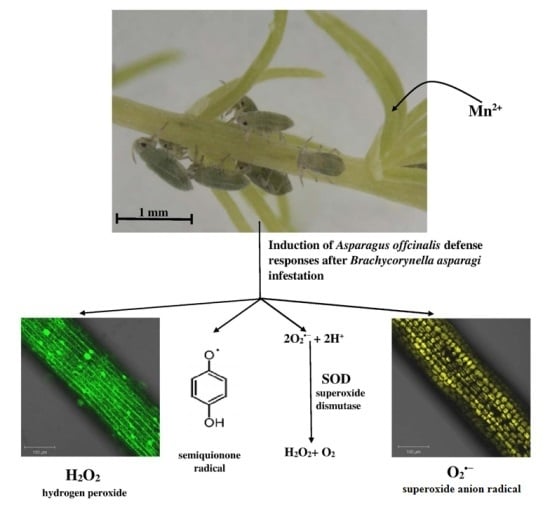
Brachycorynella asparagi (Mordv.) Induced—Oxidative Stress and Antioxidative Defenses of Asparagus officinalis L.
Abstract
:1. Introduction
2. Results
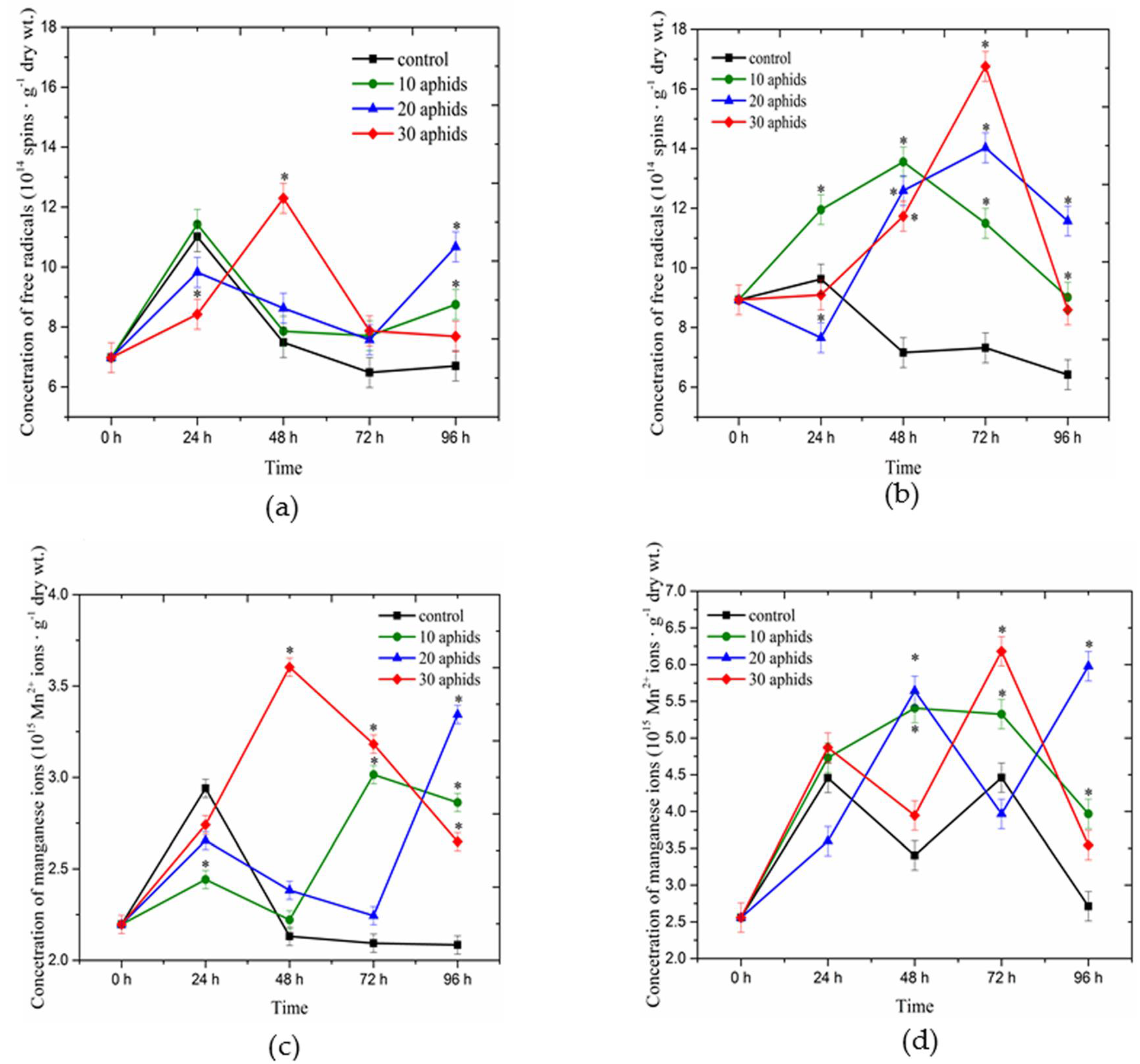
2.1. Concentrations of Free Radicals and Mn2+ Ions Detected Using Electron Paramagnetic Resonance Spectroscopy in Asparagus officinalis Infested by Brachycorynella asparagi
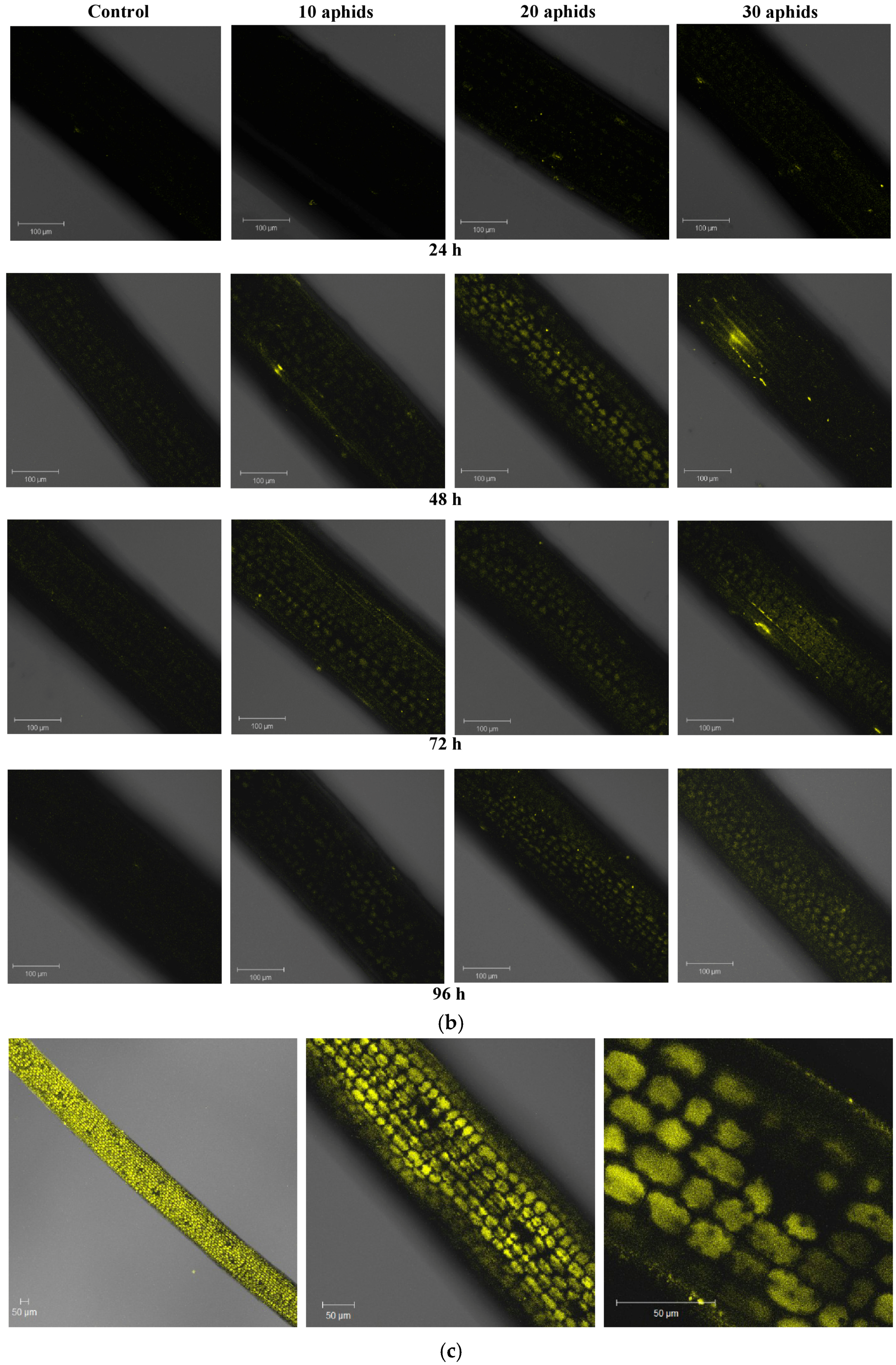
2.2. The Effect of Brachycorynella asparagi Infestation on the Generation of the Superoxide Anion Radical in Asparagus officinalis
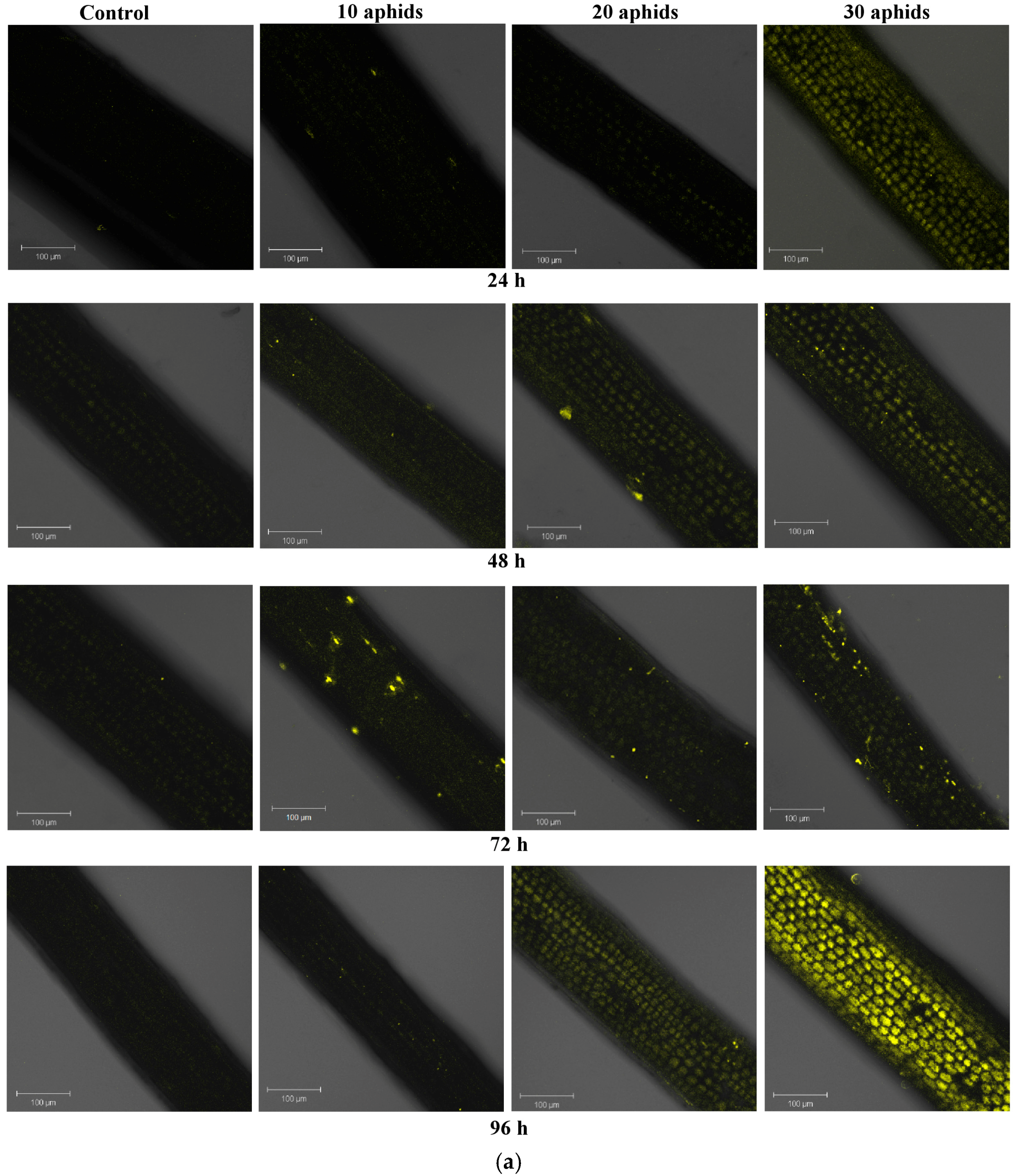
2.3. The Effect of Brachycorynella asparagi Infestation on the Level of Hydrogen Peroxide in Asparagus officinalis
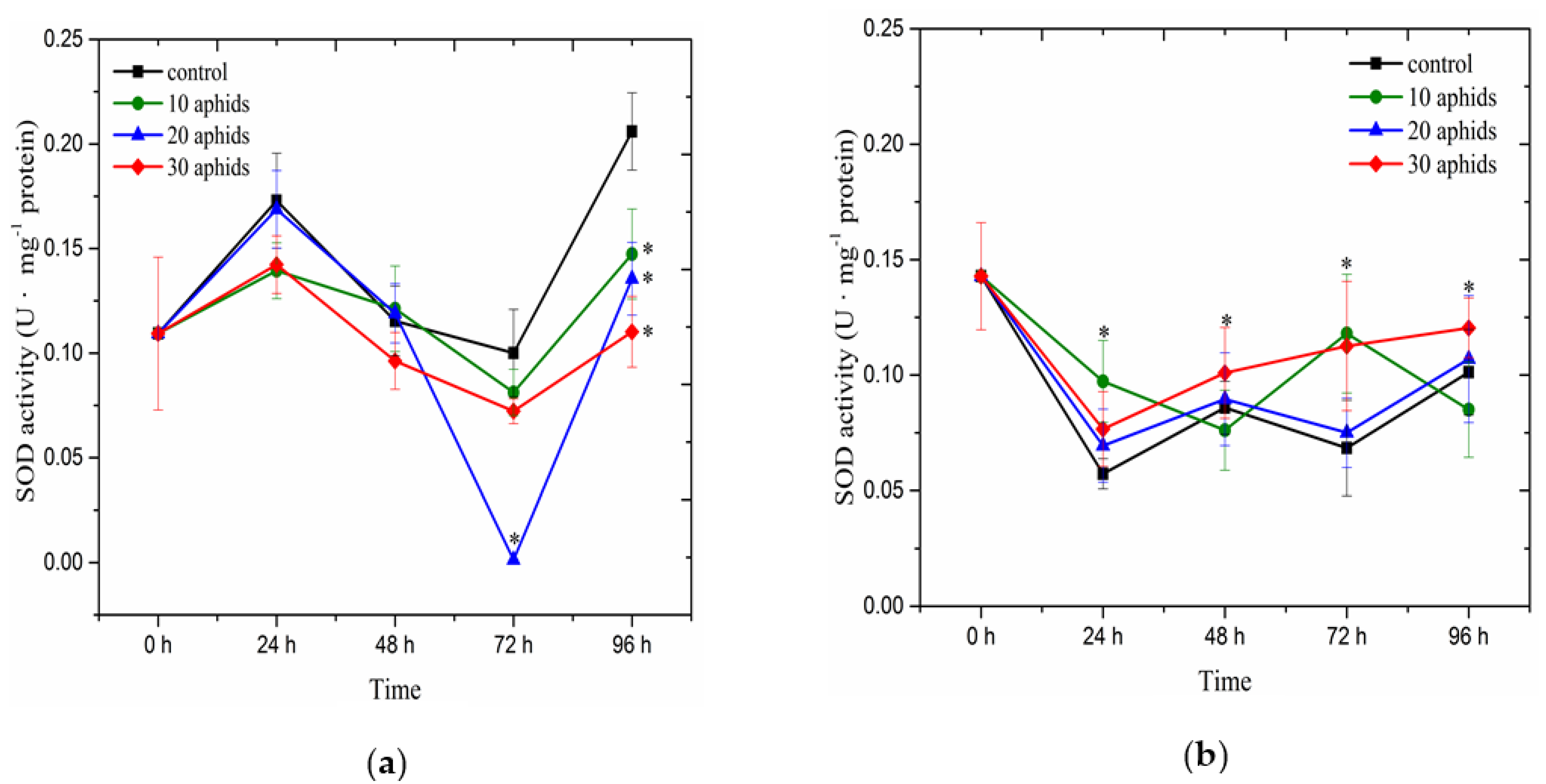
2.4. The Effect of Brachycorynella asparagi Infestation on the Antioxidant Enzyme Activity in Asparagus officinalis
2.5. Symptoms of Brachycorynella asparagi Infestation on Asparagus officinalis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Aphids and the Infestation Experiment
4.3. Electron Paramagnetic Resonance (EPR)
4.4. Detection of Hydrogen Peroxide Release
4.5. Detection of Superoxide Anion Radical Release
4.6. Extraction and Assay of Superoxide Dismutase Activity
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| EPR | electron paramagnetic resonance |
| fr. wt. | fresh weight |
| dry wt. | dry weight |
| g | gram |
| g-values | the factor describing energy levels of the unpaired electron for the paramagnetic centre in an external magnetic field |
| H2O2 | hydrogen peroxide |
| h | hour |
| hpi | hours post infestation |
| Mn2+ | manganese ions |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| O2•− | superoxide anion radical |
References
- Brothwell, D.R.; Brothwell, P. Food in Antiquity: A Survey of the Diet of Early Peoples; The Johns Hopkins University Press: Baltimore, MD, USA, 1998; p. 92. [Google Scholar]
- Pitrat, M. Vegetable crops in the Mediterranean Basin with an overview of virus resistance. Adv. Virus Res. 2012, 84, 1–29. [Google Scholar] [PubMed]
- Leszczyński, B.; Cone, W.W.; Wright, L.C. Changes in the sugar metabolism of asparagus plants infested by asparagus aphid, Brachycorynella asparagi and green peach aphid, Myzus persicae. J. Agric. Entomol. 1986, 3, 25–30. [Google Scholar]
- Stoetzel, M.B. Aphids (Homoptera: Aphididae) colonizing leaves of asparagus in the United States. J. Econ. Entomol. 1990, 83, 1994–2002. [Google Scholar] [CrossRef]
- Kerchev, P.I.; Fenton, B.; Foyer, C.H.; Hancock, R.D. Plant responses to insect herbivory: Interactions between photosynthesis, reactive oxygen species and hormonal signalling pathways. Plant Cell Environ. 2012, 35, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.O. Oxidative stress, antioxidants and chemoprevention: On the role oxidant-induced signaling in cellular adaptation. In Recent Advances in Redox Active Plant and Microbial Products; Jacob, C., Kirsch, G., Slusarenko, A., Winyard, P.G., Burkholz, T., Eds.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA, 2014; pp. 119–148. [Google Scholar]
- Goggin, F.L. Plant-aphid interactions: Molecular and ecological perspectives. Curr. Opin. Plant Biol. 2007, 10, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Giordanengo, P.; Brunissen, L.; Rusterucci, C.; Vincent, C.; van Bel, A.; Dinant, S.; Girousse, C.; Faucher, M.; Bonnemain, J.L. Compatible plant-aphid interactions: How aphids manipulate plant responses. C. R. Biol. 2010, 333, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Radville, L.; Chaves, A.; Preisser, E.L. Variation in plant defense against invasive herbivores: Evidence for a hypersensitive response in eastern hemlocks (Tsuga canadensis). J. Chem. Ecol. 2011, 37, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Prince, D.C.; Drurey, C.; Zipfel, C.; Hogenhout, S.A. The leucine-rich repeat receptor-like kinase brassinosteroid insensitive1-associated kinase1 and the cytochrome P450 phytoalexin deficient3 contribute to innate immunity to aphids in Arabidopsis. Plant Physiol. 2014, 164, 2207–2219. [Google Scholar] [CrossRef] [PubMed]
- Jaouannet, M.; Morris, J.A.; Hedley, P.E.; Bos, J.I.B. Characterization of arabidopsis transcriptional responses to different aphid species reveals genes that contribute to host susceptibility and non-host resistance. PLoS Pathog. 2015. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Brugger, A.; Lamotte, O.; Vandelle, E.; Bourque, S.; Lecourieux, D.; Poinssot, B.; Wendehenne, D.; Pugin, A. Early signaling events induced by elicitors of plant defenses. Mol. Plant-Microbe Interact. 2006, 19, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Moloi, M.J.; van der Westhuizen, A.J. The reactive oxygen species are involved in resistance responses of wheat to the Russian wheat aphid. J. Plant Physiol. 2006, 163, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Kuśnierczyk, A.; Winge, P.; Jørstad, T.S.; Troczyńska, J.; Rossiter, J.T.; Bones, A.M. Towards global understanding of plant defence against aphids—Timing and dynamics of early Arabidopsis defence responses to cabbage aphid (Brevicoryne brassicae) attack. Plant Cell Environ. 2008, 31, 1097–1115. [Google Scholar] [CrossRef] [PubMed]
- Kerchev, P.I.; Fenton, B.; Foyer, C.H.; Hancock, R.D. Infestation of potato (Solanum tuberosum L.) by the peach-potato aphid (Myzus persicae Sulzer) alters cellular redox status and is influenced by ascorbate. Plant Cell Environ. 2012, 35, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Mai, V.C.; Bednarski, W.; Borowiak-Sobkowiak, B.; Wilkaniec, B.; Samardakiewicz, S.; Morkunas, I. Oxidative stress in pea seedling leaves in response to Acyrthosiphon pisum infestation. Phytochemistry 2013, 93, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Liu, M.; Hu, Q.; He, M.; Qi, X.; Xu, Q.; Zhou, F.; Chen, X. Identification of differentially expressed genes related to aphid resistance in cucumber (Cucumis sativus L.). Sci. Rep. 2015. [Google Scholar] [CrossRef] [PubMed]
- Saxena, I.; Srikanth, S.; Chen, Z. Cross talk between H2O2 and interacting signal molecules under plant stress response. Front. Plant Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E.; Mithofer, A.; Boland, W. Insects feeding on plants: Rapid signals and responses preceding the induction of phytochemical release. Phytochemistry 2007, 68, 2946–2959. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E.; Mithofer, A.; Arimura, G.I.; Uchtenhagen, H.; Bossi, S.; Bertea, C.M.; Cucuzza, L.S.; Novero, M.; Volpe, V.; Quadro, S.; et al. Effects of feeding Spodoptera littoralis on lima bean leaves. III. Membrane depolarization and involvement of hydrogen peroxide. Plant Physiol. 2006, 140, 1022–1035. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Woźniak, A.; Formela, M.; Mai, V.C.; Marczak, Ł.; Narożna, D.; Borowiak-Sobkowiak, B.; Kühn, C.; Grimm, B. Pea aphid infestation induces changes in flavonoids, antioxidative defence, soluble sugars and sugar transporter expression in leaves of pea seedlings. Protoplasma 2015. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Yan, Y.; Peng, L.; Guo, Y.J.; Wan, F.H. Plant defense responses induced by phloem-feeding insects. Acta Entomol. Sin. 2012, 55, 736–748. [Google Scholar]
- Menendez, A.I.; Folcia, A.M.; Vizgarra, L.; Romero, A.M.; Martínez-Ghersa, M.A. Impact of plant and aphid stress history on infestation in arugula plants. Entomol. Exp. Appl. 2013, 149, 128–137. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ignacimuthu, S.; Sharma, H.C. Defensive responses in groundnut against chewing and sap-sucking insects. J. Plant Growth Regul. 2013, 32, 259–272. [Google Scholar] [CrossRef]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.Y.; Chen, J.L.; Cheng, D.F.; Sun, J.R.; Liu, Y.; Tian, Z. Biochemical and molecular characterizations of Sitobion avenae-induced wheat defense responses. Crop Prot. 2009, 28, 435–442. [Google Scholar] [CrossRef]
- Wu, J.; Baldwin, I.T. New insights into plant responses to the attack from insect herbivores. Annu. Rev. Genet. 2010, 44, 1–24. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, F.; Chen, S.; Lv, G.; Deng, Y.; Fang, W.; Guan, Z.; He, C. Chrysanthemum leaf epidermal surface morphology and antioxidant and defense enzyme activity in response to aphid infestation. J. Plant Physiol. 2011, 168, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Barbehenn, R.V.; Poopat, U.; Spencer, B. Semiquinone and ascorbyl radicals in the gut fluids of caterpillars measured with EPR spectrometry. Insect Biochem. Mol. Boil. 2003, 33, 125–130. [Google Scholar] [CrossRef]
- Morkunas, I.; Garnczarska, M.; Bednarski, W.; Ratajczak, W.; Waplak, S. Metabolic and ultrastructural responses of lupine embryo axes to sugar starvation. J. Plant Physiol. 2003, 160, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Bednarski, W.; Kozłowska, M. Response of embryo axes of germinating seeds of yellow lupine to Fusarium oxysporum. Plant Physiol. Biochem. 2004, 42, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Bednarski, W.; Kopyra, M. Defense strategies of pea embryo axes with different levels of sucrose to Fusarium oxysporum and Ascochyta pisi. Physiol. Mol. Plant Pathol. 2008, 72, 167–178. [Google Scholar] [CrossRef]
- Morkunas, I.; Formela, M.; Floryszak-Wieczorek, J.; Marczak, Ł.; Narożna, D.; Nowak, W.; Bednarski, W. Cross-talk interactions of exogenous nitric oxide and sucrose modulates phenylpropanoid metabolism in yellow lupine embryo axes infected with Fusarium oxysporum. Plant Sci. 2013, 211, 102–121. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Bednarski, W. Fusarium oxysporum-induced oxidative stress and antioxidative defenses of yellow lupine embryo axes with different sugar levels. J. Plant Physiol. 2008, 165, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Bednarski, W.; Ostrowski, A.; Waplak, S. Low temperature short-range ordering caused by Mn2+ doping of Rb3H(SO4)2. J. Phys. Condens. Matter 2010. [Google Scholar] [CrossRef] [PubMed]
- Formela, M.; Samardakiewicz, S.; Marczak, Ł.; Nowak, W.; Narożna, D.; Bednarski, W.; Kasprowicz-Maluśki, A.; Morkunas, I. Effects of endogenous signals and Fusarium oxysporum on the mechanism regulating genistein synthesis and accumulation in yellow lupine and their impact on plant cell cytoskeleton. Molecules 2014, 19, 13392–13421. [Google Scholar] [CrossRef] [PubMed]
- Mai, V.C.; Drzewiecka, K.; Jeleń, H.; Narożna, D.; Rucińska-Sobkowiak, R.; Kęsy, J.; Floryszak-Wieczorek, J.; Gabryś, B.; Morkunas, I. Differential induction of Pisum sativum defense signaling molecules in response to pea aphid infestation. Plant Sci. 2014, 221, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Del Río, L.A. ROS and RNS in plant physiology: An overview. J. Exp. Bot. 2015, 66, 2827–2837. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, H.; Philippe, F.; Domon, J.M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef] [PubMed]
- Wrzaczek, M.; Brosché, M.; Kangasjärvi, J. ROS signaling loops—Production, perception, regulation. Curr. Opin. Plant Biol. 2013, 16, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Lidon, F.C.; Barreiro, M.G.; Ramalho, J.C. Manganese accumulation in rice: Implications for photosynthetic functioning. J. Plant Physiol. 2004, 161, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Shenker, M.; Plessner, O.E.; Tel-Or, E. Manganese nutrition effects on tomato growth, chlorophyll concentration, and superoxide dismutase activity. J. Plant Physiol. 2004, 161, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Corona, M.; Robinson, G.E. Genes of the antioxidant system of the honey bee: Annotation and phylogeny. Insect Mol. Biol. 2006, 15, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Allan, A.C.; Fluhr, R. Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell 1997, 9, 1559–1572. [Google Scholar] [CrossRef] [PubMed]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Moran, P.J.; Cheng, Y.; Cassell, J.L.; Thompson, G.A. Gene expression profiling of Arabidopsis thaliana in compatible plant-aphid interactions. Arch. Insect Biochem. Physiol. 2002, 51, 182–203. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Shao, Y.; Jiang, J.; Ren, L.; Chen, F.; Fang, W.; Guan, Z.; Chen, S. Gene expression profiles responses to aphid feeding in chrysanthemum (Chrysanthemum morifolium). BMC Genom. 2014. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Zhu-Salzman, K. Enhanced aphid detoxification when confronted by a host with elevated ROS production. Plant Signal. Behav. 2015, 10, e1010936. [Google Scholar] [CrossRef] [PubMed]
- Sytykiewicz, H. Transcriptional responses of catalase genes in maize seedlings exposed to cereal aphids′ herbivory. Biochem. Syst. Ecol. 2015, 60, 131–142. [Google Scholar] [CrossRef]
- Mai, V.C.; Tran, N.T.; San Nguyen, D. The involvement of peroxidases in soybean seedlings′ defense against infestation of cowpea aphid. Arthropod-Plant Interact. 2016, 10, 283–292. [Google Scholar] [CrossRef]
- Ren, G.; Wang, X.; Chen, D.; Wang, X.; Liu, X. Effects of aphids Myzus persicae on the changes of Ca2+ and H2O2 flux and enzyme activities in tobacco. J. Plant Interact. 2014, 9, 883–888. [Google Scholar] [CrossRef]
- Bray, E.A.; Baily-Serres, J.; Weretilnyk, E. Responses to Abiotic Stresses. In Biochemistry and Molecular Biology of Plants; Buchanan, B., Gruissem, W., Jones, R., Eds.; American Society of Plant Physiologists: Rockville, MD, USA, 2000; pp. 1158–1203. [Google Scholar]
- Gaspar, T.; Franck, T.; Bisbis, B.; Kevers, C.; Jouve, L.; Hausman, J.F.; Dommes, J. Concepts in plant stress physiology. Application to plant tissue cultures. Plant Growth Regul. 2002, 37, 263–285. [Google Scholar] [CrossRef]
- Donovan, M.P.; Nabity, P.D.; DeLucia, E.H. Salicylic acid-mediated reductions in yield in Nicotiana attenuata challenged by aphid herbivory. Arthropod-Plant Interact. 2013, 7, 45–52. [Google Scholar] [CrossRef]
- Rucińska, R.; Waplak, S.; Gwóźdź, E.A. Free radical formation and activity of antioxidant enzymes in lupin roots exposed to lead. Plant Physiol. Biochem. 1999, 37, 187–194. [Google Scholar] [CrossRef]
- Garnczarska, M.; Bednarski, W.; Morkunas, I. Re-aeration-induced oxidative defenses in hypoxically pretreated lupine roots. J. Plant Physiol. 2004, 16, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Małecka, A.; Piechalak, A.; Tomaszewska, B. Reactive oxygen species production and antioxidative defense system in pea root tissues treated with lead ions: The whole roots level. Acta Physiol. Plant 2009, 31, 1053–1063. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
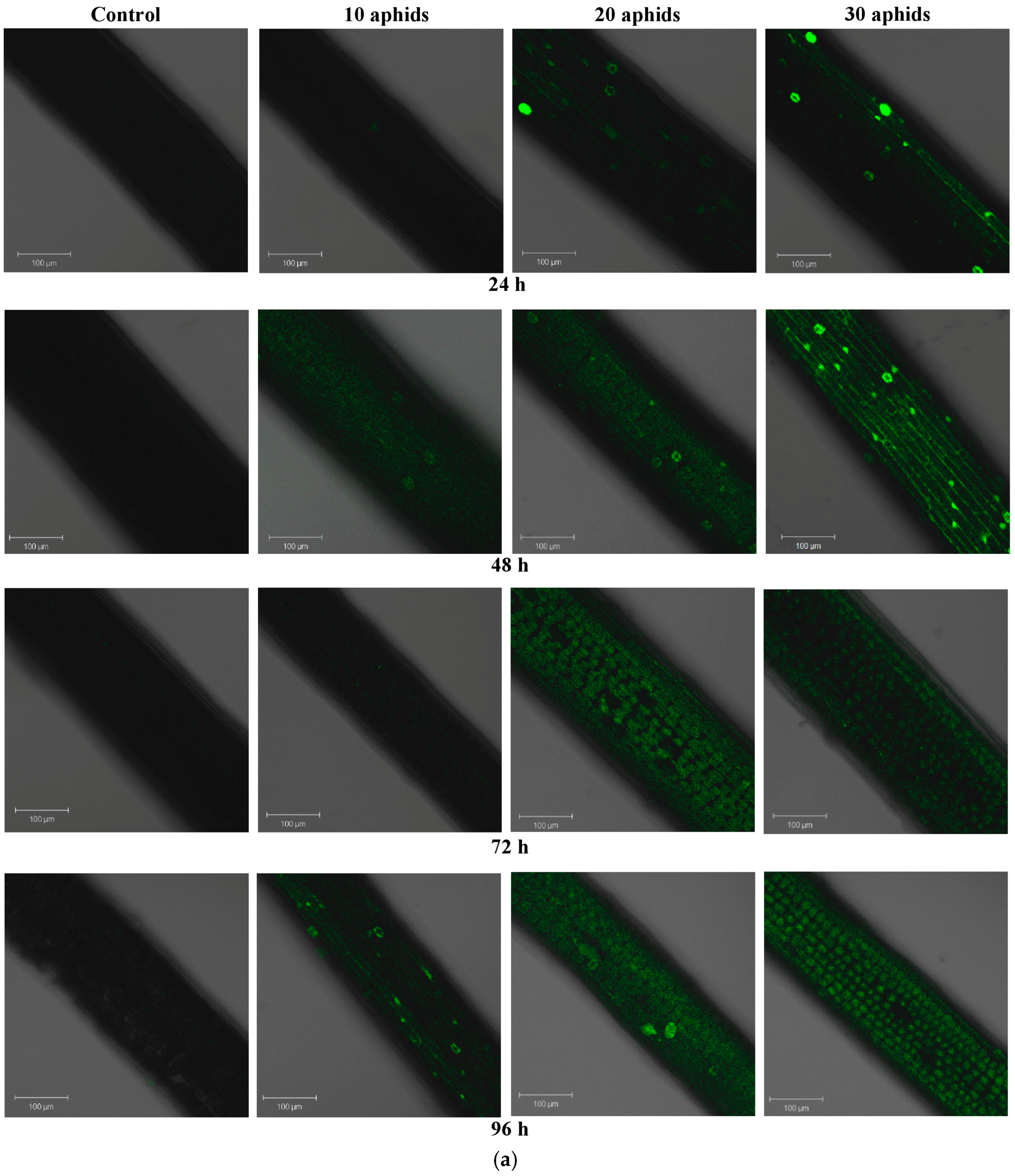
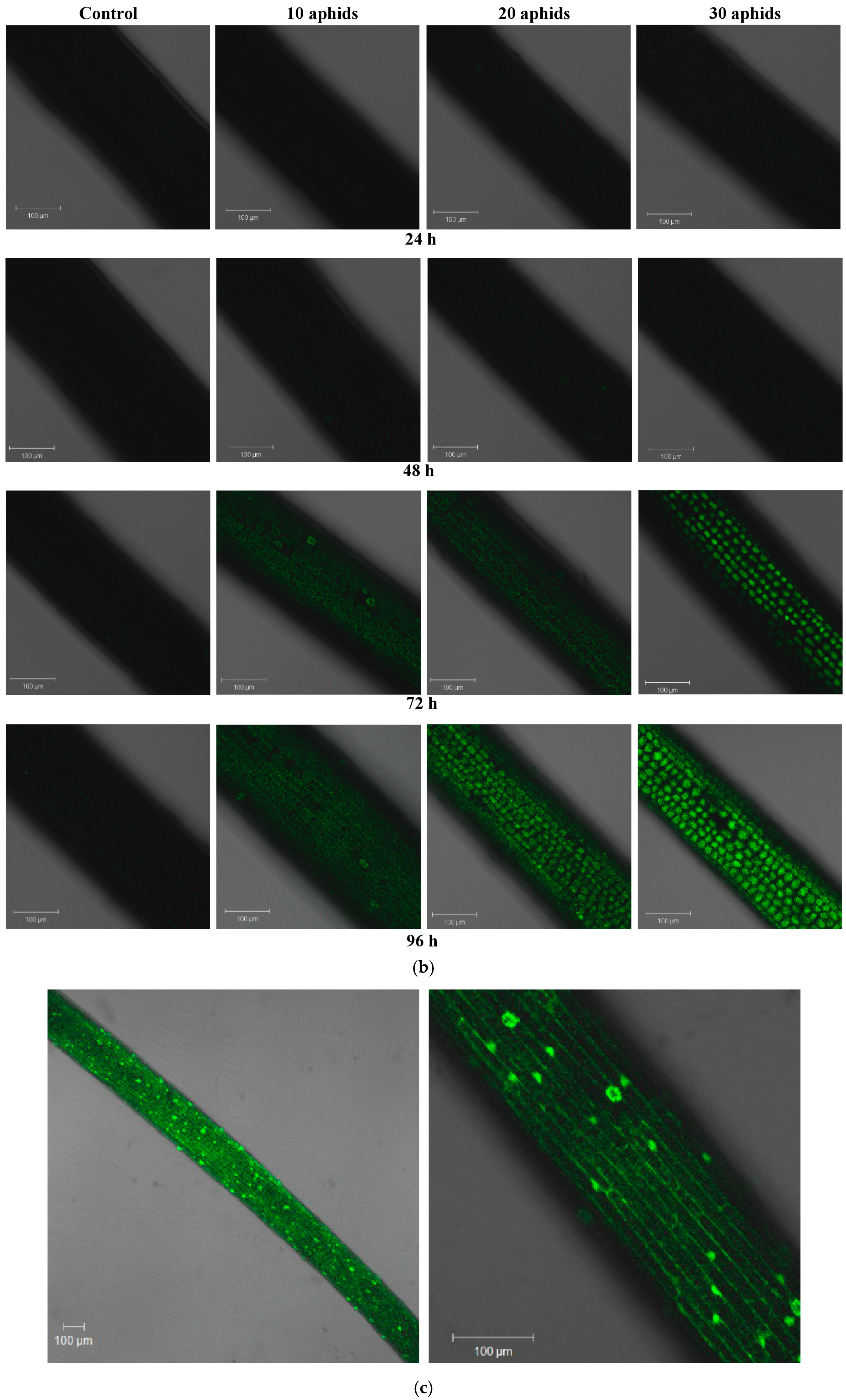
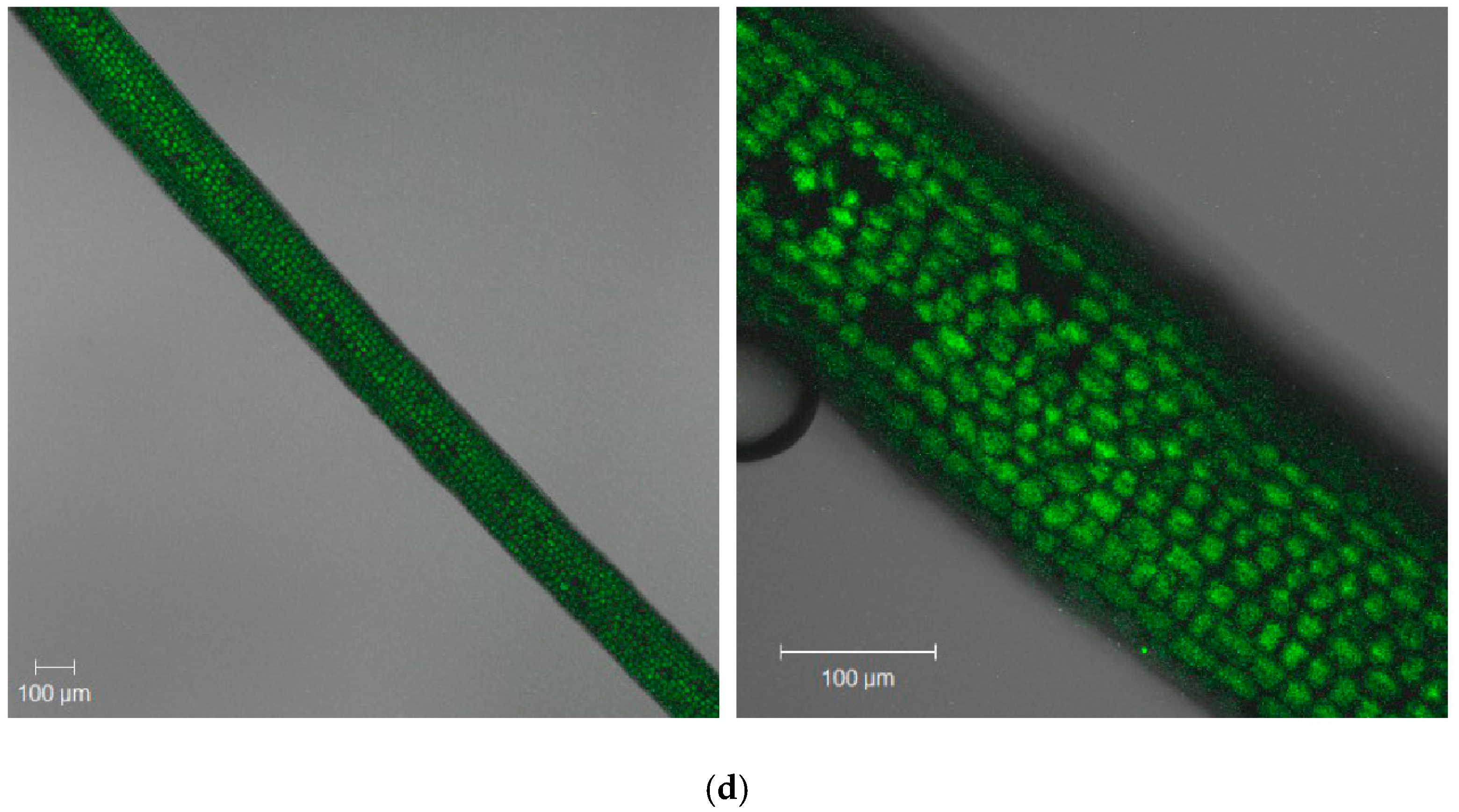
| Symptoms | Variant | ||
|---|---|---|---|
| 10 | 20 | 30 | |
| Aphids colonize the tops of the ferns | ● ♦ | ● ♦ | ● ♦ |
| Gently crimp the tops of the ferns | ● | ● ♦ | ● ♦ |
| Shorter internodes | ● | ● ♦ | |
| Aphids colonize whole ferns | ● | ● ♦ | |
| The top of the ferns are twisted, with shortening internodes, “rosetting” and the plants turning yellow. The leaves are shortened and turned blue green | ● | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borowiak-Sobkowiak, B.; Woźniak, A.; Bednarski, W.; Formela, M.; Samardakiewicz, S.; Morkunas, I. Brachycorynella asparagi (Mordv.) Induced—Oxidative Stress and Antioxidative Defenses of Asparagus officinalis L. Int. J. Mol. Sci. 2016, 17, 1740. https://doi.org/10.3390/ijms17101740
Borowiak-Sobkowiak B, Woźniak A, Bednarski W, Formela M, Samardakiewicz S, Morkunas I. Brachycorynella asparagi (Mordv.) Induced—Oxidative Stress and Antioxidative Defenses of Asparagus officinalis L. International Journal of Molecular Sciences. 2016; 17(10):1740. https://doi.org/10.3390/ijms17101740
Chicago/Turabian StyleBorowiak-Sobkowiak, Beata, Agnieszka Woźniak, Waldemar Bednarski, Magda Formela, Sławomir Samardakiewicz, and Iwona Morkunas. 2016. "Brachycorynella asparagi (Mordv.) Induced—Oxidative Stress and Antioxidative Defenses of Asparagus officinalis L." International Journal of Molecular Sciences 17, no. 10: 1740. https://doi.org/10.3390/ijms17101740
APA StyleBorowiak-Sobkowiak, B., Woźniak, A., Bednarski, W., Formela, M., Samardakiewicz, S., & Morkunas, I. (2016). Brachycorynella asparagi (Mordv.) Induced—Oxidative Stress and Antioxidative Defenses of Asparagus officinalis L. International Journal of Molecular Sciences, 17(10), 1740. https://doi.org/10.3390/ijms17101740