Targeted Therapies for Brain Metastases from Breast Cancer
Abstract
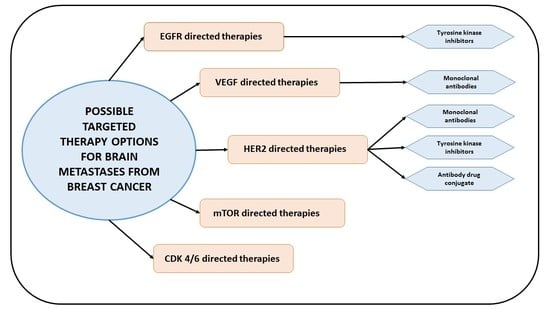
:1. Introduction
2. HER2 Pathway in Breast Cancer Brain Metastases (BCBM)
3. Vascular Endothelial Growth Factor (VEGF) Pathway in BCBM
4. PI3K/Akt/mTOR Pathway in BCBM
5. Epidermal Growth Factor Receptor (EGFR) Pathway in BCBM
6. CDK-4/6 Inhibitors in BCBM
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lin, N.U.; Amiri-Kordestani, L.; Palmieri, D.; Liewehr, D.J.; Steeg, P.S. CNS metastases in breast cancer: Old challenge, new frontiers. Clin. Cancer Res. 2013, 19, 6404–6418. [Google Scholar] [CrossRef] [PubMed]
- Arslan, C.; Dizdar, O.; Altundag, K. Systemic treatment in breast-cancer patients with brain metastasis. Expert Opin. Pharmacother. 2010, 11, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Smid, M.; Wang, Y.; Zhang, Y.; Sieuwerts, A.M.; Yu, J.; Klijn, J.G.M.; Foekens, J.A.; Martens, J.W.M. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008, 68, 3108–3114. [Google Scholar] [CrossRef] [PubMed]
- Leone, J.P.; Leone, B.A. Breast cancer brain metastases: The last frontier. Exp. Hematol. Oncol. 2015, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Venur, V.A.; Ahluwalia, M.S. Prognostic scores for brain metastasis patients: Use in clinical practice and trial design. Chin. Clin. Oncol. 2015, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Sperduto, P.W.; Berkey, B.; Gaspar, L.E.; Mehta, M.; Curran, W. A new prognostic index and comparison to three other indices for patients with brain metastases: An analysis of 1,960 patients in the rtog database. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Sperduto, P.W.; Kased, N.; Roberge, D.; Xu, Z.; Shanley, R.; Luo, X.; Sneed, P.K.; Chao, S.T.; Weil, R.J.; Suh, J.; et al. Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Sperduto, P.W.; Kased, N.; Roberge, D.; Xu, Z.; Shanley, R.; Luo, X.; Sneed, P.K.; Chao, S.T.; Weil, R.J.; Suh, J.; et al. Summary report on the graded prognostic assessment: An accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J. Clin. Oncol. 2012, 30, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Patchell, R.A.; Tibbs, P.A.; Walsh, J.W.; Dempsey, R.J.; Maruyama, Y.; Kryscio, R.J.; Markesbery, W.R.; Macdonald, J.S.; Young, B. A randomized trial of surgery in the treatment of single metastases to the brain. N. Engl. J. Med. 1990, 322, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Vecht, C.J.; Haaxma-Reiche, H.; Noordijk, E.M.; Padberg, G.W.; Voormolen, J.H.; Hoekstra, F.H.; Tans, J.T.; Lambooij, N.; Metsaars, J.A.; Wattendorff, A.R.; et al. Treatment of single brain metastasis: Radiotherapy alone or combined with neurosurgery? Ann. Neurol. 1993, 33, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Dempsey, R.J.; Mohiuddin, M.; Kryscio, R.J.; Markesbery, W.R.; Foon, K.A.; Young, B. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA 1998, 280, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Asher, A.L.; Ballman, K.V.; Farace, E.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Barker, F.G.; Deming, R.L.; Burri, S.; et al. NCCTG N0574 (alliance): A phase III randomized trial of whole brain radiation therapy (WBRT) in addition to radiosurgery (SRS) in patients with 1 to 3 brain metastases. In Proceedings of the 2015 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, USA, 29 May–2 June 2015.
- Deeken, J.F.; Loscher, W. The blood-brain barrier and cancer: Transporters, treatment, and trojan horses. Clin. Cancer Res. 2007, 13, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- De Vries, N.A.; Zhao, J.; Kroon, E.; Buckle, T.; Beijnen, J.H.; van Tellingen, O. P-glycoprotein and breast cancer resistance protein: Two dominant transporters working together in limiting the brain penetration of topotecan. Clin. Cancer Res. 2007, 13, 6440–6449. [Google Scholar] [CrossRef] [PubMed]
- Yonemori, K.; Tsuta, K.; Ono, M.; Shimizu, C.; Hirakawa, A.; Hasegawa, T.; Hatanaka, Y.; Narita, Y.; Shibui, S.; Fujiwara, Y. Disruption of the blood brain barrier by brain metastases of triple-negative and basal-type breast cancer but not HER2/neu-positive breast cancer. Cancer 2010, 116, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Witzel, I.; Oliveira-Ferrer, L.; Pantel, K.; Muller, V.; Wikman, H. Breast cancer brain metastases: Biology and new clinical perspectives. Breast Cancer Res. 2016, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, A.S.; Venur, V.A.; Preusser, M.; Ahluwalia, M.S. Immune checkpoint inhibitors in brain metastases: From biology to treatment. In Proceedings of the 2016 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, USA, 3–7 June 2016; pp. 116–122.
- Romond, E.H.; Perez, E.A.; Bryant, J.; Suman, V.J.; Geyer, C.E., Jr.; Davidson, N.E.; Tan-Chiu, E.; Martino, S.; Paik, S.; Kaufman, P.A.; et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 2005, 353, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- Leyland-Jones, B. Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases. J. Clin. Oncol. 2009, 27, 5278–5286. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, D.; Bronder, J.L.; Herring, J.M.; Yoneda, T.; Weil, R.J.; Stark, A.M.; Kurek, R.; Vega-Valle, E.; Feigenbaum, L.; Halverson, D.; et al. HER-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 2007, 67, 4190–4198. [Google Scholar] [CrossRef] [PubMed]
- Pestalozzi, B.C.; Brignoli, S. Trastuzumab in CSF. J. Clin. Oncol. 2000, 18, 2349–2351. [Google Scholar] [PubMed]
- Stemmler, H.J.; Schmitt, M.; Willems, A.; Bernhard, H.; Harbeck, N.; Heinemann, V. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs 2007, 18, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Dijkers, E.C.; Oude Munnink, T.H.; Kosterink, J.G.; Brouwers, A.H.; Jager, P.L.; de Jong, J.R.; van Dongen, G.A.; Schroder, C.P.; Lub-de Hooge, M.N.; de Vries, E.G. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther. 2010, 87, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Kurihara, H.; Yonemori, K.; Tsuda, H.; Suzuki, J.; Kono, Y.; Honda, N.; Kodaira, M.; Yamamoto, H.; Yunokawa, M.; et al. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J. Nucl. Med. 2013, 54, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Le Scodan, R.; Jouanneau, L.; Massard, C.; Gutierrez, M.; Kirova, Y.; Cherel, P.; Gachet, J.; Labib, A.; Mouret-Fourme, E. Brain metastases from breast cancer: Prognostic significance of HER-2 overexpression, effect of trastuzumab and cause of death. BMC Cancer 2011, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Park, I.H.; Ro, J.; Lee, K.S.; Nam, B.H.; Kwon, Y.; Shin, K.H. Trastuzumab treatment beyond brain progression in her2-positive metastatic breast cancer. Ann. Oncol. 2009, 20, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Baselga, J.; Kim, S.B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Baselga, J.; Miles, D.; Im, Y.H.; Quah, C.; Lee, L.F.; Cortes, J. Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: Results from the randomized phase III study CLEOPATRA. Ann. Oncol. 2014, 25, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Nieder, C.; Andratschke, N.; Grosu, A.L.; Molls, M. Recursive partitioning analysis (rpa) class does not predict survival in patients with four or more brain metastases. Strahlenther. Onkol. 2003, 179, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Krop, I.E.; Lin, N.U.; Blackwell, K.; Guardino, E.; Huober, J.; Lu, M.; Miles, D.; Samant, M.; Welslau, M.; Dieras, V. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: A retrospective, exploratory analysis in emilia. Ann. Oncol. 2015, 26, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Carey, L.A.; Liu, M.C.; Younger, J.; Come, S.E.; Ewend, M.; Harris, G.J.; Bullitt, E.; van den Abbeele, A.D.; Henson, J.W.; et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J. Clin. Oncol. 2008, 26, 1993–1999. [Google Scholar] [CrossRef] [PubMed]
- Bachelot, T.; Romieu, G.; Campone, M.; Dieras, V.; Cropet, C.; Dalenc, F.; Jimenez, M.; Le Rhun, E.; Pierga, J.Y.; Goncalves, A.; et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (landscape): A single-group phase 2 study. Lancet Oncol. 2013, 14, 64–71. [Google Scholar] [CrossRef]
- Cortes, J.; Dieras, V.; Ro, J.; Barriere, J.; Bachelot, T.; Hurvitz, S.; Le Rhun, E.; Espie, M.; Kim, S.B.; Schneeweiss, A.; et al. Afatinib alone or afatinib plus vinorelbine versus investigator’s choice of treatment for HER2-positive breast cancer with progressive brain metastases after trastuzumab, lapatinib, or both (LUX-breast 3): A randomised, open-label, multicentre, phase 2 trial. Lancet Oncol. 2015, 16, 1700–1710. [Google Scholar] [PubMed]
- Freedman, R.A.; Bullitt, E.; Sun, L.; Gelman, R.; Harris, G.; Ligibel, J.A.; Krop, I.E.; Partridge, A.H.; Eisenberg, E.; Winer, E.P.; et al. A phase II study of sagopilone (ZK 219477; ZK-EPO) in patients with breast cancer and brain metastases. Clin. Breast Cancer 2011, 11, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.Y.; Dieras, V.; Guardino, E.; et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Borges, V.F.; Ferrario, C.; Aucoin, N.; Falkson, C.I.; Khan, Q.J.; Krop, I.E.; Welch, S.; Bedard, P.L.; Conlin, A.K.; Chaves, J.; et al. Efficacy results of a phase 1b study of ont-380, a CNS-penetrant TKI, in combination with T-DM1 in HER2+ metastatic breast cancer (MBC), including patients (PTS) with brain metastases. In Proceedings of the 2016 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, USA, 3–7 June 2016.
- Mehta, M.P.; Paleologos, N.A.; Mikkelsen, T.; Robinson, P.D.; Ammirati, M.; Andrews, D.W.; Asher, A.L.; Burri, S.H.; Cobbs, C.S.; Gaspar, L.E.; et al. The role of chemotherapy in the management of newly diagnosed brain metastases: A systematic review and evidence-based clinical practice guideline. J. Neurooncol. 2010, 96, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Taskar, K.S.; Rudraraju, V.; Mittapalli, R.K.; Samala, R.; Thorsheim, H.R.; Lockman, J.; Gril, B.; Hua, E.; Palmieri, D.; Polli, J.W.; et al. Lapatinib distribution in HER2 overexpressing experimental brain metastases of breast cancer. Pharm. Res. 2012, 29, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Polli, J.W.; Humphreys, J.E.; Harmon, K.A.; Castellino, S.; O’mara, M.J.; Olson, K.L.; John-Williams, L.S.; Koch, K.M.; Serabjit-Singh, C.J. The role of efflux and uptake transporters in N-{3-chloro-4-[(3-fluorobenzyl) oxy] phenyl}-6-[5-({[2-(methylsulfonyl) ethyl] amino} methyl)-2-furyl]-4-quinazolinamine (GW572016, lapatinib) disposition and drug interactions. Drug Metab. Dispos. 2008, 36, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Dieras, V.; Paul, D.; Lossignol, D.; Christodoulou, C.; Stemmler, H.J.; Roche, H.; Liu, M.C.; Greil, R.; Ciruelos, E.; et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin. Cancer Res. 2009, 15, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; Lin, N.U. Updates on the management of breast cancer brain metastases. Oncology 2014, 28, 572–578. [Google Scholar] [PubMed]
- Freedman, R.A.; Gelman, R.S.; Wefel, J.S.; Melisko, M.E.; Hess, K.R.; Connolly, R.M.; van Poznak, C.H.; Niravath, P.A.; Puhalla, S.L.; Ibrahim, N.; et al. Translational breast cancer research consortium (TBCRC) 022: A phase II trial of neratinib for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J. Clin. Oncol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Freedman, R.A.; Miller, K.; Jhaveri, K.L.; Eiznhamer, D.A.; Berger, M.S.; Hamilton, E.P. Determination of the maximum tolerated dose (MTD) of the CNS penetrant tyrosine kinase inhibitor (TKI) tesevatinib administered in combination with trastuzumab in HER2+ patients with metastatic breast cancer (BC). In Proceedings of the 2016 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, USA, 3–7 June 2016.
- Miller, K.; Wang, M.; Gralow, J.; Dickler, M.; Cobleigh, M.; Perez, E.A.; Shenkier, T.; Cella, D.; Davidson, N.E. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N. Engl. J. Med. 2007, 357, 2666–2676. [Google Scholar] [CrossRef] [PubMed]
- Miles, D.W.; Dieras, V.; Cortes, J.; Duenne, A.A.; Yi, J.; O'Shaughnessy, J. First-line bevacizumab in combination with chemotherapy for HER2-negative metastatic breast cancer: Pooled and subgroup analyses of data from 2447 patients. Ann. Oncol. 2013, 24, 2773–2780. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Gelman, R.S.; Younger, W.J.; Sohl, J.; Freedman, R.A.; Sorensen, A.G.; Bullitt, E.; Harris, G.J.; Morganstern, D.; Schneider, B.P.; et al. Phase II trial of carboplatin (C) and bevacizumab (BEV) in patients (pts) with breast cancer brain metastases (BCBM). In Proceedings of the 2013 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, USA, 31 May–4 June 2013.
- Lu, Y.S.; Chen, T.W.; Lin, C.H.; Yeh, D.C.; Tseng, L.M.; Wu, P.F.; Rau, K.M.; Chen, B.B.; Chao, T.C.; Huang, S.M.; et al. Bevacizumab preconditioning followed by etoposide and cisplatin is highly effective in treating brain metastases of breast cancer progressing from whole-brain radiotherapy. Clin. Cancer Res. 2015, 21, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Samuels, Y.; Ericson, K. Oncogenic PI3K and its role in cancer. Curr. Opin. Oncol. 2006, 18, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Song, M.S.; Salmena, L.; Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor. Nat. Rev. Mol. Cell Biol. 2012, 13, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Loh, K.; Yap, Y.S. PI3K/Akt/mTOR inhibitors in breast cancer. Cancer Biol. Med. 2015, 12, 342–354. [Google Scholar] [PubMed]
- Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar]
- Baselga, J.; Campone, M.; Piccart, M.; Burris, H.A., III; Rugo, H.S.; Sahmoud, T.; Noguchi, S.; Gnant, M.; Pritchard, K.I.; Lebrun, F.; et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N. Engl. J. Med. 2012, 366, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Andre, F.; O’Regan, R.; Ozguroglu, M.; Toi, M.; Xu, B.; Jerusalem, G.; Masuda, N.; Wilks, S.; Arena, F.; Isaacs, C.; et al. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014, 15, 580–591. [Google Scholar] [CrossRef]
- Franz, D.N.; Belousova, E.; Sparagana, S.; Bebin, E.M.; Frost, M.; Kuperman, R.; Witt, O.; Kohrman, M.H.; Flamini, J.R.; Wu, J.Y.; et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2013, 381, 125–132. [Google Scholar] [CrossRef]
- Cox, M.C.; Dan, T.D.; Swain, S.M. Emerging drugs to replace current leaders in first-line therapy for breast cancer. Expert Opin. Emerg. Drugs 2006, 11, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Walshe, J.M.; Denduluri, N.; Berman, A.W.; Rosing, D.R.; Swain, S.M. A phase II trial with trastuzumab and pertuzumab in patients with HER2-overexpressed locally advanced and metastatic breast cancer. Clin. Breast Cancer 2006, 6, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Anders, L. Signaling through cyclin D-dependent kinases. Oncogene 2014, 33, 1890–1903. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.; Infante, J.R. Targeting CDK4/6 in patients with cancer. Cancer Treat. Rev. 2016, 45, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Ro, J.; André, F.; Loi, S.; Verma, S.; Iwata, H.; Harbeck, N.; Loibl, S.; Huang Bartlett, C.; Zhang, K.; et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 2015, 373, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Sahebjam, S.; Le Rhun, E.; Kulanthaivel, P.; Turner, P.K.; Klise, S.; Wang, H.T.; Tolaney, S.M. Assessment of concentrations of abemaciclib and its major active metabolites in plasma, CSF, and brain tumor tissue in patients with brain metastases secondary to hormone receptor positive (HR+) breast cancer. In Proceedings of the 2016 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, USA, 3–7 June 2016.
- Stemmler, H.J.; Heinemann, V. Central nervous system metastases in HER-2-overexpressing metastatic breast cancer: A treatment challenge. Oncology 2008, 13, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Kumthekar, P.; Tang, S.-C.; Brenner, A.J.; Kesari, S.; Piccioni, D.E.; Anders, C.K.; Carrillo, J.A.; Chalasani, P.; Kabos, P.; Puhalla, S.; et al. ANG1005, a novel brain-penetrant taxane derivative, for the treatment of recurrent brain metastases and leptomeningeal carcinomatosis from breast cancer. In Proceedings of the 2016 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, USA, 3–7 June 2016.
- Ramakrishna, N.; Temin, S.; Chandarlapaty, S.; Crews, J.R.; Davidson, N.E.; Esteva, F.J.; Giordano, S.H.; Gonzalez-Angulo, A.M.; Kirshner, J.J.; Krop, I.; et al. Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2014, 32, 2100–2108. [Google Scholar] [CrossRef] [PubMed]
| Study | Targeted Therapy Used | Trial Characteristics | Number of Patients | Local Control (%) | PFS (Months) | OS (Months) |
|---|---|---|---|---|---|---|
| Krop et al. [30] | Trastuzumab Emtasine (T-DM1) vs. lapatinib-capecitabine (XL) | Retrospective analysis of patients with CNS metastases in EMILIA trial | T-DM1: 45 | Not reported | 5.9 | 26.8 |
| XL: 50 | Not reported | 5.7 | 12.9 | |||
| Lin et al. [31] | Lapatinib | Phase II study of progressive brain metastases, all received prior trastuzumab | 39 | Not reported | PFS at 4 months: 18% | NR |
| Bachelot et al. (LANDSCAPE) [32] | Lapatinib + capecitabine | Phase II study for Newly diagnosed brain metastases | 45 | 65.9% | 5.5 | 17 |
| Cortes et al. [33] | Afatinib | Phase II three arm study. Arm A: afatinib; Arm B: afatinib plus vinorelbine; Arm C: investigators’ choice | Arm A: 40 | 12/40 (30%) | - | - |
| Arm B: 38 | 13/38 (34.2%) | - | - | |||
| Arm C: 43 | 18/43 (41.9%) | - | - | |||
| Freedman et al. [34] | Neratinib | Single arm phase II study in previously treated patients | 40 | - | 1.9 | 8.7 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venur, V.A.; Leone, J.P. Targeted Therapies for Brain Metastases from Breast Cancer. Int. J. Mol. Sci. 2016, 17, 1543. https://doi.org/10.3390/ijms17091543
Venur VA, Leone JP. Targeted Therapies for Brain Metastases from Breast Cancer. International Journal of Molecular Sciences. 2016; 17(9):1543. https://doi.org/10.3390/ijms17091543
Chicago/Turabian StyleVenur, Vyshak Alva, and José Pablo Leone. 2016. "Targeted Therapies for Brain Metastases from Breast Cancer" International Journal of Molecular Sciences 17, no. 9: 1543. https://doi.org/10.3390/ijms17091543
APA StyleVenur, V. A., & Leone, J. P. (2016). Targeted Therapies for Brain Metastases from Breast Cancer. International Journal of Molecular Sciences, 17(9), 1543. https://doi.org/10.3390/ijms17091543