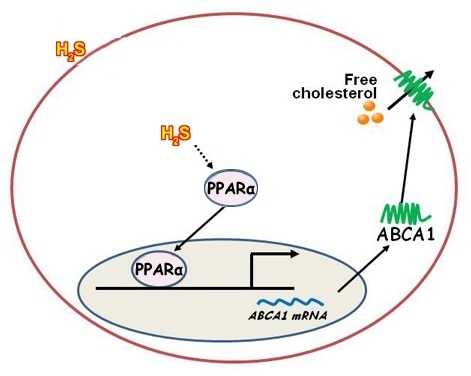
Hydrogen Sulfide Up-Regulates the Expression of ATP-Binding Cassette Transporter A1 via Promoting Nuclear Translocation of PPARα
Abstract
:1. Introduction
2. Results
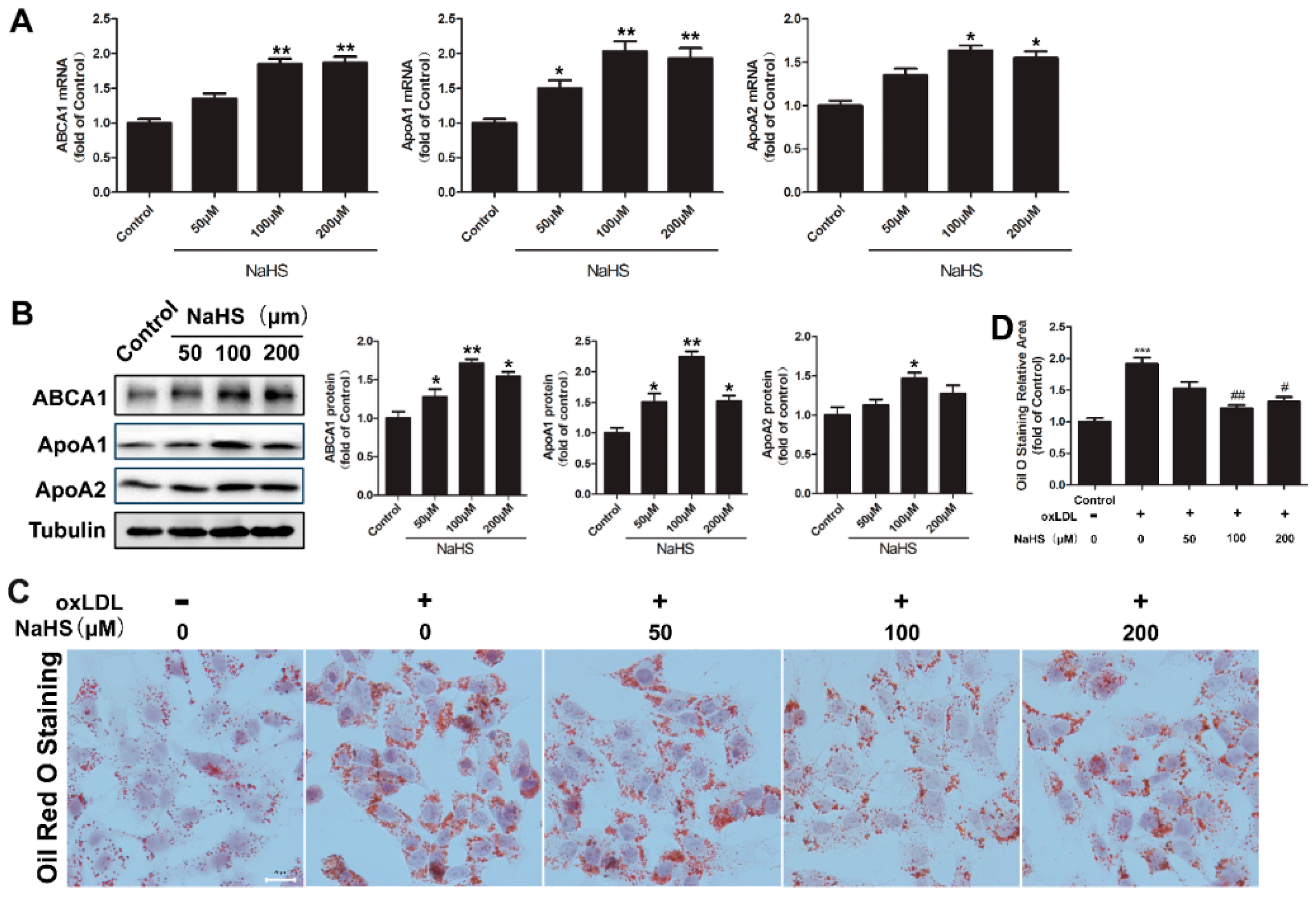
2.1. NaHS Up-Regulates ABCA1, ApoA1, and ApoA2 Expression and Decreases Lipid Accumulation in HepG2 Cells
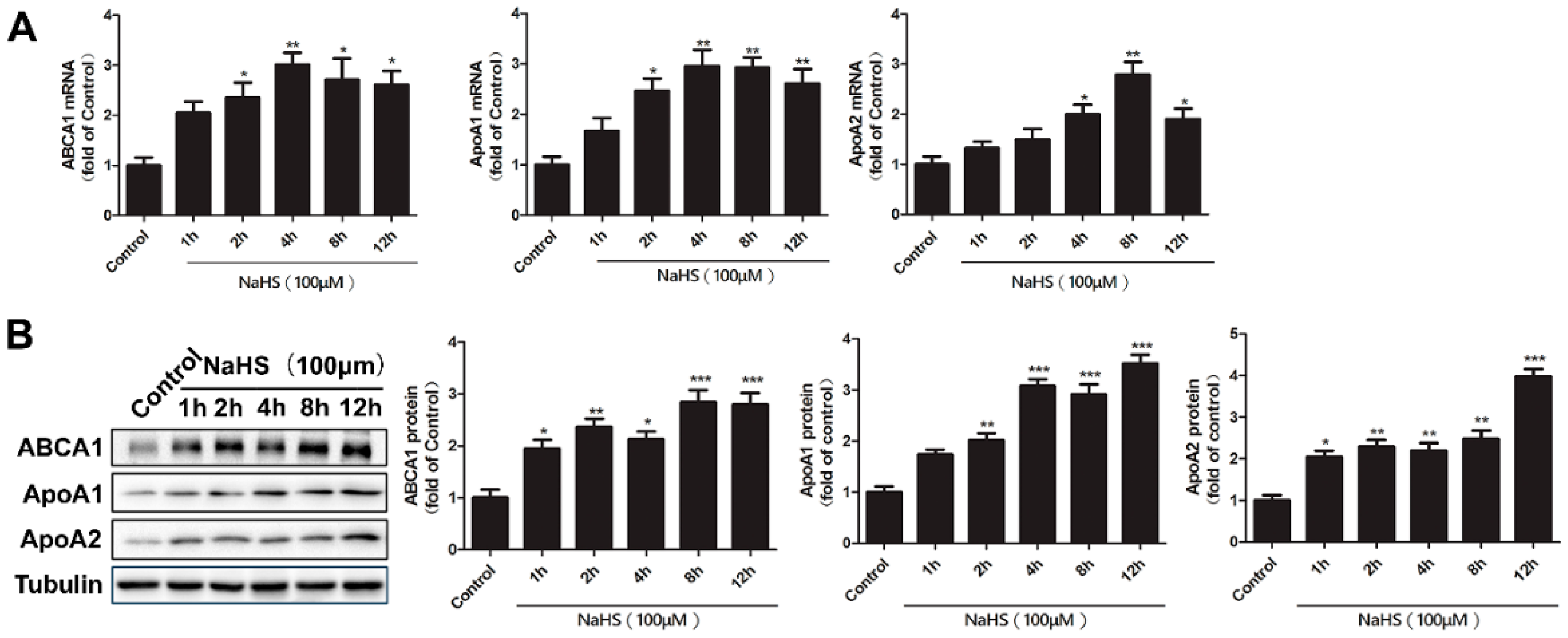
2.2. Time Course of NaHS Effects on ABCA1, ApoA1, and ApoA2 Gene and Protein Expression
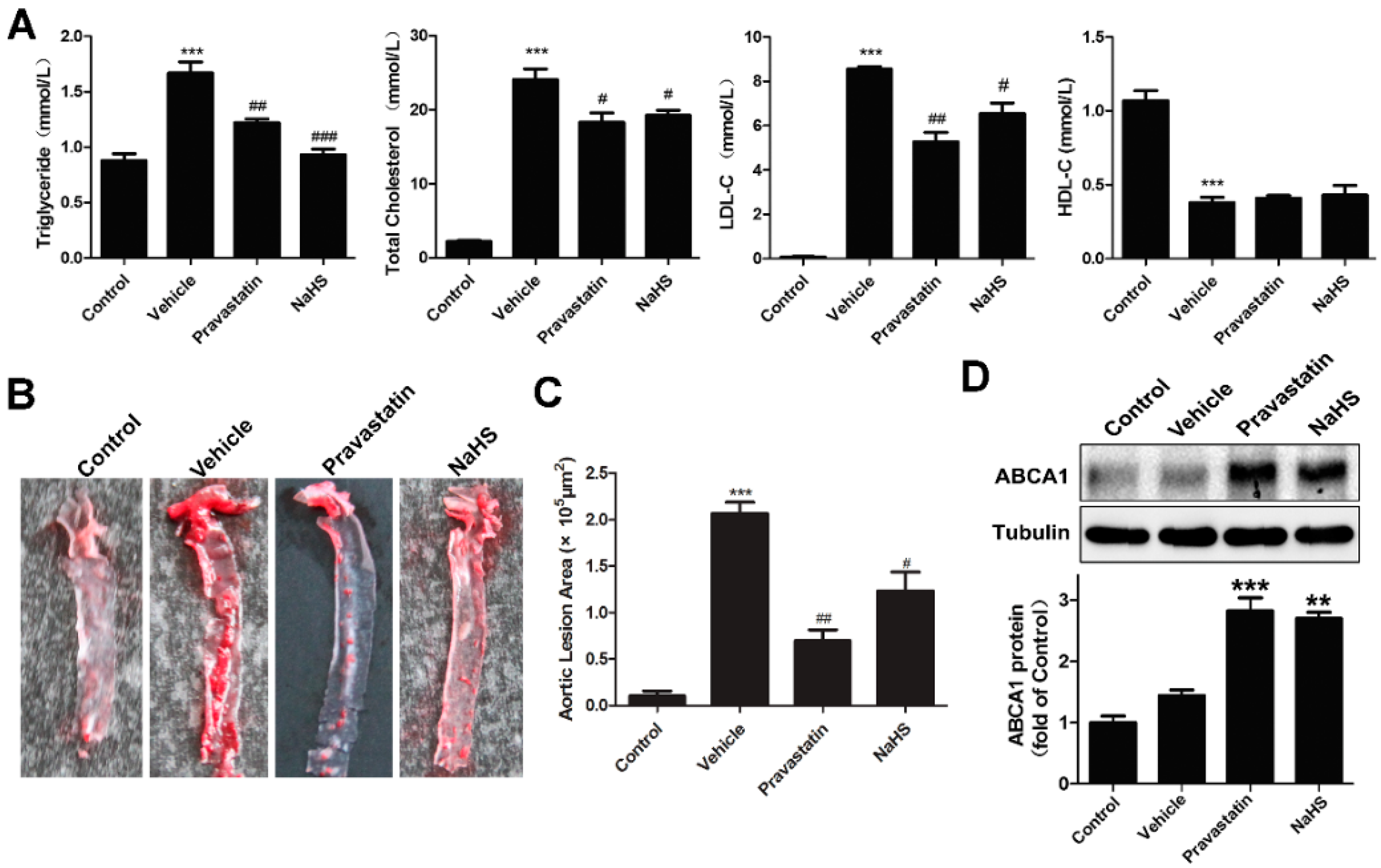
2.3. NaHS Alleviated Atherogenesis in High-Fat Dieted ApoE−/− Mice with Increased Liver ABCA1 Expression
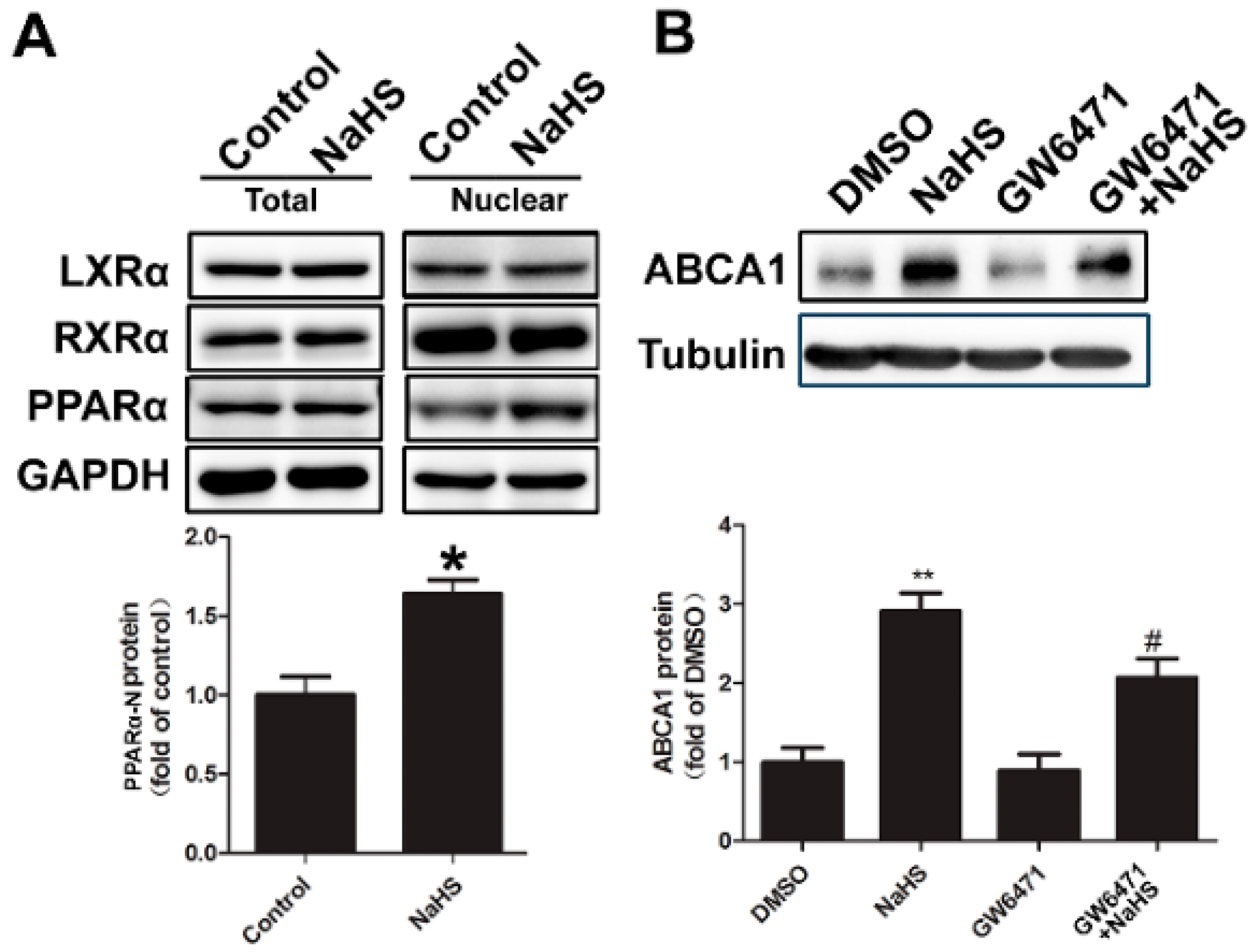
2.4. NaHS Upregulated ABCA1 Expression by Promoting PPARα Nuclear Translocation
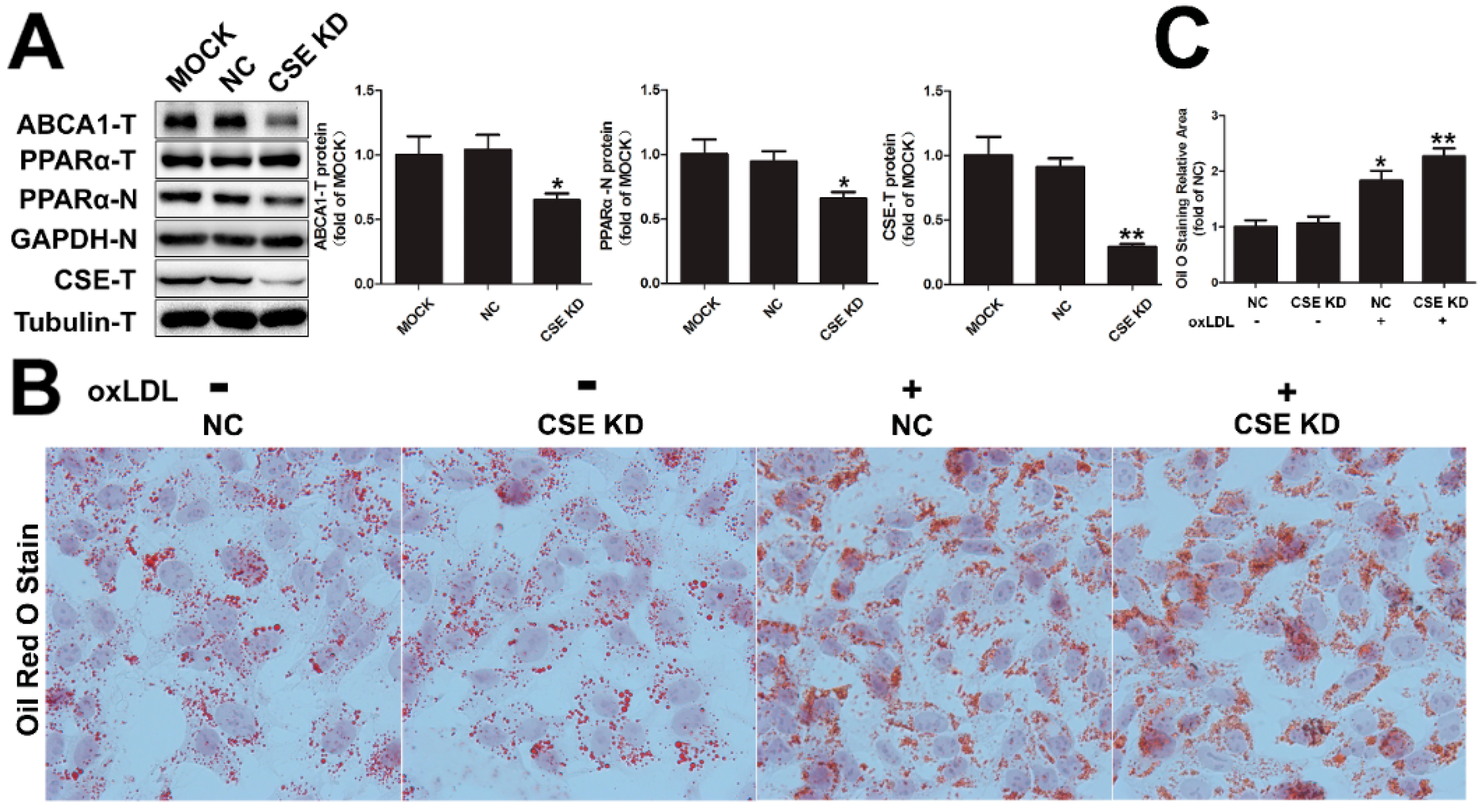
2.5. CSE Knockdown Decreased the Protein Level of ABCA1 by Inhibiting PPARα Nuclear Translocation
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture and NaHS Treatment
4.3. Animals and Biochemical Analysis
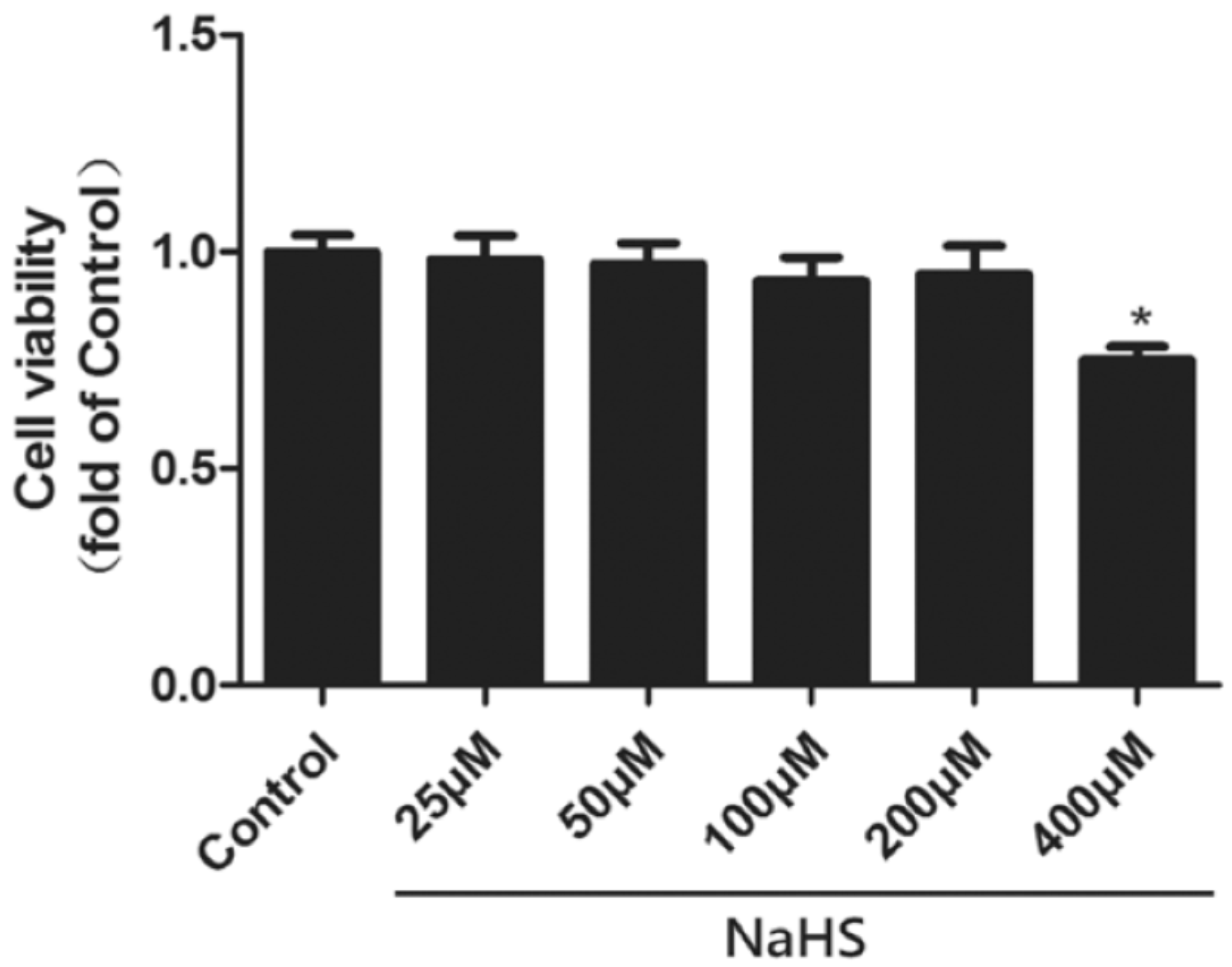
4.4. Cell Viability Assay
4.5. RNA Isolation and RT-Quantitative-PCR
4.6. Western Blotting Analysis
4.7. Oil Red O Staining
4.8. Small Interfering RNA
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
Appendix A
References
- Oram, F.J.; Vaughan, A.M. ATP-Binding cassette cholesterol transporters and cardiovascular disease. Circ. Res. 2006, 99, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Attie, A.D. ABCA1: At the nexus of cholesterol, HDL and atherosclerosis. Trends Biochem. Sci. 2007, 32, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, E.W.; Piehler, A.; Wenzel, J.J. ABCA-subfamily transporters: Structure, function and disease. Biochim. Biophys. Acta 2006, 1762, 510–524. [Google Scholar] [CrossRef] [PubMed]
- Oram, J.F.; Lawn, R.M.; Garvin, M.R.; Wade, D.P. ABCA1 is the cAMP-inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J. Biol. Chem. 2000, 275, 34508–34511. [Google Scholar] [CrossRef] [PubMed]
- Bodzioch, M.; Orsó, E.; Klucken, J.; Langmann, T.; Böttcher, A.; Diederich, W.; Drobnik, W.; Barlage, S.; Büchler, C.; Porsch-Ozcürümez, M.; et al. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat. Genet. 1999, 22, 347–351. [Google Scholar] [PubMed]
- Aiello, J.R.; Brees, D.; Francone, O.L. ABCA1-deficient mice: Insights into the role of monocyte lipid efflux in HDL formation and inflammation. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Vaisman, B.L.; Lambert, G.; Amar, M.; Joyce, C.; Ito, T.; Shamburek, R.D.; Cain, W.J.; Fruchart-Najib, J.; Neufeld, E.D.; Remaley, A.T.; et al. ABCA1 overexpression leads to hyperalphalipoproteinemia and increased biliary cholesterol excretion in transgenic mice. J. Clin. Investig. 2001, 108, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Wellington, C.L.; Brunham, L.R.; Zhou, S.; Singaraja, R.R.; Visscher, H.; Gelfer, A.; Ross, C.; James, E.; Liu, G.; Huber, M.T.; et al. Alterations of plasma lipids in mice via adenoviral-mediated hepatic overexpression of human ABCA1. J. Lipid Res. 2003, 44, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007, 6, 917–935. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Two’s company, three’s a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 2002, 16, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.; Cao, W.; Wu, L.; Wang, R. Hydrogen sulfide and the liver. Nitric Oxide 2014, 41, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.H.; Kwok, W.K.; To, K.F.; Lau, J.Y. Anti-atherogenic effect of hydrogen sulfide by over-expression of cystathionineγ-lyase (CSE) gene. PLoS ONE 2014, 9, e113038. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Han, Y.; Li, L.; Lu, H.; Meng, G.; Li, X.; Shirhan, M.; Peh, M.T.; Xie, L.; Zhou, S.; et al. The hydrogen sulfide donor, GYY4137, exhibits anti-atherosclerotic activity in high fat fed apolipoprotein E (−/−) mice. Br. J. Pharmacol. 2013, 169, 1795–1809. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.; Untereiner, A.; Wu, L.; Wang, R. Hydrogen sulfide and the pathogenesis of atherosclerosis. Antioxid. Redox Signal. 2014, 20, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Chaoshu, T.; Hongfang, J.; Junbao, D. Endogenous hydrogen sulfide is involved in the pathogenesis of atherosclerosis. Biochem. Biophys. Res. Commun. 2010, 396, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Vacek, T.P.; Rehman, S.; Neamtu, D.; Yu, S.; Givimani, S.; Tyagi, S.C. Matrix metalloproteinases in atherosclerosis: Role of nitric oxide, hydrogen sulfide, homocysteine, and polymorphisms. Vasc. Health Risk Manag. 2015, 11, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, X.; Jin, H.; Wei, H.; Li, W.; Bu, D.; Tang, X.; Ren, Y.; Tang, C.; Du, J. Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, C.; Wu, D.; Zhang, A.; Gu, T.; Wang, L.; Wang, C. Hydrogen sulfide inhibits the development of atherosclerosis with suppressing CX3CR1 and CX3CL1 expression. PLoS ONE 2012, 7, e41147. [Google Scholar] [CrossRef] [PubMed]
- Getz, G.S.; Reardon, C.A. ApoE knockout and knockin mice: The history of their contribution to the understanding of atherogenesis. J. Lipid Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Oram, J.F. Unsaturated fatty acids inhibit cholesterol efflux from macrophages by increasing degradation of ATP-binding cassette transporter A1. J. Biol. Chem. 2002, 277, 5692–5697. [Google Scholar] [CrossRef] [PubMed]
- Lund, G.E.; Menke, J.G.; Sparrow, C.P. Liver X receptor agonists as potential therapeutic agents for dyslipidemia and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Nagao, K.; Tomioka, M.; Ueda, K. Function and regulation of ABCA1—membrane meso-domain organization and reorganization. FEBS J. 2011, 278, 3190–3203. [Google Scholar] [CrossRef] [PubMed]
- Repa, J.J.; Turley, S.D.; Lobaccaro, J.A.; Medina, J.; Li, L.; Lustig, K.; Shan, B.; Heyman, R.A.; Dietschy, J.M.; Mangelsdorf, D.J. Regulation of absorption and abc1-mediated efflux of cholesterol by rxr heterodimers. Science 2000, 289, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Sartippour, R.M.; Renier, G. Differential regulation of macrophage peroxisome proliferator–activated receptor expression by glucose: Role of peroxisome proliferator–activated receptors in lipoprotein lipase gene expression. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Langmann, T. Transcriptional regulatory networks in lipid metabolism control ABCA1 expression. Biochim. Biophys. Acta 2005, 1735, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wellington, C.L.; Walker, E.K.; Suarez, A.; Kwok, A.; Bissada, N.; Singaraja, R.; Yang, Y.Z.; Zhang, L.H.; James, E.; Wilson, J.E.; et al. ABCA1 mRNA and protein distribution patterns predict multiple different roles and levels of regulation. Lab. Investig. 2002, 82, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Jeney, V.; Komódi, E.; Nagy, E.; Zarjou, A.; Vercellotti, G.M.; Eaton, J.W.; Balla, G.; Balla, J. Supression of hemin-mediated oxidation of low-density lipoprotein and subsequent endothelial reactions by hydrogen sulfide (H2S). Free Radic. Biol. Med. 2009, 46, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tang, C.; Jin, H.; Du, J. Regulatory effects of sulfur dioxide on the development of atherosclerotic lesions and vascular hydrogen sulfide in atherosclerotic rats. Atherosclerosis 2011, 215, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.; Li, H.; Untereiner, A.; Wu, L.; Yang, G.; Austin, R.C.; Dickhout, J.G.; Lhoták, Š.; Meng, Q.H.; Wang, R. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation 2013, 127, 2523–2534. [Google Scholar] [CrossRef] [PubMed]
- Peh, M.T.; Anwar, A.B.; Ng, D.S.; Atan, M.S.; Kumar, S.D.; Moore, P.K. Effect of feeding a high fat diet on hydrogen sulfide (H2S) metabolism in the mouse. Nitric Oxide 2014, 41, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, C.; Zhang, A.; Fan, Y.; Gu, T.; Wu, D.; Sparatore, A.; Wang, C. Effect of S-aspirin, a novel hydrogen-sulfide-releasing aspirin (ACS14), on atherosclerosis in apoE-deficient mice. Eur. J. Pharmacol. 2012, 697, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wu, L.; Bryan, S.; Khaper, N.; Mani, S.; Wang, R. Cystathionine γ-lyase deficiency and overproliferation of smooth muscle cells. Cardiovasc. Res. 2010, 86, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Z.; Wang, Z.; Li, G.H.; Wang, R.; Tan, J.M.; Cao, X.; Suo, R.; Jiang, Z.S. Hydrogen sulfide inhibits macrophage-derived foam cell formation. Exp. Biol. Med. 2011, 236, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Muzaffar, S.; Shukla, N.; Bond, M.; Newby, A.C.; Angelini, G.D.; Sparatore, A.; del Soldato, P.; Jeremy, J.Y. Exogenous hydrogen sulfide inhibits superoxide formation, nox-1 expression and rac1 activity in human vascular smooth muscle cells. J. Vasc. Res. 2008, 45, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Cheng, H.P.; Xie, W.; Zhang, M.; Liu, D.; Lan, G.; Huang, C.; Zhao, Z.W.; Chen, L.Y.; Yao, F. Cystathionine γ-lyase (CSE)/hydrogen sulfide system is regulated by miR-216a and influences cholesterol efflux in macrophages via the PI3K/AKT/ABCA1 pathway. Biochem. Biophys. Res. Commun. 2016, 470, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.J.; Plutzky, J. Peroxisome proliferator–Activated receptors as transcriptional nodal points and therapeutic targets. Circulation 2007, 115, 518–533. [Google Scholar] [CrossRef] [PubMed]
- Auboeuf, D.; Rieusset, J.; Fajas, L.; Vallier, P.; Frering, V.; Riou, J.P.; Staels, B.; Auwerx, J.; Laville, M.; Vidal, H. Tissue Distribution and quantification of the expression of mRNAs of peroxisome proliferator–activated receptors and liver X Receptor-α in humans: No alteration in adipose tissue of obese and NIDDM patients. Diabetes 1997, 46, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Chandra, V.; Huang, P.; Hamuro, Y.; Raghuram, S.; Wang, Y.; Burris, T.P.; Rastinejad, F. Structure of the intact PPAR-γ–RXR-α nuclear receptor complex on DNA. Nature 2008, 456, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Chinetti, G.; Lestavel, S.; Bocher, V.; Remaley, A.T.; Neve, B.; Torra, I.P.; Teissier, E.; Minnich, A.; Jaye, M.; Duverger, N.; et al. PPAR-αand PPAR-γ activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat. Med. 2001, 7, 53–58. [Google Scholar] [PubMed]
- Kockx, M.; Princen, H.M.G.; Kooistra, T. Fibrate-modulated expression of fibrinogen, plasminogen activator inhibitor-1 and apolipoprotein A-I in cultured cynomolgus monkey hepatocytes role of the peroxisome proliferator-activated receptor-α. Thromb. Haemost. 1998, 80, 942–948. [Google Scholar] [PubMed]
- Duez, H.; Chao, Y.S.; Hernandez, M.; Torpier, G.; Poulain, P.; Mundt, S.; Mallat, Z.; Teissier, E.; Burton, C.A.; Tedgui, A.; et al. Reduction of atherosclerosis by the peroxisome proliferator-activated receptor alpha agonist fenofibrate in mice. J. Biol. Chem. 2002, 277, 48051–48057. [Google Scholar] [CrossRef] [PubMed]
- Denis, M.; Haidar, B.; Marcil, M.; Bouvier, M.; Krimbou, L.; Genest, J., Jr. Molecular and cellular physiology of apolipoprotein A-I Lipidation by the ATP-binding cassette transporter A1 (ABCA1). J. Biol. Chem. 2004, 279, 7384–7394. [Google Scholar] [CrossRef] [PubMed]
- Krimbou, L.; Hajj Hassan, H.; Blain, S.; Rashid, S.; Denis, M.; Marcil, M.; Genest, J. Biogenesis and speciation of nascent apoA-I-containing particles in various cell lines. J. Lipid Res. 2005, 46, 1668–1677. [Google Scholar] [CrossRef] [PubMed]
- Basso, F.; Freeman, L.; Knapper, C.L.; Remaley, A.; Stonik, J.; Neufeld, E.B.; Tansey, T.; Amar, M.J.; Fruchart-Najib, J.; Duverger, N. Role of the hepatic ABCA1 transporter in modulating intrahepatic cholesterol and plasma HDL cholesterol concentrations. J. Lipid Res. 2003, 44, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Rothblat, H.G.; Phillips, M.C. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr. Opin. Lipidol. 2010, 21, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Vertommen, J.; van Gaal, L.; de Leeuw, I. Effects of pravastatin on lipid levels, in vitro oxidizability of non-HDL lipoproteins and microalbuminuria in IDDM patients. Diabetes Res. Clin. Pract. 1995, 29, 189–194. [Google Scholar] [CrossRef]
- Pan, L.L.; Liu, X.H.; Shen, Y.Q.; Wang, N.Z.; Xu, J.; Wu, D.; Xiong, Q.H.; Deng, H.Y.; Huang, G.Y.; Zhu, Y.Z. Inhibition of NADPH oxidase 4-related signaling by sodium hydrosulfide attenuates myocardial fibrotic response. Int. J. Cardiol. 2013, 168, 3770–3778. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Hao, D.D.; Sun, J.P.; Li, W.W.; Zhao, M.M.; Li, X.H.; Chen, Y.; Zhu, J.H.; Ding, Y.J.; Liu, J.; et al. Hydrogen sulfide treatment promotes glucose uptake by increasing insulin receptor sensitivity and Ameliorates kidney lesions in Type 2 diabetes. Antioxid. Redox Signal. 2013, 19, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.L.; Wang, X.L.; Wang, X.L.; Zhu, Y.Z. Sodium hydrosulfide prevents myocardial dysfunction through modulation of extracellular matrix accumulation and vascular density. Int. J. Mol. Sci. 2014, 15, 23212–23226. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Xiong, Q.; Peng, J.; Hu, B.; Li, W.; Zhu, Y.; Shen, X. Hydrogen Sulfide Up-Regulates the Expression of ATP-Binding Cassette Transporter A1 via Promoting Nuclear Translocation of PPARα. Int. J. Mol. Sci. 2016, 17, 635. https://doi.org/10.3390/ijms17050635
Li D, Xiong Q, Peng J, Hu B, Li W, Zhu Y, Shen X. Hydrogen Sulfide Up-Regulates the Expression of ATP-Binding Cassette Transporter A1 via Promoting Nuclear Translocation of PPARα. International Journal of Molecular Sciences. 2016; 17(5):635. https://doi.org/10.3390/ijms17050635
Chicago/Turabian StyleLi, Dong, Qinghui Xiong, Jin Peng, Bin Hu, Wanzhen Li, Yizhun Zhu, and Xiaoyan Shen. 2016. "Hydrogen Sulfide Up-Regulates the Expression of ATP-Binding Cassette Transporter A1 via Promoting Nuclear Translocation of PPARα" International Journal of Molecular Sciences 17, no. 5: 635. https://doi.org/10.3390/ijms17050635
APA StyleLi, D., Xiong, Q., Peng, J., Hu, B., Li, W., Zhu, Y., & Shen, X. (2016). Hydrogen Sulfide Up-Regulates the Expression of ATP-Binding Cassette Transporter A1 via Promoting Nuclear Translocation of PPARα. International Journal of Molecular Sciences, 17(5), 635. https://doi.org/10.3390/ijms17050635