Non-Coding RNAs in Saliva: Emerging Biomarkers for Molecular Diagnostics
Abstract
:1. Introduction: Saliva as a Liquid Biopsy
2. Salivary Non-Coding RNAs Associated with Physiological and Pathological States
2.1. Characterization of Salivary Non-Coding RNAs
2.2. Salivary Exosomes as a Source of Non-Coding RNAs
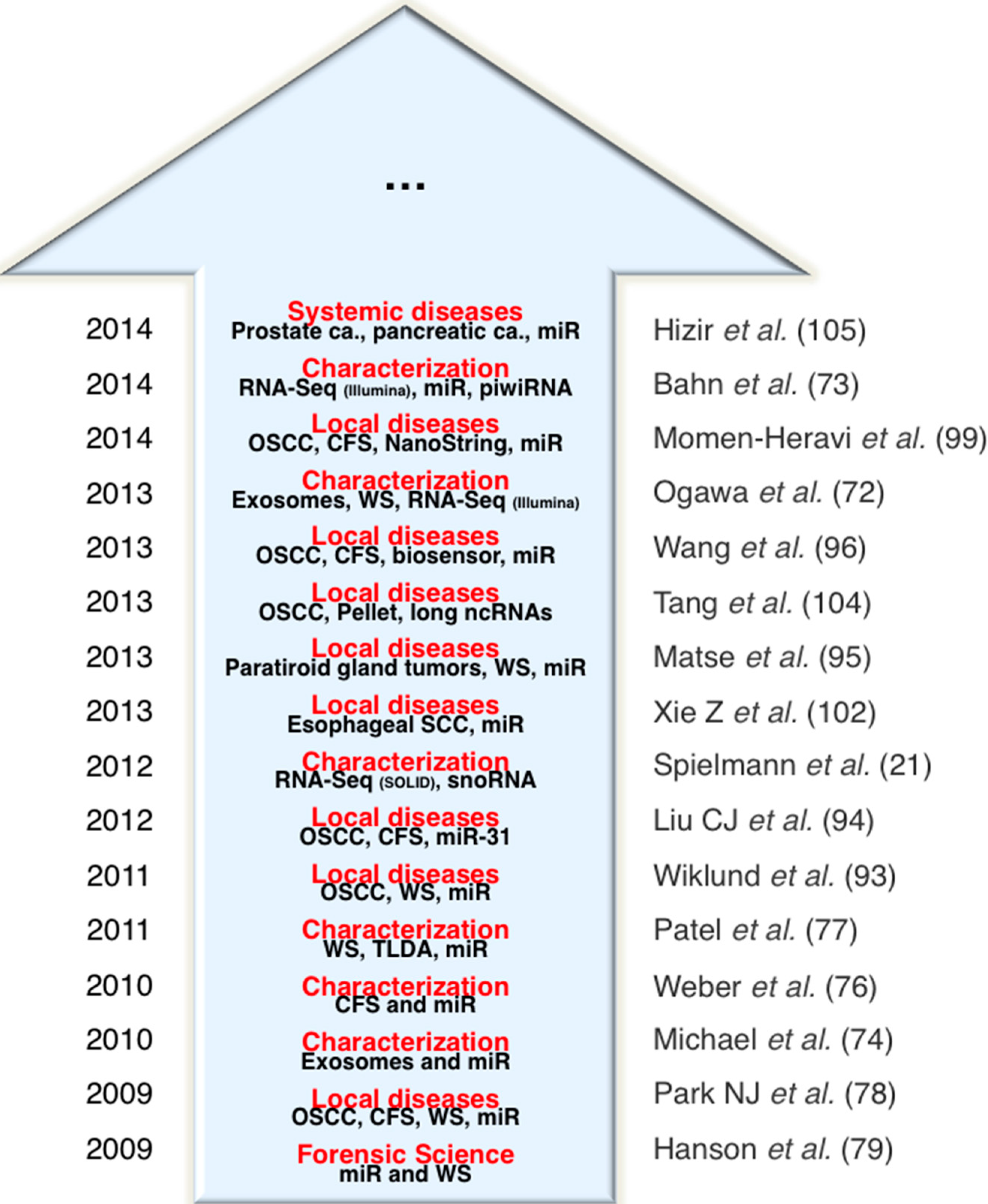
2.3. Salivary Non-Coding RNAs as a Source of Biomarkers for Local and Systemic Diseases
| Ref. | Study | Saliva Fraction | Disease | Study Cohort | Technique | Molecular Profile |
|---|---|---|---|---|---|---|
| Characterization | ||||||
| [76] | Weber et al., Clin. Chem. 2010 | CFS | characterization | 5 healthy donors | Human miScript Assay panel (Qiagen)—714 miRNA | miR-182*, miR-450b-5p, miR-622, miR-141, miR-26a, miR-145*, miR-135b*, miR-381, miR-96*, miR-1228, miR-431* |
| [74] | Michael et al., Oral Dis. 2010 | exosomes | characterization | 2 healthy donors | miRCURY LNA microRNA Array, v.10.0, (Exiqon, Denmark) | let-7b, let-7c*, miR-128, miR-150*, miR-17, miR-1908, miR-212, miR-27b*, miR-29b, miR-29c, (Top-10) |
| [77] | Patel et al., Arch Oral Biol. 2011 | WS | characterization | 20 healthy donors | TaqMan1 Low Density Array Card (TLDA) Human miRNA Panel v2.0 (Applied Biosystems) | miR-223, miR-191, miR-16, miR-203, and miR-24 |
| [21] | Spielmann et al., Clin. Chem. 2012 | CFS and WS | characterization | 8 healthy donors | SOLiDTM Total RNA-Seq Kit and Barcoding Kit (modules 1–16) (Applied Biosystems) | 224 snoRNAs |
| [91] | Gallo et al., PLoS ONE 2012 | exosomes | characterization | Healthy donors (# N/A) | TaqMan MicroRNA Assay, PN 4427975, Applied Biosystems | miR-22, miR202, miR-203, miR-1273d |
| [72] | Ogawa et al., Biol. Pharm. Bull. 2013 | exosomes and WS | characterization | 1 healthy donor (7 saliva collection replicates) | Illumina Genome Analyzer Iix by Hokkaido System Sciences Co., Ltd. (Japan) | miR-378a, miR-143, let-7c, miR-146b, miR-21, let-7f-1, let-7f-2, miR-30a, miR-9-1, miR-9-2, miR-9-3, let-7a-1, let-7a-2, miR-20a, miR-30d, miR-30e; piR-39980, piR-48209, piR-52207, piR-38581, piR-36095, piR-59293, piR-61648, piR-55361; U78, U44, U21, U31, U104, U15A, snR39B |
| [73] | Bahn et al., Clin. Chem. 2014 | CFS | characterization | 8 healthy donors | Illumina HiSeq 50SE | 127–418 miRNAs (Top-2: miR-223-3p and miR-148a-3p) |
| Local & Systemic Diseases | ||||||
| [78] | Park NJ et al., Clin. Cancer Res. 2009 | CFS and WS | oral squamous cell carcinoma | 50 OSCC patients and 50 healthy matched controls | RT-preamp-qPCR | miR-125a and miR-200a |
| [93] | Wiklund et al., PLoS ONE 2011 | WS | oral squamous cell carcinoma | 15 OSCC patients and 7 healthy controls | TaqManH qRT-PCR assays (Applied Biosystems) | miR-375 and miR-200a expression and miR-200c-141 methylation |
| [94] | Liu CJ et al., Head Neck 2012 | CFS | oral squamous cell carcinoma | 45 oral carcinoma, 10 oral verrucous leukoplakia, and 24 healthy controls | TaqMan miRNA assay system (Applied Biosystems, Foster City, CA, USA) | miR-31 |
| [95] | Matse et al., Clin. Cancer Res. 2013 | WS | paratiroid gland tumors | 38 malignant tumors and 29 benign parotid gland tumors | TaqMan Human MicroRNA Cards (Applied Biosystems) and RTqPCR | hsa-miR-132, hsa-miR-15b, mmu-miR-140, and hsa-miR-22 |
| [96] | Tang et al., Mol. Med. Rep. 2013 | SP | oral squamous cell carcinoma | 4 OSCC saliva samples and 12 healthy controls | RT-qPCR of six lncRNAs found in OSCC tissue | MALALT-1, HOTAIR |
| [97] | Wang et al., Biosens. Bioelectron. 2013 | CFS | oral squamous cell carcinoma | 5 artificial saliva samples (spiked) | Novel home-made electrochemical biosensor magnetic-controllable gold electrode | miR- 200a, miR-142-3p, miR-93 and miR-125a |
| [98] | Xie Z et al., PLoS ONE 2013 | CFS and WS | esophageal squamous cell carcinoma | NA | Agilent miRNA microarray | miR-144, miR-10b*, miR-21 and miR-451 |
| [99] | Yang et al., BMC Cancer 2013 | SP | oral squamous cell carcinoma | 7 non-progressing LGD, 8 progressing LGD into OSCC and 7 healthy controls | The TaqManW low density array (TLDA) qRT-PCR system (Applied Biosystems, Foster City, CA, USA) | miR-10b, miR-660, miR-708, miR-30e, miR-145, miR-99b, miR-181c and miR-197 |
| [100] | Salazar et al., Cell Oncol. 2014 | WS | head and neck cancer | 61 HNSCC patients and 61 healthy controls | miScriptTM miRNA microarray, RTqPCR, TCGA | miR-9, miR-134 and miR-191 |
| [101] | Wang et al., Tumor Biol. 2014 | 2 saliva data sets, 6 plasma/serum data sets (meta-analysis) | esophageal squamous cell carcinoma | 995 ESCC patients and 733 healthy controls | Bioinformatic and Statistics tools | miR-144, miR-10, miR-451 |
| [102] | Momen-Heravi et al., J. Dent. Res. 2014 | CFS | oral squamous cell carcinoma | 9 OSCC patients before treatment, 8 patients with OSCC in remission, and 9 healthy controls | NanoString nCounter miRNA expression assay (NanoString Technologies, Seattle, WA, USA) | miRNA-136, miRNA-147, miRNA-1250, miRNA-148a, miRNA- 632, miRNA-646, miRNA668, miRNA- 877, miRNA-503, miRNA-220a, miRNA-323-5p, miRNA-24, miRNA-27b |
| [103] | Hizir et al., ACS Appl. Mater. Interfaces 2014 | CFS | prostate cancer | NA | nanographene oxide system | miR-21, miR141 |
| [104] | Gao et al., BioMed. Res. Int. 2014 | CFS | pancreatic cancer | 30 PC patients and 32 healthy controls | miScript miRNA PCR array human miRNome (384-well plate) (Qiagen) | miR-17, miR-21, miR-181b, miR-196a |
| Forensic Science | ||||||
| [79] | Hanson et al., Anal. Biochem. 2009 | WS | body fluid identification | Healthy donors (# NA) | RTqPCR | miR-658, miR-205 |
| [105] | Zubakov et al., Int. J. Legal. Med. 2010 | WS | body fluid identification | Healthy donors (# NA) | Microarray LNATM-modified oligo-nucleotides (Exiqon, Vedbæk, Denmark), and RTqPCR | miR-583, miR-518c*, miR-208b |
| [106] | Courts et al., J. Forensic Sci. 2011 | WS | body fluid identification | Healthy donors (# NA) | Microarray Geniom Biochips (Heidelberg, Germany) and RTqPCR | miR-200c, miR-203, miR-205 |
| [107] | Wang et al., Forensic Sci. Int. Genet. 2012 | WS | body fluid identification | 10 healthy donors | RTqPCR | miR-658, miR-205 |
| [108] | Omelia et al., Anal. Biochem. 2013 | WS | body fluid identification | 6 healthy donors | RTqPCR | miR-205 |
| [109] | Park JL et al., Electrophoresis 2014 | WS | body fluid identification | 60 healthy donors | Affymetrix Gene Chip miRNA 3.0 array and RTqPCR | miR-203, miR-205 |
| [110] | Silva et al., Forensic Sci. Int. Genet. 2015 | – | body fluid identification | – | – | (Review) |
2.3.1. Non-Coding RNAs for Local Diseases
2.3.2. Non-Coding RNAs for Systemic Diseases
2.4. Forensic Science and Body Fluid Identification
3. Salivary Non-Coding RNAs as a Diagnostic Test Tool
4. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Loo, J.A.; Yan, W.; Ramachandran, P.; Wong, D.T. Comparative human salivary and plasma proteomes. J. Dent. Res. 2010, 89, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Apweiler, R.; Balgley, B.M.; Boontheung, P.; Bundy, J.L.; Cargile, B.J.; Cole, S.; Fang, X.; Gonzalez-Begne, M.; Griffin, T.J.; et al. Systematic comparison of the human saliva and plasma proteomes. Proteomics Clin. Appl. 2009, 3, 116–134. [Google Scholar] [CrossRef] [PubMed]
- Mandel, I.D. The role of saliva in maintaining oral homeostasis. J. Am. Dent. Assoc. 1989, 119, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Amerongen, A.V.N.; Veerman, E.C.I. Saliva—The defender of the oral cavity. Oral Dis. 2002, 8, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Teshima, K.; Murakami, R.; Tomitaka, E.; Nomura, T.; Toya, R.; Hiraki, A.; Nakayama, H.; Hirai, T.; Shinohara, M.; Oya, N.; et al. Radiation-induced parotid gland changes in oral cancer patients: Correlation between parotid volume and saliva production. Jpn. J. Clin. Oncol. 2010, 40, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Wong, D.T. Saliva: An emerging biofluid for early detection of diseases. Am. J. Dent. 2009, 22, 241–248. [Google Scholar] [PubMed]
- Baum, B.J.; Yates, J.R.; Srivastava, S.; Wong, D.T.W.; Melvin, J.E. Scientific frontiers: Emerging technologies for salivary diagnostics. Adv. Dent. Res. 2011, 23, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Segal, A.; Wong, D.T. Salivary diagnostics: Enhancing disease detection and making medicine better. Eur. J. Dent. Educ. 2008, 12, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Li, Y.; Wang, J.; Xie, Y.; Tjon, K.; Wolinsky, L.; Loo, R.R.O.; Loo, J.A.; Wong, D.T. Human saliva proteome and transcriptome. J. Dent. Res. 2006, 85, 1129–1133. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.; Boontheung, P.; Xie, Y.; Sondej, M.; Wong, D.T.; Loo, J.A. Identification of N-linked glycoproteins in human saliva by glycoprotein capture and mass spectrometry. J. Proteome Res. 2006, 5, 1493–1503. [Google Scholar] [CrossRef] [PubMed]
- Whitelegge, J.P.; Zabrouskov, V.; Halgand, F.; Souda, P.; Bassilian, S.; Yan, W.; Wolinsky, L.; Loo, J.A.; Wong, D.T.W.; Faull, K.F. Protein-sequence polymorphisms and post-translational modifications in proteins from human saliva using top–down fourier-transform ion cyclotron resonance mass spectrometry. Int. J. Mass Spectrom. 2007, 268, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Sondej, M.; Denny, P.A.; Xie, Y.; Ramachandran, P.; Si, Y.; Takashima, J.; Shi, W.; Wong, D.T.; Loo, J.A.; Denny, P.C. Glycoprofiling of the human salivary proteome. Clin. Proteomics 2009, 5, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Jiang, J.; Wong, D.T. Proteomic analysis of saliva: 2D gel electrophoresis, LC–MS/MS, and Western blotting. Methods Mol. Biol. 2010, 666, 31–41. [Google Scholar] [PubMed]
- Halgand, F.; Zabrouskov, V.; Bassilian, S.; Souda, P.; Loo, J.A.; Faull, K.F.; Wong, D.T.; Whitelegge, J.P. Defining intact protein primary structures from saliva: A step toward the human proteome project. Anal. Chem. 2012, 84, 4383–4395. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, X.; St John, M.A.R.; Wong, D.T.W. RNA profiling of cell-free saliva using microarray technology. J. Dent. Res. 2004, 83, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Park, N.J.; Li, Y.; Yu, T.; Brinkman, B.M.N.; Wong, D.T. Characterization of RNA in saliva. Clin. Chem. 2006, 52, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Park, N.J.; Zhou, X.; Yu, T.; Brinkman, B.M.N.; Zimmermann, B.G.; Palanisamy, V.; Wong, D.T. Characterization of salivary RNA by cDNA library analysis. Arch. Oral Biol. 2007, 52, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zimmermann, B.G.; Zhou, H.; Wang, J.; Henson, B.S.; Yu, W.; Elashoff, D.; Krupp, G.; Wong, D.T. Exon-level expression profiling: A comprehensive transcriptome analysis of oral fluids. Clin. Chem. 2008, 54, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Wang, J.; Liao, W.; Zimmermann, B.G.; Wong, D.T.; Ho, C.M. Electrochemical detection of low-copy number salivary RNA based on specific signal amplification with a hairpin probe. Nucleic Acids Res. 2008, 36, e65. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, V.; Wong, D.T. Transcriptomic analyses of saliva. Methods Mol. Biol. 2010, 666, 43–51. [Google Scholar] [PubMed]
- Spielmann, N.; Ilsley, D.; Gu, J.; Lea, K.; Brockman, J.; Heater, S.; Setterquist, R.; Wong, D.T.W. The human salivary RNA transcriptome revealed by massively parallel sequencing. Clin. Chem. 2012, 58, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Farrell, J.J.; Zhang, L.; Zhou, H.; Chia, D.; Elashoff, D.; Akin, D.; Paster, B.J.; Joshipura, K.; Wong, D.T.W. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 2012, 61, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Wong, D.T.; Hirayama, A.; Soga, T.; Tomita, M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics 2010, 6, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.A.; Alex, S.; Routray, S. Incorporation of salivary metabolomics in oral cancer diagnostics. Oral Oncol. 2014, 50, e53–e54. [Google Scholar] [CrossRef] [PubMed]
- Barnes, V.M.; Kennedy, A.D.; Panagakos, F.; Devizio, W.; Trivedi, H.M.; Jönsson, T.; Guo, L.; Cervi, S.; Scannapieco, F.A. Global metabolomic analysis of human saliva and plasma from healthy and diabetic subjects, with and without periodontal disease. PLoS ONE 2014, 9, e105181. [Google Scholar] [CrossRef] [PubMed]
- Salivaomics Knowledge Base. Available online: http// www.skb.ucla.edu (accessed on 23 July 2010).
- Ai, J.; Smith, B.; Wong, D.T. Saliva ontology: An ontology-based framework for a salivaomics knowledge base. BMC Bioinform. 2010, 11, 302. [Google Scholar] [CrossRef]
- Wong, D.T.W. Salivaomics. J. Am. Dent. Assoc. 2012, 143, S19–S24. [Google Scholar] [CrossRef]
- Navazesh, M. Methods for collecting saliva. Ann. N. Y. Acad. Sci. 1993, 694, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Henson, B.S.; Wong, D.T. Collection, storage, and processing of saliva samples for downstream molecular applications. Methods Mol. Biol. 2010, 666, 21–30. [Google Scholar] [PubMed]
- Lee, Y.H.; Zhou, H.; Reiss, J.K.; Yan, X.; Zhang, L.; Chia, D.; Wong, D.T.W. Direct saliva transcriptome analysis. Clin. Chem. 2011, 57, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Park, N.J.; Yu, T.; Nabili, V.; Brinkman, B.M.N.; Henry, S.; Wang, J.; Wong, D.T. RNAprotect saliva: An optimal room-temperature stabilization reagent for the salivary transcriptome. Clin. Chem. 2006, 52, 2303–2304. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Park, N.J.; Hu, S.; Wong, D.T. A universal pre-analytic solution for concurrent stabilization of salivary proteins, RNA and DNA at ambient temperature. Arch. Oral Biol. 2009, 54, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Zhou, H.; Nabili, V.; Wang, M.B.; Abemayor, E.; Wong, D.T.W. Utility of multiple sampling in reducing variation of salivary interleukin-8 and interleukin-1β mRNA levels in healthy adults. Head Neck 2013, 35, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Wong, D.T.W. Method development for proteome stabilization in human saliva. Anal. Chim. Acta 2012, 722, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, J.; Meijer, J.; Ieong, S.; Xie, Y.; Yu, T.; Zhou, H.; Henry, S.; Vissink, A.; Pijpe, J.; et al. Salivary proteomic and genomic biomarkers for primary Sjögren’s syndrome. Arthritis Rheumatol. 2007, 56, 3588–3600. [Google Scholar] [CrossRef]
- Hu, S.; Gao, K.; Pollard, R.; Arellano-Garcia, M.; Zhou, H.; Zhang, L.; Elashoff, D.; Kallenberg, C.G.M.; Vissink, A.; Wong, D.T. Preclinical validation of salivary biomarkers for primary Sjögren’s syndrome. Arthritis Care Res. (Hoboken) 2010, 62, 1633–1638. [Google Scholar] [CrossRef]
- Hu, S.; Vissink, A.; Arellano, M.; Roozendaal, C.; Zhou, H.; Kallenberg, C.G.M.; Wong, D.T. Identification of autoantibody biomarkers for primary Sjögren’s syndrome using protein microarrays. Proteomics 2011, 11, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- St John, M.A.R.; Li, Y.; Zhou, X.; Denny, P.; Ho, C.M.; Montemagno, C.; Shi, W.; Qi, F.; Wu, B.; Sinha, U.; et al. Interleukin 6 and interleukin 8 as potential biomarkers for oral cavity and oropharyngeal squamous cell carcinoma. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; St John, M.A.R.; Zhou, X.; Kim, Y.; Sinha, U.; Jordan, R.C.K.; Eisele, D.; Abemayor, E.; Elashoff, D.; Park, N.H.; et al. Salivary transcriptome diagnostics for oral cancer detection. Clin. Cancer Res. 2004, 10, 8442–8450. [Google Scholar] [CrossRef] [PubMed]
- Arellano-Garcia, M.E.; Hu, S.; Wang, J.; Henson, B.; Zhou, H.; Chia, D.; Wong, D.T. Multiplexed immunobead-based assay for detection of oral cancer protein biomarkers in saliva. Oral Dis. 2008, 14, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Arellano, M.; Boontheung, P.; Wang, J.; Zhou, H.; Jiang, J.; Elashoff, D.; Wei, R.; Loo, J.A.; Wong, D.T. Salivary proteomics for oral cancer biomarker discovery. Clin. Cancer Res. 2008, 14, 6246–6252. [Google Scholar] [CrossRef] [PubMed]
- Elashoff, D.; Zhou, H.; Reiss, J.; Wang, J.; Xiao, H.; Henson, B.; Hu, S.; Arellano, M.; Sinha, U.; Le, A.; et al. Prevalidation of salivary biomarkers for oral cancer detection. Cancer Epidemiol. Biomark. Prev. 2012, 21, 664–672. [Google Scholar] [CrossRef]
- Tamashiro, H.; Constantine, N.T. Serological diagnosis of HIV infection using oral fluid samples. Bull. World Health Organ. 1994, 72, 135–143. [Google Scholar] [PubMed]
- Mortimer, P.P.; Parry, J. V Detection of antibody to HIV in saliva: A brief review. Clin. Diagn. Virol. 1994, 2, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Hodinka, R.L.; Nagashunmugam, T.; Malamud, D. Detection of human immunodeficiency virus antibodies in oral fluids. Clin. Diagn. Lab. Immunol. 1998, 5, 419–426. [Google Scholar] [PubMed]
- Yaari, A.; Tovbin, D.; Zlotnick, M.; Mostoslavsky, M.; Shemer-Avni, Y.; Hanuka, N.; Burbea, Z.; Katzir, Z.; Storch, S.; Margalith, M. Detection of HCV salivary antibodies by a simple and rapid test. J. Virol. Methods 2006, 133, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Elsana, S.; Sikuler, E.; Yaari, A.; Shemer-Avni, Y.; Abu-Shakra, M.; Buskila, D.; Katzman, P.; Naggan, L.; Margalith, M. HCV antibodies in saliva and urine. J. Med. Virol. 1998, 55, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.J.; Park, Q.; Kang, E.S.; Yoo, B.C.; Park, K.U.; Kim, J.W.; Hwang, Y.S.; Kim, M.H. Performance evaluation of the OraQuick hepatitis C virus rapid antibody test. Ann. Lab. Med. 2013, 33, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Jurado, F.; Hernández-Galindo, V.L.; Meléndez-Mena, D.; Mendoza-Torres, M.A.; Martínez-Arroniz, F.J.; Vallejo-Ruiz, V.; Reyes-Leyva, J.; Santos-López, G. Detection of hepatitis C virus RNA in saliva of patients with active infection not associated with periodontal or liver disease severity. BMC Infect. Dis. 2014, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V; Reddy, A.P.; Lu, X.; Dasari, S.; Krishnaprasad, A.; Biggs, E.; Roberts, C.T.; Nagalla, S.R. Proteomic identification of salivary biomarkers of type-2 diabetes. J. Proteome Res. 2009, 8, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Desai, G.S.; Mathews, S.T. Saliva as a non-invasive diagnostic tool for inflammation and insulin-resistance. World J. Diabetes 2014, 5, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Mackay, D.F.; Newby, D.E.; Pell, J.P. Association between salivary cotinine and cardiovascular biomarkers among nonsmokers and current smokers: Cross-sectional study of 10,081 participants. Eur. J. Vasc. Endovasc. Surg. 2014, 48, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Li, R.; Zhang, J.; Zhou, S.; Ma, Q.; Zhou, Y.; Chen, F.; Lin, J. Salivary biomarkers indicate obstructive sleep apnea patients with cardiovascular diseases. Sci. Rep. 2014, 4, 7046. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Zhou, H.; Zhang, L.; Lee, J.W.; Zhou, Q.; Hu, S.; Wolinsky, L.E.; Farrell, J.; Eibl, G.; Wong, D.T. Systemic disease-induced salivary biomarker profiles in mouse models of melanoma and non-small cell lung cancer. PLoS ONE 2009, 4, e5875. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Zhang, L.; Zhou, H.; Lee, J.M.; Garon, E.B.; Wong, D.T.W. Proteomic analysis of human saliva from lung cancer patients using two-dimensional difference gel electrophoresis and mass spectrometry. Mol. Cell. Proteomics 2012, 11. M111.012112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiao, H.; Zhou, H.; Santiago, S.; Lee, J.M.; Garon, E.B.; Yang, J.; Brinkmann, O.; Yan, X.; Akin, D.; et al. Development of transcriptomic biomarker signature in human saliva to detect lung cancer. Cell. Mol. Life Sci. 2012, 69, 3341–3350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Farrell, J.J.; Zhou, H.; Elashoff, D.; Akin, D.; Park, N.H.; Chia, D.; Wong, D.T. Salivary transcriptomic biomarkers for detection of resectable pancreatic cancer. Gastroenterology 2010, 138, 949–957.e7. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; Kim, Y.; Chia, D.; Spielmann, N.; Eibl, G.; Elashoff, D.; Wei, F.; Lin, Y.L.; Moro, A.; Grogan, T.; et al. Role of pancreatic cancer-derived exosomes in salivary biomarker development. J. Biol. Chem. 2013, 288, 26888–26897. [Google Scholar] [CrossRef] [PubMed]
- Brooks, M.N.; Wang, J.; Li, Y.; Zhang, R.; Elashoff, D.; Wong, D.T. Salivary protein factors are elevated in breast cancer patients. Mol. Med. Rep. 2008, 1, 375–378. [Google Scholar] [PubMed]
- Zhang, L.; Xiao, H.; Karlan, S.; Zhou, H.; Gross, J.; Elashoff, D.; Akin, D.; Yan, X.; Chia, D.; Karlan, B.; et al. Discovery and preclinical validation of salivary transcriptomic and proteomic biomarkers for the non-invasive detection of breast cancer. PLoS ONE 2010, 5, e15573. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kim, J.H.; Zhou, H.; Kim, B.W.; Wong, D.T. Salivary transcriptomic biomarkers for detection of ovarian cancer: For serous papillary adenocarcinoma. J. Mol. Med. 2012, 90, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S. Non-coding RNAs: The architects of eukaryotic complexity. EMBO Rep. 2001, 2, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Morris, K.V.; Mattick, J.S. The rise of regulatory RNA. Nat. Rev. Genet. 2014, 15, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Orozco, A.F.; Lewis, D.E. Flow cytometric analysis of circulating microparticles in plasma. Cytometry A 2010, 77, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, C.A.; Voinnet, O. The long and the short of noncoding RNAs. Curr. Opin. Cell Biol. 2009, 21, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Esteller, M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011, 12, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Reis, E.M.; Verjovski-Almeida, S. Perspectives of long non-coding RNAs in cancer diagnostics. Front. Genet. 2012, 3, 32. [Google Scholar] [PubMed]
- Mercer, T.R.; Dinger, M.E.; Mattick, J.S. Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 2009, 10, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Dalmay, T. MicroRNAs and cancer. J. Intern. Med. 2008, 263, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Tandon, M.; Gallo, A.; Jang, S.I.; Illei, G.G.; Alevizos, I. Deep sequencing of short RNAs reveals novel microRNAs in minor salivary glands of patients with Sjögren’s syndrome. Oral Dis. 2012, 18, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Taketomi, Y.; Murakami, M.; Tsujimoto, M.; Yanoshita, R. Small RNA transcriptomes of two types of exosomes in human whole saliva determined by next generation sequencing. Biol. Pharm. Bull. 2013, 36, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Bahn, J.H.; Zhang, Q.; Li, F.; Chan, T.M.; Lin, X.; Kim, Y.; Wong, D.T.W.; Xiao, X. The landscape of microRNA, Piwi-interacting RNA, and circular RNA in human saliva. Clin. Chem. 2015, 61, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.; Bajracharya, S.D.; Yuen, P.S.T.; Zhou, H.; Star, R.A; Illei, G.G.; Alevizos, I. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010, 16, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.; Kiriakidou, M.; Sharma, A.; Maniataki, E.; Mourelatos, Z. The microRNA world: Small is mighty. Trends Biochem. Sci. 2003, 28, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.S.; Jakymiw, A.; Yao, B.; Pauley, B.A.; Carcamo, W.C.; Katz, J.; Cheng, J.Q.; Chan, E.K.L. High resolution of microRNA signatures in human whole saliva. Arch. Oral Biol. 2011, 56, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Park, N.J.; Zhou, H.; Elashoff, D.; Henson, B.S.; Kastratovic, D.A.; Abemayor, E.; Wong, D.T. Salivary microRNA: Discovery, characterization, and clinical utility for oral cancer detection. Clin. Cancer Res. 2009, 15, 5473–5477. [Google Scholar] [CrossRef] [PubMed]
- Hanson, E.K.; Lubenow, H.; Ballantyne, J. Identification of forensically relevant body fluids using a panel of differentially expressed microRNAs. Anal. Biochem. 2009, 387, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 940–948. [Google Scholar] [CrossRef]
- Pisitkun, T.; Shen, R.; Knepper, M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef] [PubMed]
- Lässer, C.; Alikhani, V.S.; Ekström, K.; Eldh, M.; Paredes, P.T.; Bossios, A.; Sjöstrand, M.; Gabrielsson, S.; Lötvall, J.; Valadi, H. Human saliva, plasma and breast milk exosomes contain RNA: Uptake by macrophages. J. Transl. Med. 2011, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Mittelbrunn, M.; Gutiérrez-Vázquez, C.; Villarroya-Beltri, C.; González, S.; Sánchez-Cabo, F.; González, M.Á.; Bernad, A.; Sánchez-Madrid, F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011, 2. [Google Scholar] [CrossRef]
- Berckmans, R.J.; Sturk, A.; van Tienen, L.M.; Schaap, M.C.L.; Nieuwland, R. Cell-derived vesicles exposing coagulant tissue factor in saliva. Blood 2011, 117, 3172–3180. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Miura, Y.; Harazono, A.; Kanai-Azuma, M.; Akimoto, Y.; Kawakami, H.; Yamaguchi, T.; Toda, T.; Endo, T.; Tsubuki, M.; et al. Proteomic analysis of two types of exosomes in human whole saliva. Biol. Pharm. Bull. 2011, 34, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, V.; Sharma, S.; Deshpande, A.; Zhou, H.; Gimzewski, J.; Wong, D.T. Nanostructural and transcriptomic analyses of human saliva derived exosomes. PLoS ONE 2010, 5, e8577. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Rasool, H.I.; Palanisamy, V.; Mathisen, C.; Schmidt, M.; Wong, D.T.; Gimzewski, J.K. Structural-mechanical characterization of nanoparticle exosomes in human saliva, using correlative AFM, FESEM, and force spectroscopy. ACS Nano 2010, 4, 1921–1926. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Wong, D.T.W. Proteomic analysis of microvesicles in human saliva by gel electrophoresis with liquid chromatography–mass spectrometry. Anal. Chim. Acta 2012, 723, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Tandon, M.; Alevizos, I.; Illei, G.G. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS ONE 2012, 7, e30679. [Google Scholar] [CrossRef] [PubMed]
- Boja, E.; Hiltke, T.; Rivers, R.; Kinsinger, C.; Rahbar, A.; Mesri, M.; Rodriguez, H. Evolution of clinical proteomics and its role in medicine. J. Proteome Res. 2011, 10, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, E.D.; Gao, S.; Hulf, T.; Sibbritt, T.; Nair, S.; Costea, D.E.; Villadsen, S.B.; Bakholdt, V.; Bramsen, J.B.; Sørensen, J.A.; et al. MicroRNA alterations and associated aberrant DNA methylation patterns across multiple sample types in oral squamous cell carcinoma. PLoS ONE 2011, 6, e27840. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Lin, S.C.; Yang, C.C.; Cheng, H.W.; Chang, K.W. Exploiting salivary miR-31 as a clinical biomarker of oral squamous cell carcinoma. Head Neck 2012, 34, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Matse, J.H.; Yoshizawa, J.; Wang, X.; Elashoff, D.; Bolscher, J.G.M.; Veerman, E.C.I.; Bloemena, E.; Wong, D.T.W. Discovery and prevalidation of salivary extracellular microRNA biomarkers panel for the noninvasive detection of benign and malignant parotid gland tumors. Clin. Cancer Res. 2013, 19, 3032–3038. [Google Scholar] [PubMed]
- Tang, H.; Wu, Z.; Zhang, J.; Su, B. Salivary lncRNA as a potential marker for oral squamous cell carcinoma diagnosis. Mol. Med. Rep. 2013, 7, 761–766. [Google Scholar] [PubMed]
- Wang, Z.; Zhang, J.; Guo, Y.; Wu, X.; Yang, W.; Xu, L.; Chen, J.; Fu, F. A novel electrically magnetic-controllable electrochemical biosensor for the ultra sensitive and specific detection of attomolar level oral cancer-related microRNA. Biosens. Bioelectron. 2013, 45, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Chen, G.; Zhang, X.; Li, D.; Huang, J.; Yang, C.; Zhang, P.; Qin, Y.; Duan, Y.; Gong, B.; Li, Z. Salivary microRNAs as promising biomarkers for detection of esophageal cancer. PLoS ONE 2013, 8, e57502. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Y.; Yang, X.; Jiang, L.; Zhou, Z.; Zhu, Y. Progress risk assessment of oral premalignant lesions with saliva miRNA analysis. BMC Cancer 2013, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Salazar, C.; Nagadia, R.; Pandit, P.; Cooper-White, J.; Banerjee, N.; Dimitrova, N.; Coman, W.B.; Punyadeera, C. A novel saliva-based microRNA biomarker panel to detect head and neck cancers. Cell. Oncol. 2014, 37, 331–338. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhang, N.; Ma, H.; Gu, Y.; Tang, H.; Xu, Z.; Gao, Y. Identification of microRNAs as novel biomarkers for detecting esophageal squamous cell carcinoma in Asians: A meta-analysis. Tumour Biol. 2014, 35, 11595–11604. [Google Scholar] [CrossRef] [PubMed]
- Momen-Heravi, F.; Trachtenberg, A.J.; Kuo, W.P.; Cheng, Y.S. Genomewide study of salivary microRNAs for detection of oral cancer. J. Dent. Res. 2014, 93, S86–S93. [Google Scholar] [CrossRef]
- Hizir, M.S.; Balcioglu, M.; Rana, M.; Robertson, N.M.; Yigit, M.V. Simultaneous detection of circulating oncomiRs from body fluids for prostate cancer staging using nanographene oxide. ACS Appl. Mater. Interfaces 2014, 6, 14772–14778. [Google Scholar] [PubMed]
- Gao, S.; Chen, L.Y.; Wang, P.; Liu, L.M.; Chen, Z. MicroRNA expression in salivary supernatant of patients with pancreatic cancer and its relationship with Zheng. Biomed Res. Int. 2014, 2014, 1–8. [Google Scholar]
- Zubakov, D.; Boersma, A.W.M.; Choi, Y.; van Kuijk, P.F.; Wiemer, E.A.C.; Kayser, M. MicroRNA markers for forensic body fluid identification obtained from microarray screening and quantitative RT-PCR confirmation. Int. J. Legal Med. 2010, 124, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Courts, C.; Madea, B. Specific micro-RNA signatures for the detection of saliva and blood in forensic body-fluid identification. J. Forensic Sci. 2011, 56, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, H.; Pan, X.; Liao, M.; Hou, Y. A model for data analysis of microRNA expression in forensic body fluid identification. Forensic Sci. Int. Genet. 2012, 6, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Omelia, E.J.; Uchimoto, M.L.; Williams, G. Quantitative PCR analysis of blood- and saliva-specific microRNA markers following solid-phase DNA extraction. Anal. Biochem. 2013, 435, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Park, J.L.; Park, S.M.; Kwon, O.H.; Lee, H.; Kim, J.; Seok, H.H.; Lee, W.S.; Lee, S.H.; Kim, Y.S.; Woo, K.M.; et al. Microarray screening and qRT-PCR evaluation of microRNA markers for forensic body fluid identification. Electrophoresis 2014, 35, 3062–3068. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.S.; Lopes, C.; Teixeira, A.L.; de Sousa, M.J.C.; Medeiros, R. Forensic miRNA: Potential biomarker for body fluids. Forensic Sci. Int. Genet. 2015, 14C, 1–10. [Google Scholar] [CrossRef] [PubMed]
- NanoString nCounter® miRNA Expression Assays. Available online: http://www.nanostring.com/products/miRNA (accessed on 19 April 2010).
- Wu, W.; Hou, W.; Wu, Z.; Wang, Y.; Yi, Y.; Lin, W. miRNA-144 in the saliva is a genetic marker for early diagnosis of esophageal cancer. Nan Fang Yi Ke Da Xue Xue Bao 2013, 33, 1783–1786. [Google Scholar] [PubMed]
- Isin, M.; Ozgur, E.; Cetin, G.; Erten, N.; Aktan, M.; Gezer, U.; Dalay, N. Investigation of circulating lncRNAs in B-cell neoplasms. Clin. Chim. Acta 2014, 431, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Wang, F.; Shen, J.; Sun, Y.; Xu, W.; Lu, J.; Wei, M.; Xu, C.; Wu, C.; Zhang, Z.; et al. Long non-coding RNA metastasis associated in lung adenocarcinoma transcript 1 derived miniRNA as a novel plasma-based biomarker for diagnosing prostate cancer. Eur. J. Cancer 2013, 49, 2949–2959. [Google Scholar] [CrossRef] [PubMed]
- Rönnau, C.G.H.; Verhaegh, G.W.; Luna-Velez, M.V.; Schalken, J.A. Noncoding RNAs as novel biomarkers in prostate cancer. Biomed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Kohls, K.; Schmidt, D.; Holdenrieder, S.; Müller, S.C.; Ellinger, J. Detection of cell-free lncRNA in serum of cancer patients. Urol. A 2014. [Google Scholar] [CrossRef]
- Juusola, J.; Ballantyne, J. Messenger RNA profiling: A prototype method to supplant conventional methods for body fluid identification. Forensic Sci. Int. 2003, 135, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Juusola, J.; Ballantyne, J. Multiplex mRNA profiling for the identification of body fluids. Forensic Sci. Int. 2005, 152, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.; Ballantyne, J. The identification of newborns using messenger RNA profiling analysis. Anal. Biochem. 2006, 357, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, J.; Lin, C.C.; Abemayor, E.; Wang, M.B.; Wong, D.T.W. The emerging landscape of salivary diagnostics. Oral Health Dent. Manag. 2014, 13, 200–210. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majem, B.; Rigau, M.; Reventós, J.; Wong, D.T. Non-Coding RNAs in Saliva: Emerging Biomarkers for Molecular Diagnostics. Int. J. Mol. Sci. 2015, 16, 8676-8698. https://doi.org/10.3390/ijms16048676
Majem B, Rigau M, Reventós J, Wong DT. Non-Coding RNAs in Saliva: Emerging Biomarkers for Molecular Diagnostics. International Journal of Molecular Sciences. 2015; 16(4):8676-8698. https://doi.org/10.3390/ijms16048676
Chicago/Turabian StyleMajem, Blanca, Marina Rigau, Jaume Reventós, and David T. Wong. 2015. "Non-Coding RNAs in Saliva: Emerging Biomarkers for Molecular Diagnostics" International Journal of Molecular Sciences 16, no. 4: 8676-8698. https://doi.org/10.3390/ijms16048676
APA StyleMajem, B., Rigau, M., Reventós, J., & Wong, D. T. (2015). Non-Coding RNAs in Saliva: Emerging Biomarkers for Molecular Diagnostics. International Journal of Molecular Sciences, 16(4), 8676-8698. https://doi.org/10.3390/ijms16048676