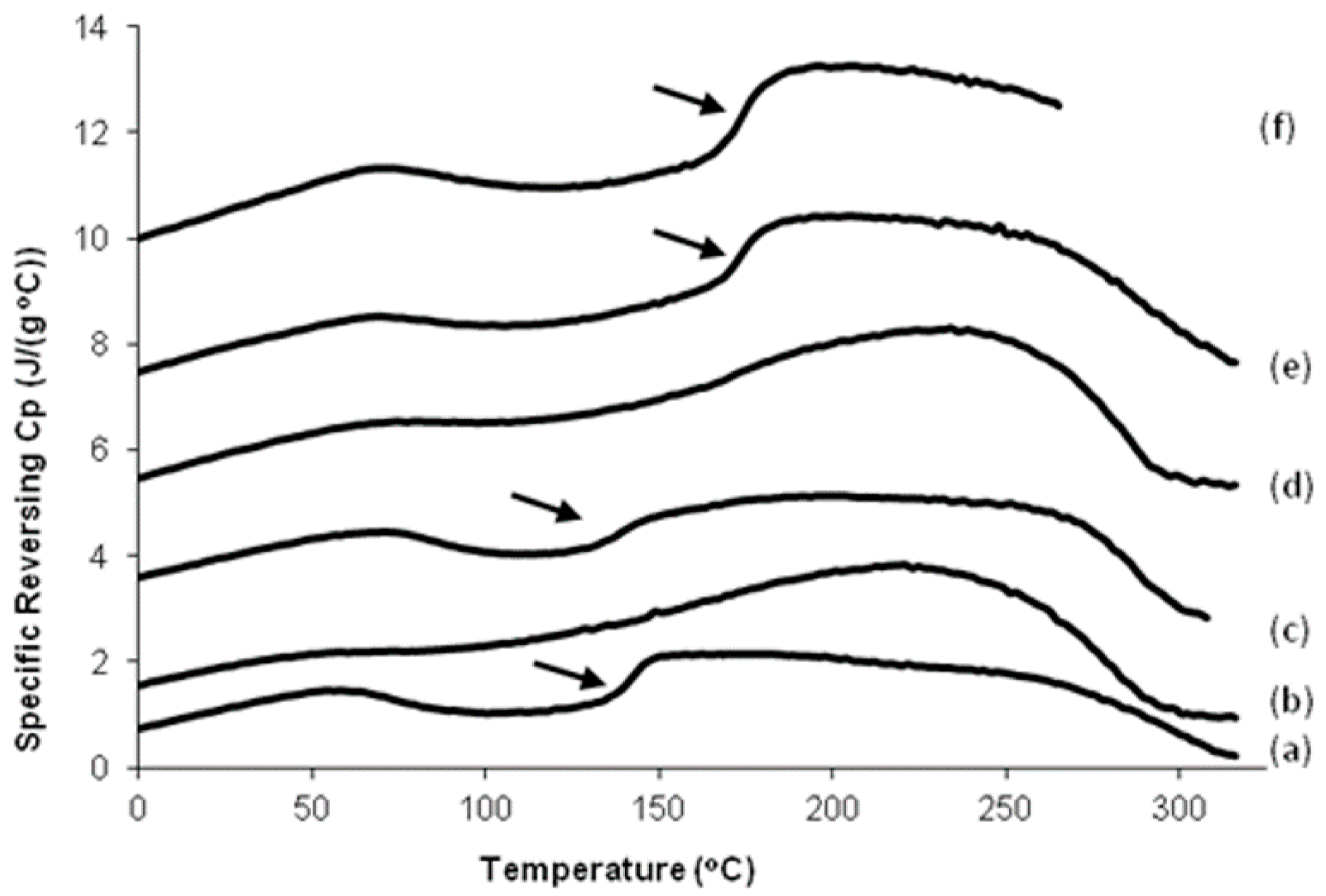


| Sample | Glass Transition Temperature (Tg °C) | Degradation Temperature (°C) |
|---|---|---|
| 100% silk | 140.5 | 283 |
| 100% silk-CBD | 172 | 279 |
| 75% silk 25% CNC | 138 | 255 |
| 75% silk-CBD 25% CNC | 174 | 269 |
| 25% silk 75% CNC | 163 | 231 |
| 25% silk-CBD 75% CNC | 178 | 237 |
| Silk | Sonicated Silk | Silk-CBD | Sonicated Silk-CBD | ||||
|---|---|---|---|---|---|---|---|
| diameter (nm) | 2.78 ± 0.04 | 3.8 ± 0.11 | 825 ± 401 | 55 ± 36 | 259 ± 30 | 96 ± 8 | 2048 ± 691 |
| % volume | 100 | 99.9 | 0.1 | 32 | 68 | 64 | 36 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meirovitch, S.; Shtein, Z.; Ben-Shalom, T.; Lapidot, S.; Tamburu, C.; Hu, X.; Kluge, J.A.; Raviv, U.; Kaplan, D.L.; Shoseyov, O. Spider Silk-CBD-Cellulose Nanocrystal Composites: Mechanism of Assembly. Int. J. Mol. Sci. 2016, 17, 1573. https://doi.org/10.3390/ijms17091573
Meirovitch S, Shtein Z, Ben-Shalom T, Lapidot S, Tamburu C, Hu X, Kluge JA, Raviv U, Kaplan DL, Shoseyov O. Spider Silk-CBD-Cellulose Nanocrystal Composites: Mechanism of Assembly. International Journal of Molecular Sciences. 2016; 17(9):1573. https://doi.org/10.3390/ijms17091573
Chicago/Turabian StyleMeirovitch, Sigal, Zvi Shtein, Tal Ben-Shalom, Shaul Lapidot, Carmen Tamburu, Xiao Hu, Jonathan A. Kluge, Uri Raviv, David L. Kaplan, and Oded Shoseyov. 2016. "Spider Silk-CBD-Cellulose Nanocrystal Composites: Mechanism of Assembly" International Journal of Molecular Sciences 17, no. 9: 1573. https://doi.org/10.3390/ijms17091573
APA StyleMeirovitch, S., Shtein, Z., Ben-Shalom, T., Lapidot, S., Tamburu, C., Hu, X., Kluge, J. A., Raviv, U., Kaplan, D. L., & Shoseyov, O. (2016). Spider Silk-CBD-Cellulose Nanocrystal Composites: Mechanism of Assembly. International Journal of Molecular Sciences, 17(9), 1573. https://doi.org/10.3390/ijms17091573








