Synthetic Human TLR9-LRR11 Peptide Attenuates TLR9 Signaling by Binding to and thus Decreasing Internalization of CpG Oligodeoxynucleotides
Abstract
:1. Introduction
2. Results and Discussion
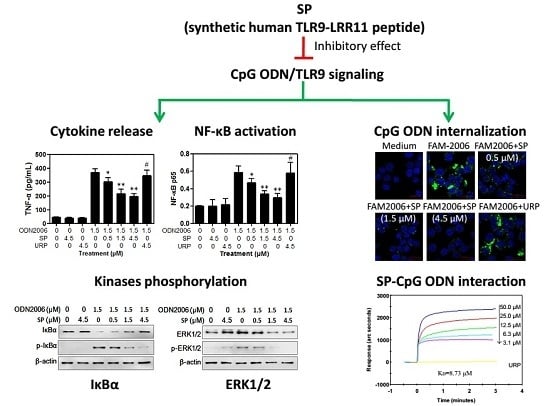
2.1. SP Inhibits CpG ODN-Induced Cytokine Release from RAW264.7 Cells
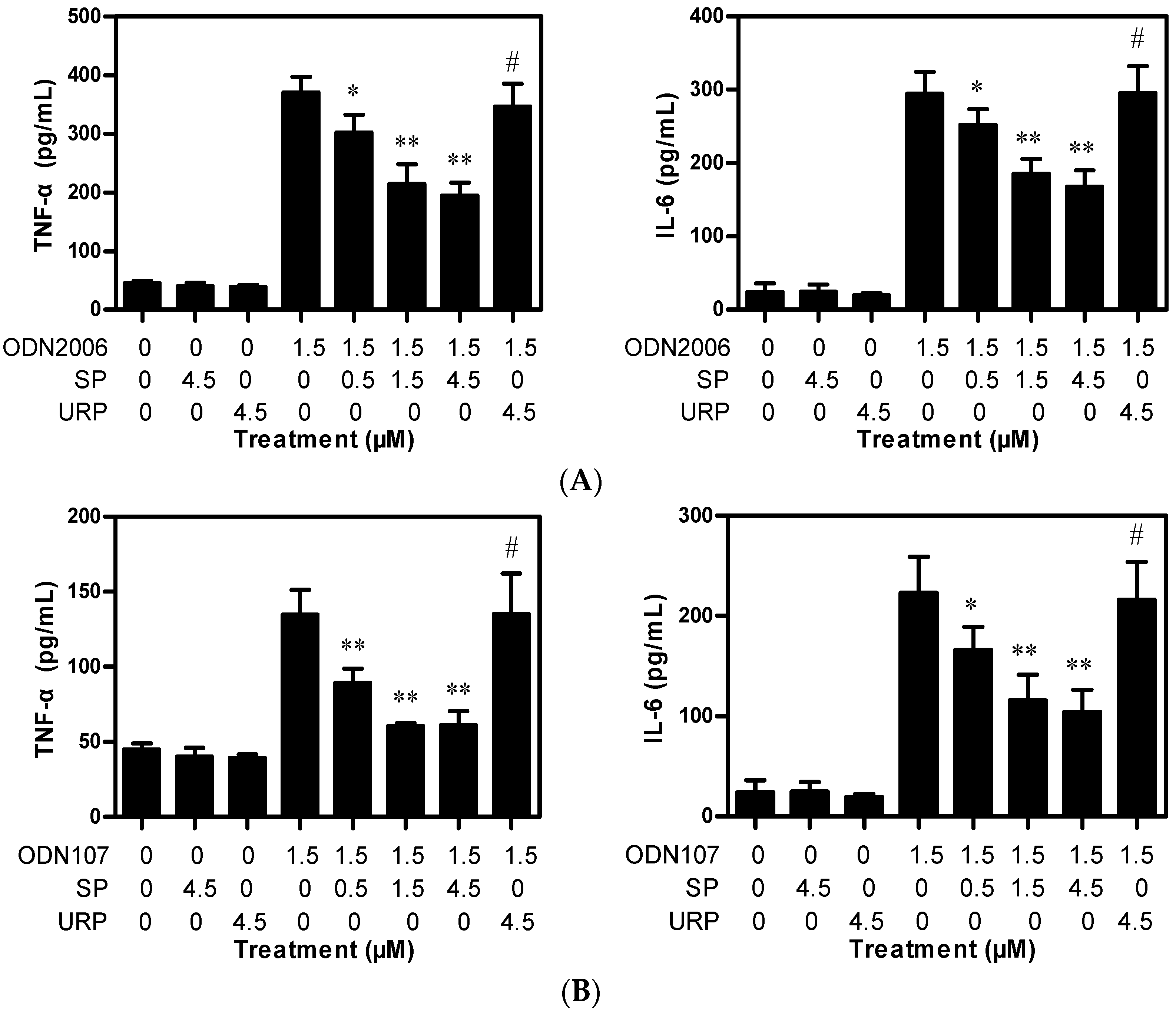
2.1.1. SP Decreases TNF-α and IL-6 Release Induced by CpG ODN from RAW264.7 Cells
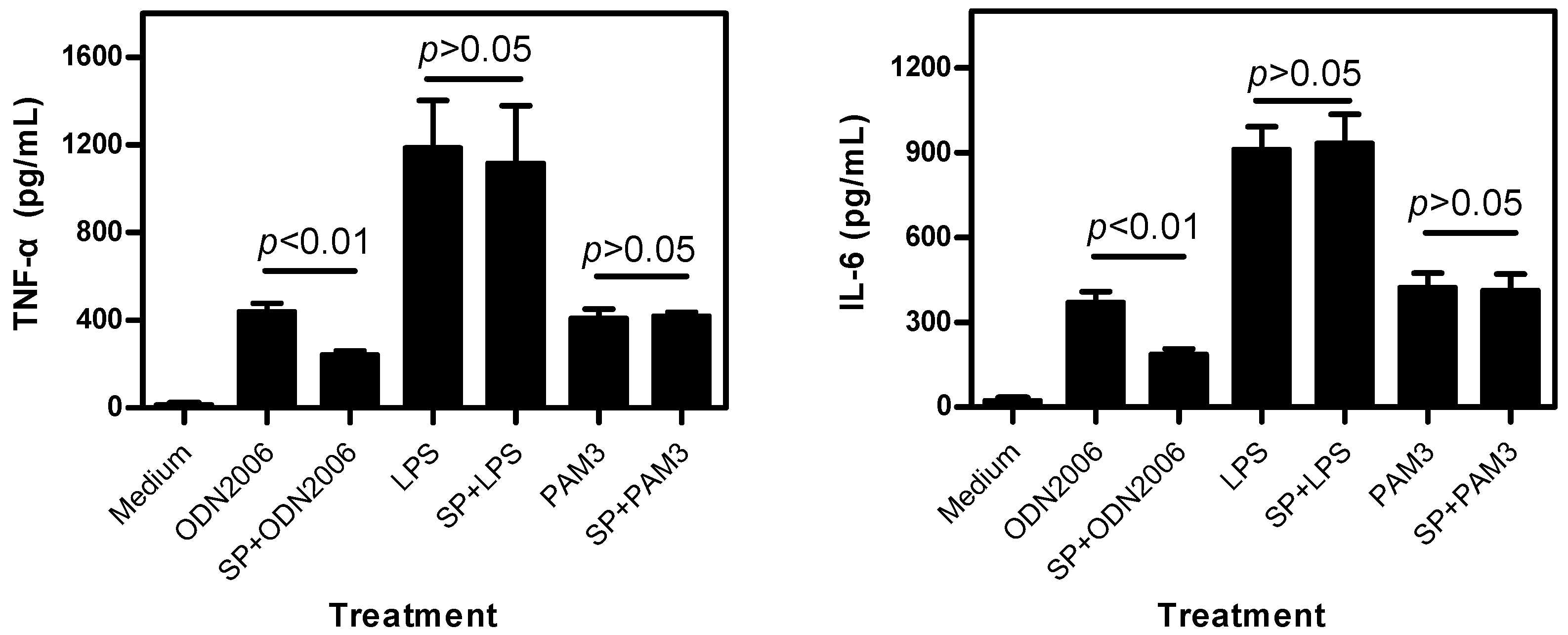
2.1.2. SP Inhibits CpG ODN-Induced, But Not LPS- and PAM3-Induced, Cytokine Release
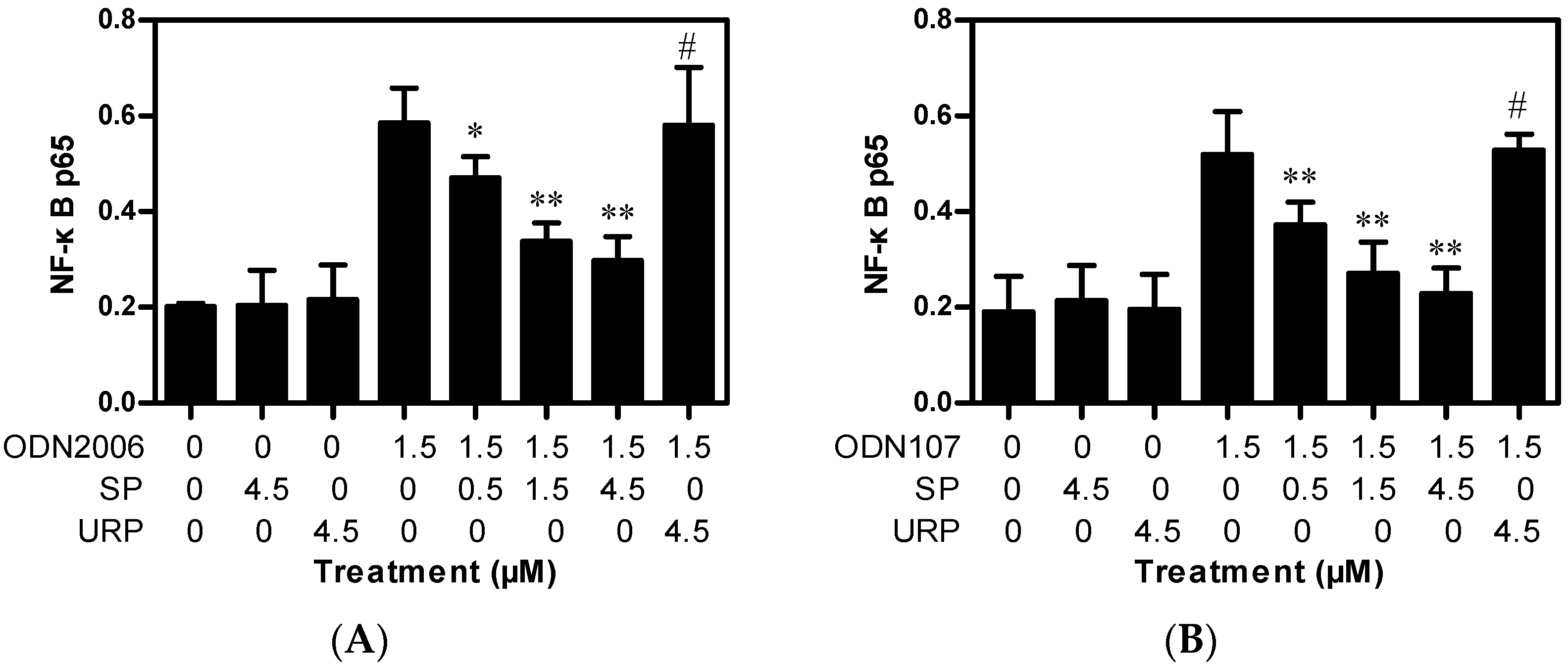
2.2. SP Inhibits CpG ODN-Induced NF-κB Activation
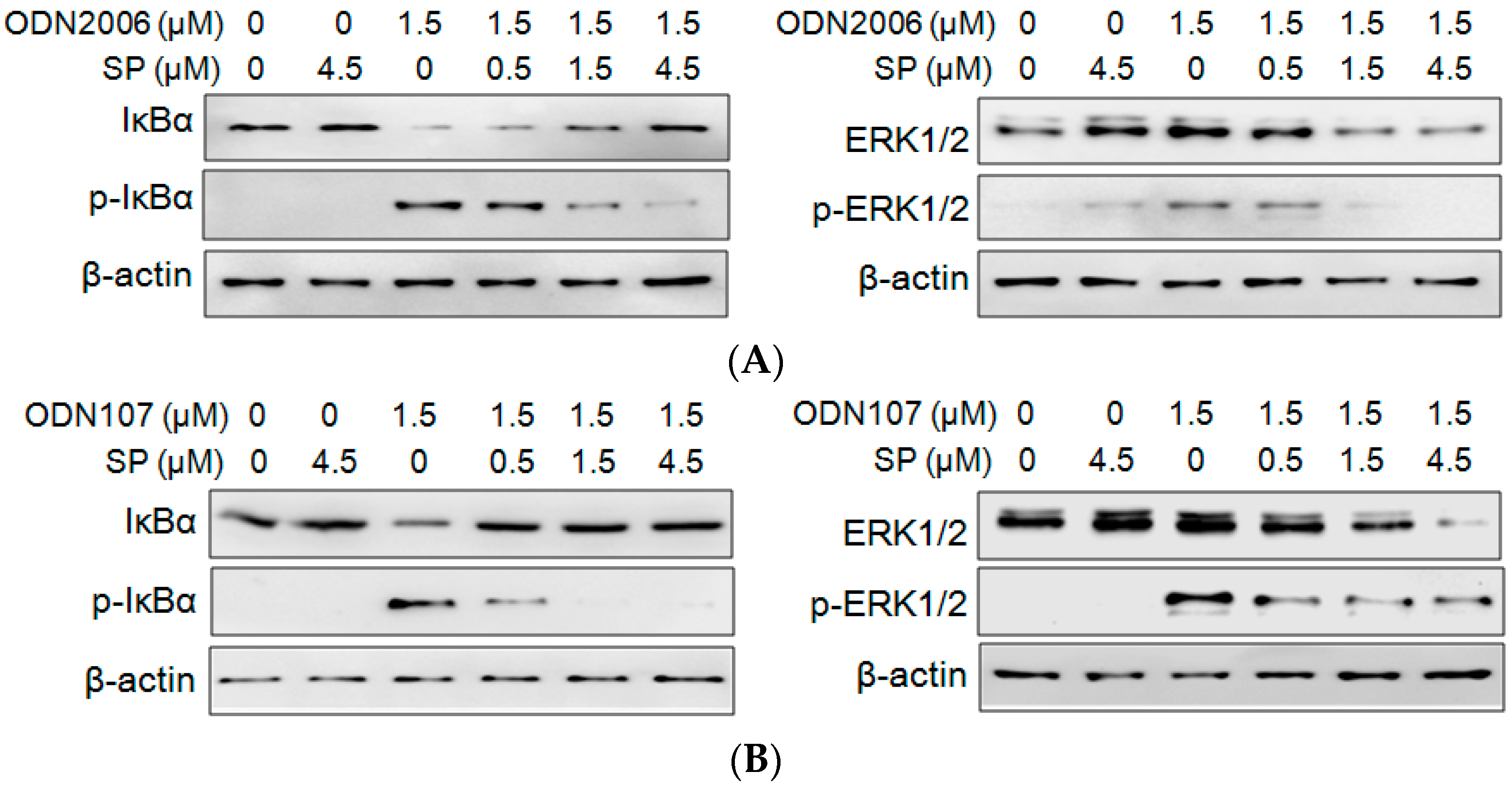
2.3. SP Inhibits CpG ODN-Induced IκBα and ERK Phosphorylation
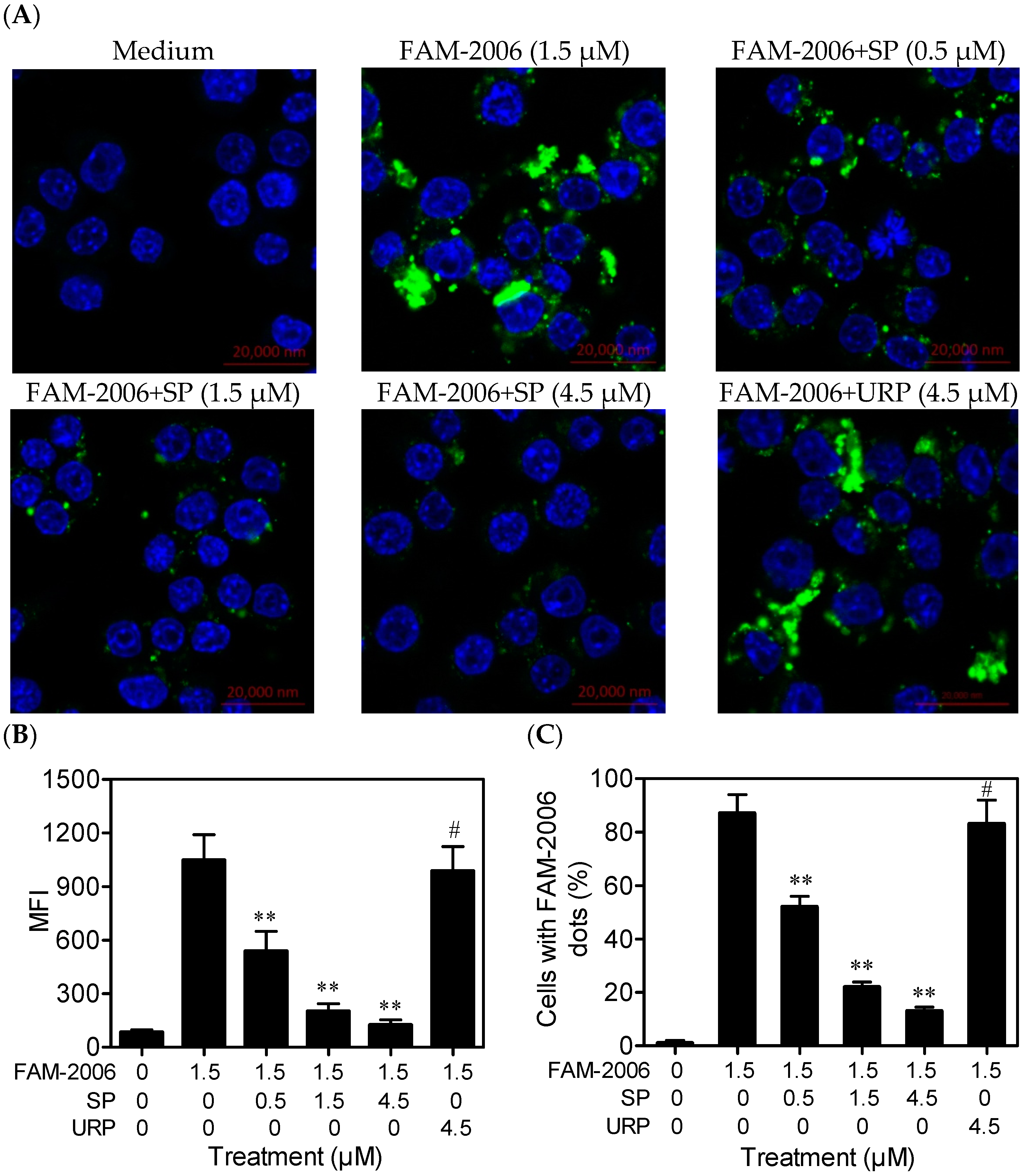
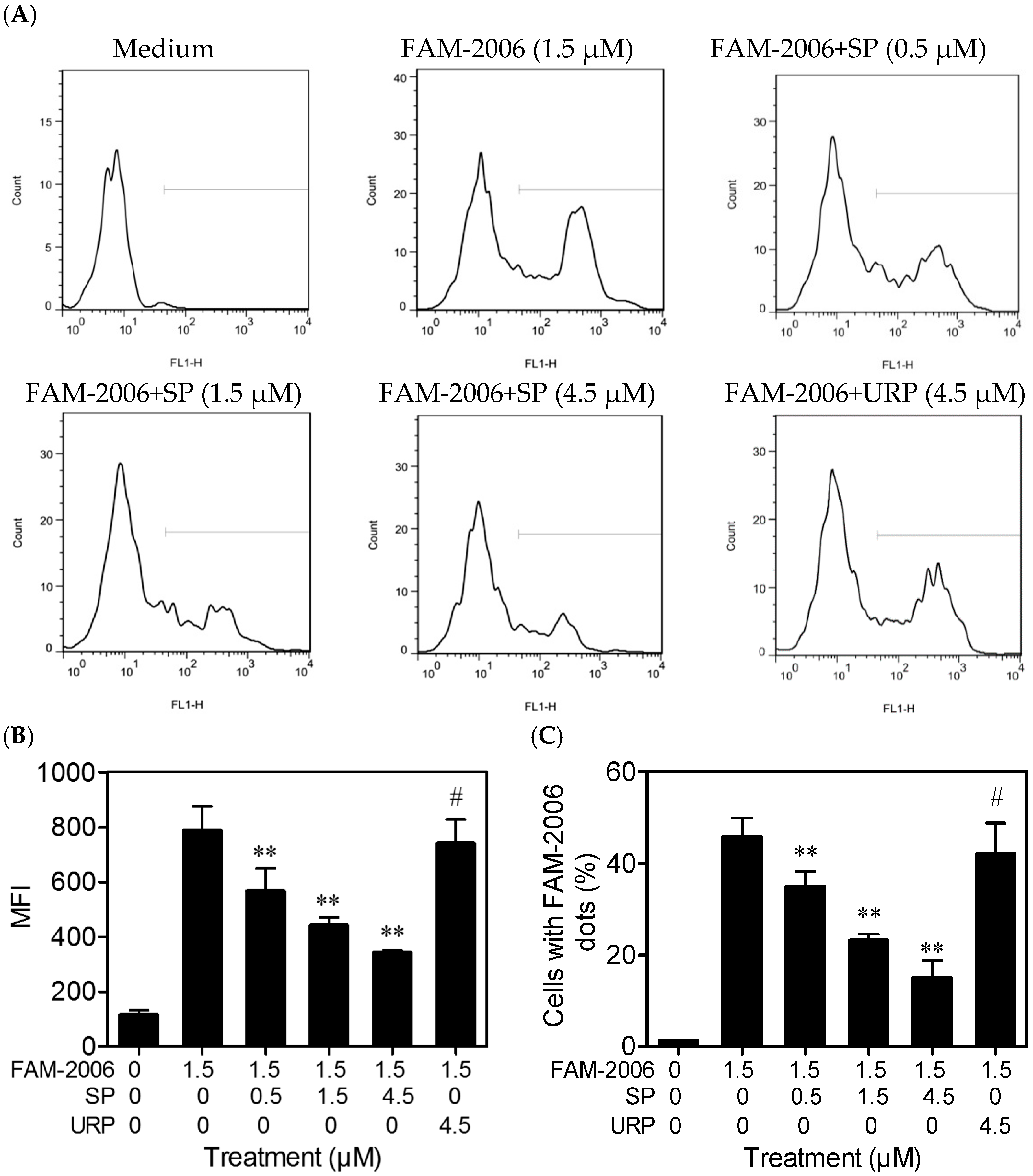
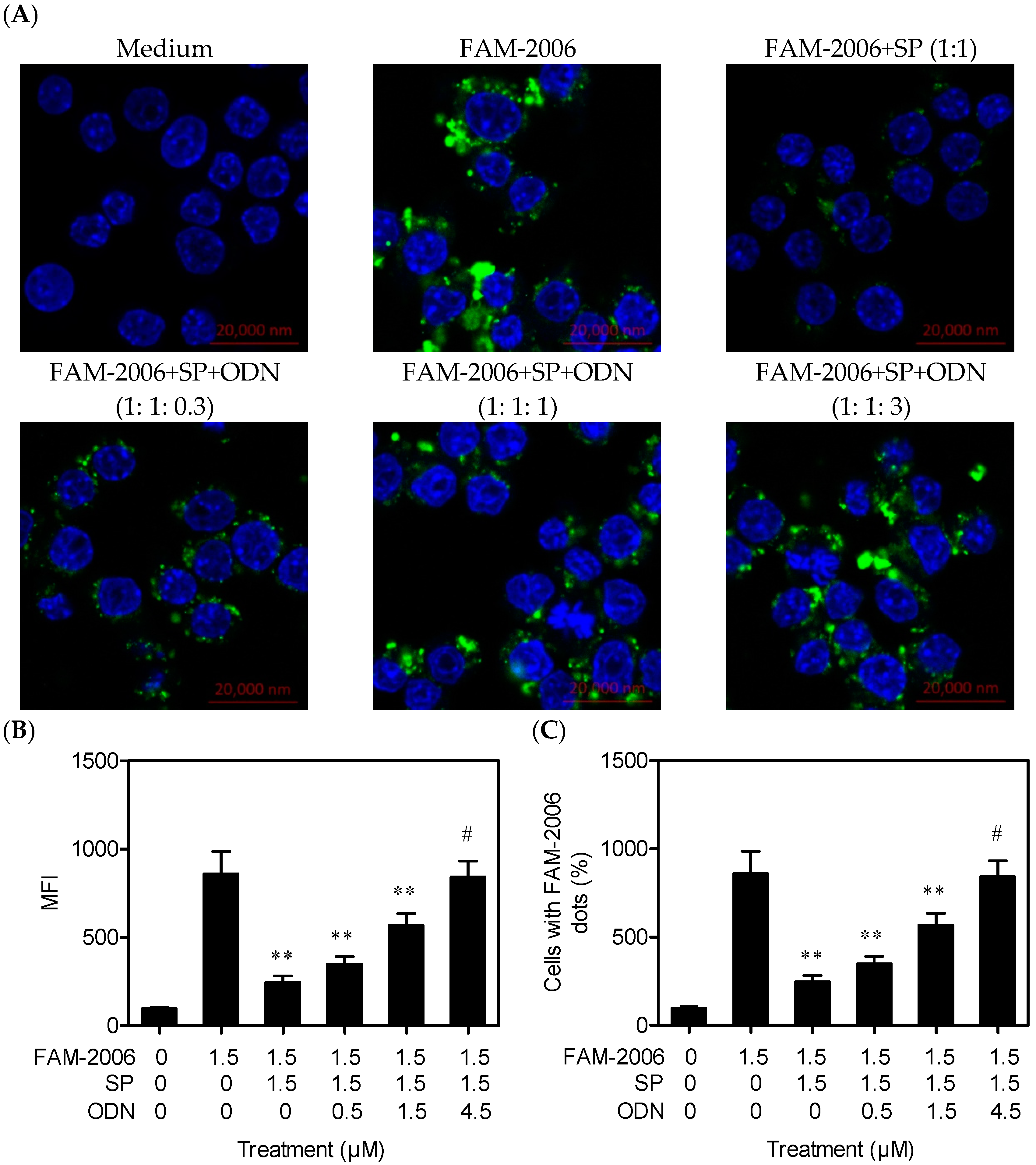
2.4. SP Inhibits CpG ODN Internalization within RAW264.7 Cells
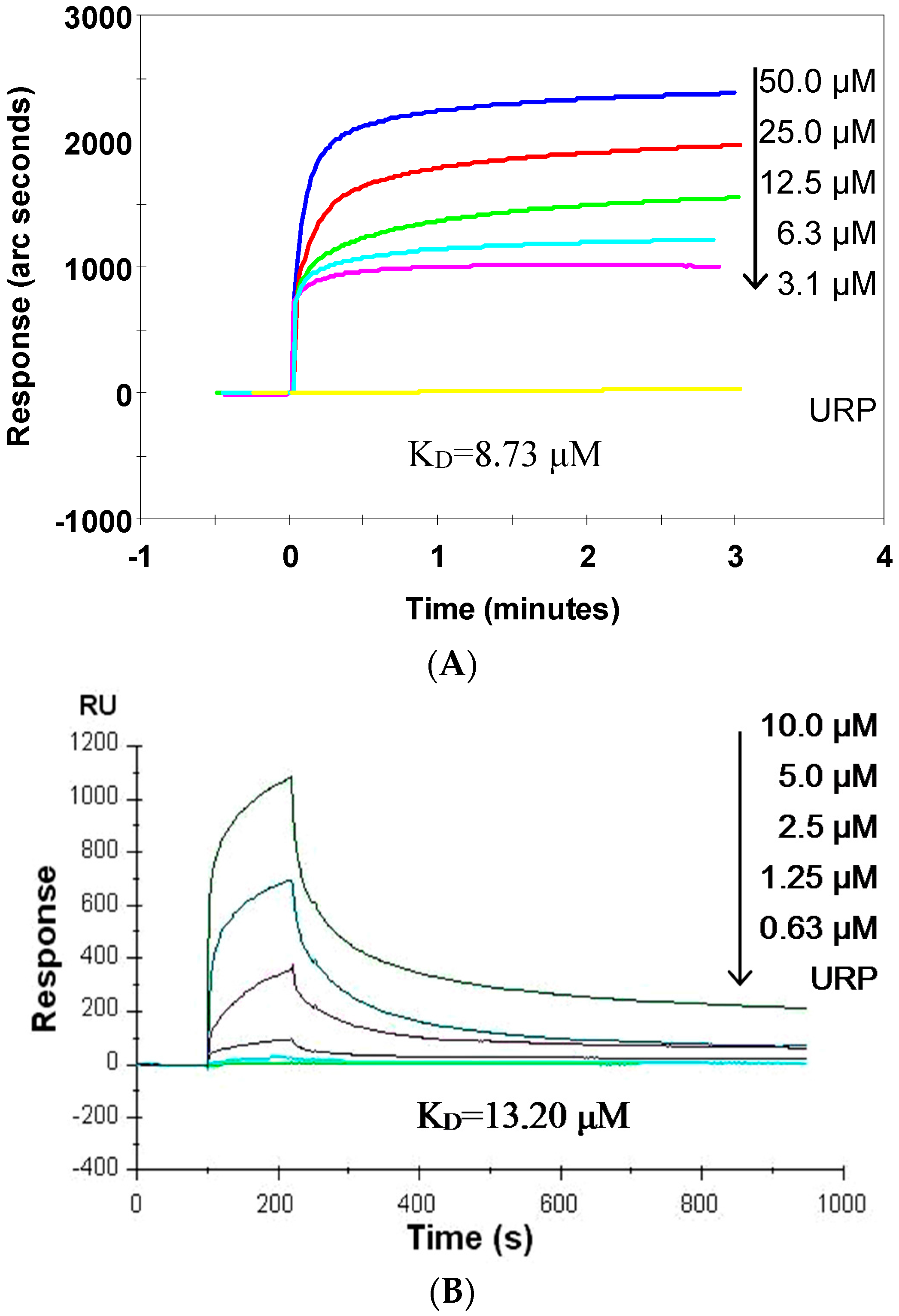
2.5. SP Binds CpG ODN with High Affinity
2.6. Discussion
3. Experimental Section
3.1. Materials
3.2. Cell Culture
3.3. Cytokines Release Assays
3.4. NF-κB Activity Assay
3.5. Western Blotting
3.6. Laser Confocal Scanning
3.7. Flow Cytometry Analysis
3.8. Binding Affinity Assays
3.9. Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brubaker, S.W.; Bonham, K.S.; Zanoni, I.; Kagan, J.C. Innate immune pattern recognition: A cell biological perspective. Annu. Rev. Immunol. 2015, 33, 257–290. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cao, H.; Wang, N.; Xiang, Y.; Lu, Y.; Zhao, K.; Zheng, J.; Zhou, H. A novel antagonist of TLR9 blocking all classes of immunostimulatory CpG-ODNs. Vaccine 2011, 29, 2193–2198. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Yue, J.; Ding, G.; Li, B.; Liu, X.; Zheng, X.; Yu, M.; Li, J.; Jiang, W.; Wu, C.; et al. Leucine-rich repeat 11 of Toll-like receptor 9 can tightly bind to CpG-containing oligodeoxynucleotides, and the positively charged residues are critical for the high affinity. J. Biol. Chem. 2012, 287, 30596–30609. [Google Scholar] [CrossRef] [PubMed]
- Savva, A.; Roger, T. Targeting toll-like receptors: Promising therapeutic strategies for the management of sepsis-associated pathology and infectious diseases. Front. Immunol. 2013, 4, 387. [Google Scholar] [CrossRef] [PubMed]
- Plitas, G.; Burt, B.M.; Nguyen, H.M.; Bamboat, Z.M.; DeMatteo, R.P. Toll-like receptor 9 inhibition reduces mortality in polymicrobial sepsis. J. Exp. Med. 2008, 205, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Celhar, T.; Magalhaes, R.; Fairhurst, A.M. TLR7 and TLR9 in SLE: When sensing self goes wrong. Immunol. Res. 2012, 53, 58–77. [Google Scholar] [CrossRef] [PubMed]
- De Pita, O.; Nardis, C.; Lupi, F.; Luci, C.A.; Frezzolini, A.; Pallotta, S. Modulation of Toll-like receptors in psoriatic patients during therapy with adalimumab. Int. J. Immunopathol. Pharmacol. 2011, 24, 185–188. [Google Scholar] [PubMed]
- Kawai, T.; Akira, S. TLR signaling. Cell Death Differ. 2006, 13, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Graham, K.L.; Lee, L.Y.; Higgins, J.P.; Steinman, L.; Utz, P.J.; Ho, P.P. Treatment with a toll-like receptor inhibitory GpG oligonucleotide delays and attenuates lupus nephritis in NZB/W mice. Autoimmunity 2010, 43, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Peter, M.E.; Kubarenko, A.V.; Weber, A.N.; Dalpke, A.H. Identification of an N-terminal recognition site in TLR9 that contributes to CpG-DNA-mediated receptor activation. J. Immunol. 2009, 182, 7690–7697. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Navajas, J.M.; Law, J.; Nguyen, K.P.; Bhargava, M.; Corr, M.P.; Varki, N.; Eckmann, L.; Hoffman, H.M.; Lee, J.; Raz, E. Interleukin 1 receptor signaling regulates DUBA expression and facilitates Toll-like receptor 9-driven antiinflammatory cytokine production. J. Exp. Med. 2010, 207, 2799–2807. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.; Bowie, A.G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 2007, 7, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Gungor, B.; Yagci, F.C.; Tincer, G.; Bayyurt, B.; Alpdundar, E.; Yildiz, S.; Ozcan, M.; Gursel, I.; Gursel, M. CpG ODN nanorings induce IFNalpha from plasmacytoid dendritic cells and demonstrate potent vaccine adjuvant activity. Sci. Trans. Med. 2014, 6, 235ra61. [Google Scholar] [CrossRef] [PubMed]
- Kissner, T.L.; Moisan, L.; Mann, E.; Alam, S.; Ruthel, G.; Ulrich, R.G.; Rebek, M.; Rebek, J., Jr.; Saikh, K.U. A small molecule that mimics the BB-loop in the Toll interleukin-1 (IL-1) receptor domain of MyD88 attenuates staphylococcal enterotoxin B-induced pro-inflammatory cytokine production and toxicity in mice. J. Biol. Chem. 2011, 286, 31385–31396. [Google Scholar] [CrossRef] [PubMed]
- Potter, C.; Cordell, H.J.; Barton, A.; Daly, A.K.; Hyrich, K.L.; Mann, D.A.; Morgan, A.W.; Wilson, A.G.; Isaacs, J.D. Association between anti-tumour necrosis factor treatment response and genetic variants within the TLR and NFκB signalling pathways. Ann. Rheum. Dis. 2010, 69, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Botos, I.; Segal, D.M.; Davies, D.R. The structural biology of Toll-like receptors. Structure 2011, 19, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.K.; Mullen, G.E.; Leifer, C.A.; Mazzoni, A.; Davies, D.R.; Segal, D.M. Leucine-rich repeats and pathogen recognition in Toll-like receptors. Trends. Immunol. 2003, 24, 528–533. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Park, H.H. Toll/interleukin-1 receptor (TIR) domain-mediated cellular signaling pathways. Apoptosis 2015, 20, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zheng, J.; Wang, L.; Ding, G.; Luo, P.; Lu, Y.; Pan, W.; Wang, M. Chloroquine protects mice from challenge with CpG ODN and LPS by decreasing proinflammatory cytokine release. Int. Immunopharmacol. 2004, 4, 223–234. [Google Scholar]
- Liu, X.; Zheng, X.; Wang, N.; Cao, H.; Lu, Y.; Long, Y.; Zhao, K.; Zhou, H.; Zheng, J. Kukoamine B, a novel dual inhibitor of LPS and CpG DNA, is a potential candidate for sepsis treatment. Br. J. Pharmacol. 2011, 162, 1274–1290. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, D.; Liu, X.; Jiang, W.; Zhou, W.; Yan, W.; Cen, Y.; Li, B.; Cao, G.; Ding, G.; et al. CpG ODN107 potentiates radiosensitivity of human glioma cells via TLR9-mediated NF-kappaB activation and NO production. Tumour Biol. 2012, 33, 1607–1618. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.J.; Chun, J.; Khan, S.; Kim, Y.S. Desoxyrhapontigenin, a potent anti-inflammatory phytochemical, inhibits LPS-induced inflammatory responses via suppressing NF-kappaB and MAPK pathways in RAW 264.7 cells. Int. Immunopharmacol. 2014, 18, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Y.; Liu, X.; Wang, N.; Cao, H.; Lu, Y.; Zhou, H.; Zheng, J. Inhibition of clathrin/dynamin-dependent internalization interferes with LPS-mediated TRAM-TRIF-dependent signaling pathway. Cell. Immunol. 2012, 274, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Rutz, M.; Schlatter, B.; Metzger, J.; Luppa, P.B.; Schmitz, F.; Haas, T.; Heit, A.; Bauer, S.; Wagner, H. CpG motif-independent activation of TLR9 upon endosomal translocation of “natural” phosphodiester DNA. Eur. J. Immunol. 2006, 36, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Blasius, A.L.; Beutler, B. Intracellular toll-like receptors. Immunity 2010, 32, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Keestra, A.M.; de Zoete, M.R.; Bouwman, L.I.; van Putten, J.P. Chicken TLR21 is an innate CpG DNA receptor distinct from mammalian TLR9. J. Immunol. 2010, 185, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Kuznik, A.; Bencina, M.; Svajger, U.; Jeras, M.; Rozman, B.; Jerala, R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J. Immunol. 2011, 186, 4794–4804. [Google Scholar] [CrossRef] [PubMed]
- Rommler, F.; Hammel, M.; Waldhuber, A.; Muller, T.; Jurk, M.; Uhlmann, E.; Wagner, H.; Vollmer, J.; Miethke, T. Guanine-modified inhibitory oligonucleotides efficiently impair TLR7- and TLR9-mediated immune responses of human immune cells. PLoS ONE 2015, 10, e0116703. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, X.; Li, B.; Kuang, M.; Liu, X.; Cen, Y.; Qin, R.; Ding, G.; Zheng, J.; Zhou, H. Synthetic Human TLR9-LRR11 Peptide Attenuates TLR9 Signaling by Binding to and thus Decreasing Internalization of CpG Oligodeoxynucleotides. Int. J. Mol. Sci. 2016, 17, 242. https://doi.org/10.3390/ijms17020242
Pan X, Li B, Kuang M, Liu X, Cen Y, Qin R, Ding G, Zheng J, Zhou H. Synthetic Human TLR9-LRR11 Peptide Attenuates TLR9 Signaling by Binding to and thus Decreasing Internalization of CpG Oligodeoxynucleotides. International Journal of Molecular Sciences. 2016; 17(2):242. https://doi.org/10.3390/ijms17020242
Chicago/Turabian StylePan, Xichun, Bin Li, Mei Kuang, Xin Liu, Yanyan Cen, Rongxin Qin, Guofu Ding, Jiang Zheng, and Hong Zhou. 2016. "Synthetic Human TLR9-LRR11 Peptide Attenuates TLR9 Signaling by Binding to and thus Decreasing Internalization of CpG Oligodeoxynucleotides" International Journal of Molecular Sciences 17, no. 2: 242. https://doi.org/10.3390/ijms17020242
APA StylePan, X., Li, B., Kuang, M., Liu, X., Cen, Y., Qin, R., Ding, G., Zheng, J., & Zhou, H. (2016). Synthetic Human TLR9-LRR11 Peptide Attenuates TLR9 Signaling by Binding to and thus Decreasing Internalization of CpG Oligodeoxynucleotides. International Journal of Molecular Sciences, 17(2), 242. https://doi.org/10.3390/ijms17020242