1. Introduction
The
in utero stage is a vulnerable window in which organisms are very sensitive to environmental factors [
1]. Increasing evidence indicates that maternal exposure to various dietary and environmental factors during pregnancy has a profound impact on the offspring’s mammary development and breast cancer risk [
2]. A notable example is the DES (diethylstilbestrol) daughter story; the daughters of women exposed to DES during pregnancy have an increased risk of vaginal adenocarcinoma and breast cancer [
3]. Animal studies have shown that
in utero exposure to a number of factors, such as bisphenol A (BPA) and
n-6 polyunsaturated fatty acids (PUFA), increase mammary tumor risk [
4,
5]. In contrast, maternal exposure to soy protein isolate and
n-3 PUFA was associated with decreased mammary tumor risk in the DMBA carcinogen model [
6,
7]. These studies underscore the significance of prenatal factors in breast cancer risk later in life.
Although alcohol intake has been associated with increased breast cancer risk, most studies were based on the risk of the individuals who consumed the alcohol [
8,
9]. Studies on the impact of mothers’ alcohol consumption during pregnancy on daughters’ mammary development and breast cancer risk, however, remain sparse. Nevertheless, the CDC reported that 7.6% of pregnant women admitted drinking alcohol during pregnancy [
10].
In utero exposure to alcohol has been associated with developmental damage or diseases such as fetal alcohol syndrome (FAS) [
11]. With increasing reports on the modification of tumor risk later in life by
in utero exposure, it is imperative to understand whether and how
in utero exposure to alcohol modifies breast cancer risk. Hilakivi-Clark
et al. first reported that
in utero exposure to alcohol via liquid diets containing 16–25 g alcohol per kg fed between days 7 and 19 of gestation promoted 7,12-dimethylbenz(a)anthracene (DMBA)-induced mammary tumor development in Sprague-Dawley rats [
12]. Polanco
et al. found that
in utero alcohol exposure via liquid diets containing 6.7% (
v/
v) alcohol increased mammary tumor susceptibility to
N-nitroso-
N-methylurea (NMU)-mediated carcinogenesis in rats, as indicated by greater multiplicity and more malignant phenotypes [
13]. The results from the above studies indicated that the modified mammary tumor risk associated with
in utero exposure to alcohol was associated with increased serum estradiol levels in alcohol-exposed mothers, as well as enhanced prepubertal mammary development and deregulated IGF-I and estradiol systems in their offspring [
12,
13,
14]. These changes appear to be similar to the alterations induced by
in utero exposure to hormonal disruptors [
12].
Although earlier studies provided “proof of concept” for that in utero exposure to alcohol could modify mammary tumor risk, the molecular mechanisms by which in utero exposure to alcohol modifies mammary development and tumor risk remain largely unknown. Factors that may affect the effects of in utero alcohol exposure, such as genetic predisposition, have not been documented. To facilitate mechanistic studies, a transgenic model with a defined genetic background and clinically relevant predisposition may provide further insight into the underlying mechanisms. We therefore tested the effect of in utero exposure to low-dose alcohol on mammary tumor risk in MMTV-erbB-2 transgenic mice.
erbB-2 (Her2/neu) is a member of the ErbB family of receptor tyrosine kinases (RTKs), which also includes EGFR, erbB-3 and erbB-4 [
15]. Aberrant expression/activation of these RTKs plays a critical role in breast cancer development. In particular, erbB-2 is amplified/overexpressed in approximately 30% of human breast cancers, and this effect has been associated with poor prognosis and therapeutic resistance [
16,
17]. Activated erbB-2 interacts with its family members to induce the activation of a plethora of pathways, such as the PI3K/Akt and MAPK/Erk pathways, that are involved in cell proliferation, survival and other activities [
18,
19,
20]. The MMTV-erbB-2 transgenic mouse is a well-established model for studies on the effect of various environmental, dietary and genetic factors on erbB-2-mediated carcinogenesis [
21,
22]. Using this model, we demonstrated an interaction between estrogen and erbB-2 induced mammary tumor development in transgenic mice [
23]. We also showed that low doses of soy isoflavones interfered with tamoxifen-mediated chemoprevention in MMTV-erbB-2 mice [
24], indicating that this model can detect subtle oncogenic factors. Moreover, Wong
et al. reported that postnatal alcohol consumption promoted mammary tumor development in MMTV-erbB-2 transgenic mice through the estrogen pathway [
25]. These studies suggest that MMTV-erbB-2 transgenic mice could be a useful model for studying the modification of mammary development and tumor risk by
in utero exposure to alcohol.
We describe here, for the first time, the use of MMTV-erbB-2 transgenic mice to examine the effects of in utero alcohol exposure on mammary development and tumor risk. We found that in utero exposure to low-dose alcohol induced prolonged ductal extension and altered differentiation of mammary glands. Although the treatment increased tumor multiplicity, tumor development was initially delayed under the given conditions. Molecular analysis indicated that in utero alcohol exposure induced the upregulation of the ER and the erbB-2 pathways in mammary tissues. These results also provide support for using the MMTV-erbB-2 transgenic mouse model for studies on in utero exposure to alcohol-modified mammary tumor development.
3. Discussion
The impact of
in utero exposure to environmental or dietary factors on adult health and disease is an emerging field in biomedical research. The increased risk of breast cancer in later life induced by
in utero alcohol exposure is of particular importance because of the common occurrence of alcohol drinking among pregnant women [
10]. Previously, two studies showed that
in utero alcohol exposure induced increases in tumor multiplicity in DMBA- or NMU-treated rats [
12,
13]. In one study, pregnant rats were fed liquid diets containing 16 g (7% alcohol of total energy) or 25 g (15% alcohol of total energy) alcohol/kg feed [
12]. Based on a study on liquid diet consumption and alcohol intake [
29], the alcohol doses used in this study would have been approximately 5 and 9 g/kg/day, respectively. In the other study, the dams were fed a liquid diet containing 6.7% ethanol from days 11 to 21 (35% of total calories) of gestation [
13,
14], which would have been a dose of approximately 16 g/kg/day. In the present study, pregnant MMTV-erbB-2 transgenic mice were exposed to 3–6 g/kg body weight of alcohol via the oral feeding of drinking water to mimic binge drinking. This is a much lower dose than in previous
in utero exposure studies. Previous studies showed that mice administered 1.12 g/kg alcohol had in a BAC of 141 mg/dL at 30 min after administration [
26]. In this study, the BAC of the pregnant erbB-2 mice 30 min after exposure to half of the daily dose was 114.5 mg/dL. Compared with previous reports, alcohol exposure in the pregnant mice in this study was in the low dose range.
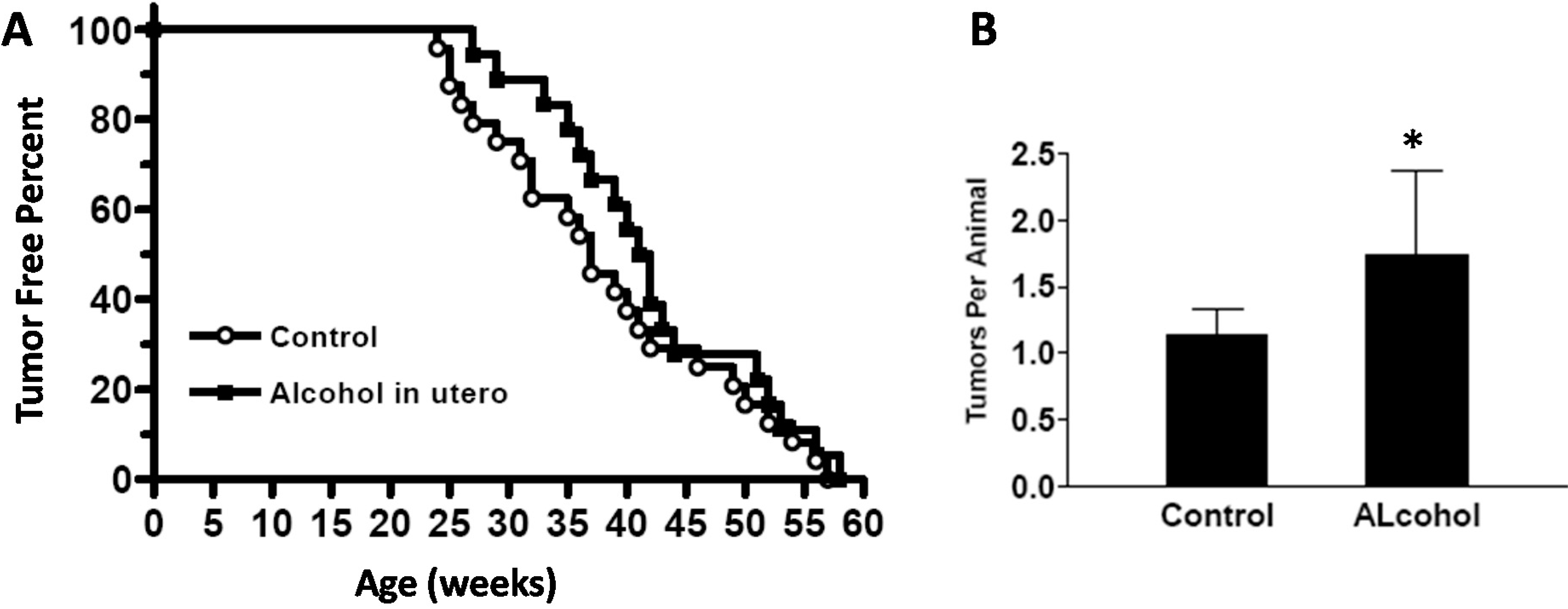
Our results showed that in utero exposure to low-dose alcohol induced a series of alterations in mammary development and tumor risk in MMTV-erbB-2 transgenic mice. The notable changes included earlier onset of mammary development at puberty, longer extension of ductal trees at 10 weeks of age, and the upregulation of ER- and erbB-2-regulated signaling in the mammary tissues. In utero alcohol exposure induced an initial delay and later an acceleration of tumor development. Nevertheless, tumor multiplicity in these animals was increased. These results suggest that in utero exposure even to low doses of alcohol induces mammary reprogramming and has profound impacts on tumor risk later in life.
We found that
in utero exposure to low doses of alcohol induced an intriguing tumor development pattern, which was an increase of tumor latency before 40 weeks of age. The rate of tumor formation was slow initially and then increased to catch up with the control group. The initial delay in tumor development was the opposite to what was expected. These data should be interpreted with caution. This delay might be partially explained by the weak proestrogenic effect induced by
in utero exposure to low-dose alcohol. Previous studies showed that subtle changes in hormonal modulation may result in opposing effects on mammary tumor risk. For example,
in utero exposure to genistein or n6 PUFA increased mammary tumor risk [
5,
30], whereas
in utero exposure to soy protein isolate or n3 PUFA was associated with a protection from mammary tumor risk [
6,
7]. We also found that MMTV-erbB-2 mice implanted with low-dose estrogen pellets at a younger age had a slight delay in tumor latency but faster tumor development in the later stage, although implantation of high-dose estradiol (E2) pellets after puberty significantly promoted tumor development [
23]. Opposite results from subtle changes of microenvironment were also observed in modified growth factor signaling. One of such examples is that inhibition of the IGF1 receptor promoted mammary tumor development in MMTV-Wnt mice [
31]. In the present study, the weak estrogen-like effect associated with
in utero exposure to a low dose of alcohol may induce altered differentiation that results in the initial delay in tumor development. Nevertheless, the modified tumor development pattern also includes relatively faster tumor development after 40 weeks and increased tumor multiplicity in mice that show a longer latency for tumor development, which could result from increased susceptibility to mutations due to altered hormonal and developmental conditions [
32]. The specific mechanisms underlying these changes warrant further investigation. Based on the findings from this report, effects of
in utero exposure to various doses of alcohol on mammary tumor risk in MMTV-erbB-2 transgenic mice will be followed.
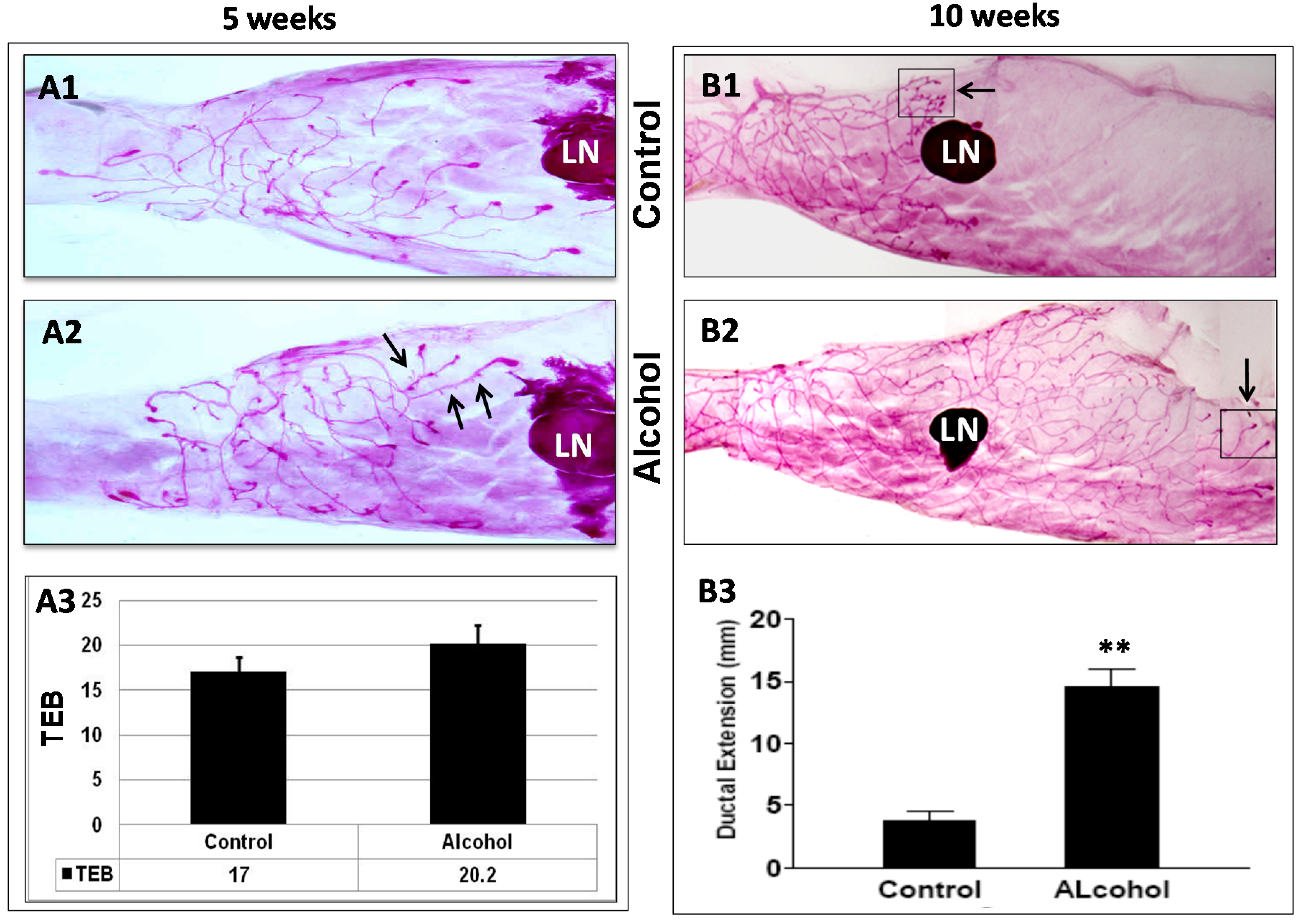
We found that
in utero exposure to alcohol induced significant changes in mammary morphogenesis in MMTV-erbB-2 mice. Previously, Hilakivi-Clarke
et al. showed that
in utero alcohol exposure induced increases in TEB numbers at puberty and mammary epithelial densities in 8- and 22-week-old rats [
12]. Polano
et al. reported that exposure to high-dose alcohol
in utero had no effect on TEB numbers, although the proliferation index measured by BrdU incorporation was greater at PND 20 in the glands of the alcohol group [
13,
14]. In the present study,
in utero alcohol exposure induced significant changes in mammary morphogenesis. Although the difference in TEB numbers in pubertal glands was not significant, mammary glands from mice that had undergone
in utero alcohol exposure displayed prolonged extension of ductal trees at 10 weeks, a striking difference between the two groups. In particular, this change was accompanied by a reduction in the number of lateral buds and an increase in pubertal TEB-like structures at the distal end of the ductal tree compared to the controls. Our results suggest that
in utero alcohol exposure not only induces ductal outgrowth but also alters mammary differentiation and the dynamics of morphogenesis, which results in the reprogramming of mammary development and tumor risk.
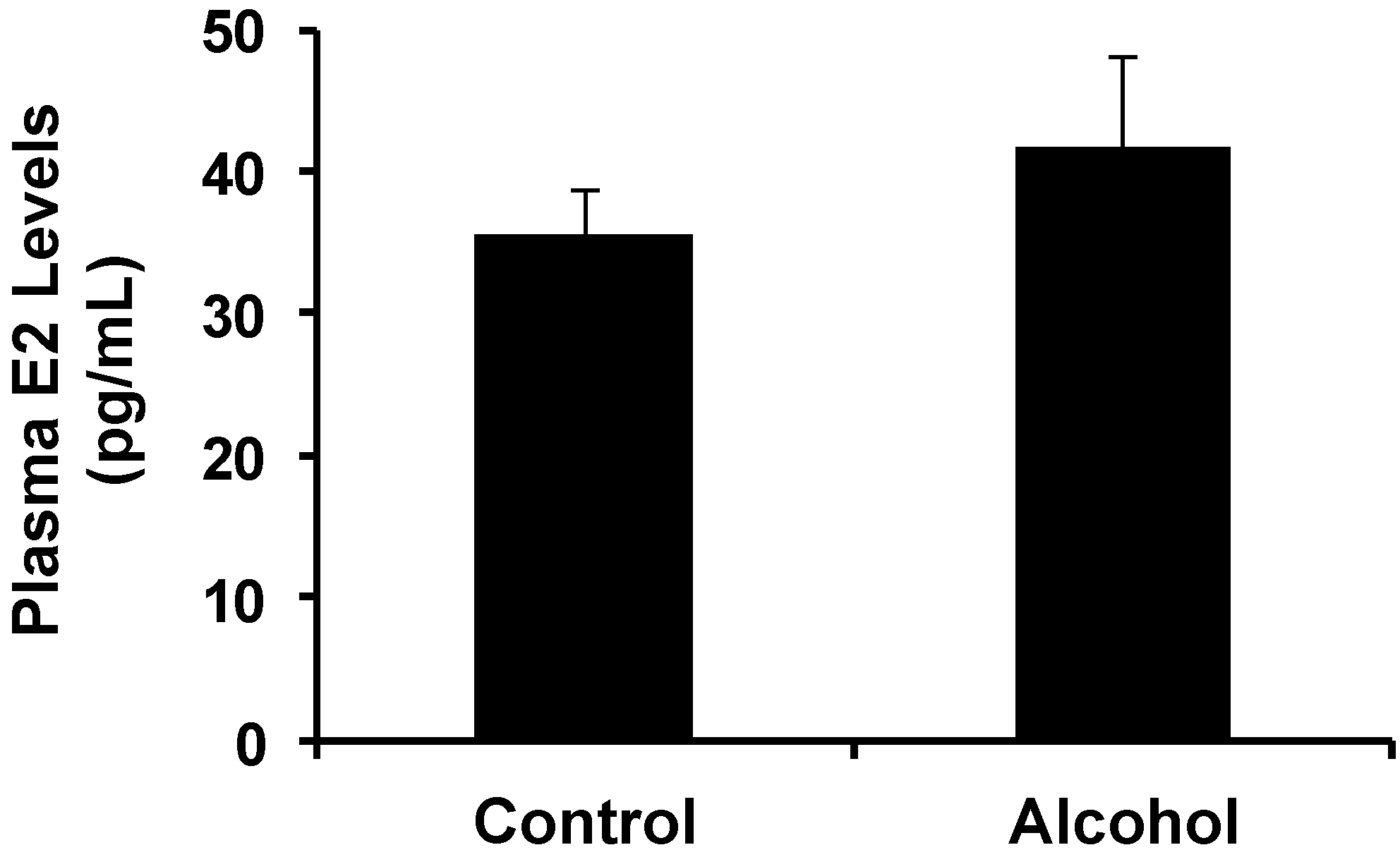
It has been reported that serum E2 levels were increased in pregnant rats exposed to alcohol and in offspring after
in utero alcohol exposure, and these changes were associated with increased tumorigenesis [
12,
13,
14]. Our data showed that increases in plasma E2 levels in alcohol-treated pregnant mice were not significant (
Figure 1), which could be related to the lower dose of alcohol. Because
in utero exposure to estrogen or estrogenic compounds has been associated with modified mammary tumor risk [
30], the correlation between
in utero exposure to alcohol-induced changes and altered E2 levels warrants further investigation. A dose-response relationship will be examined in future studies to establish this connection.
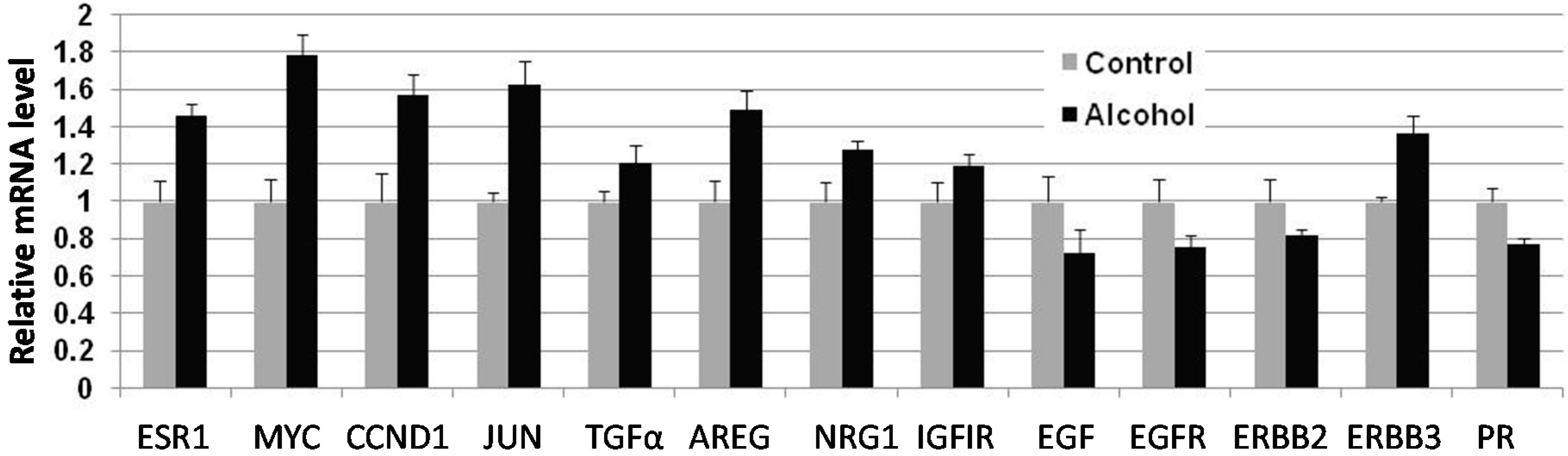
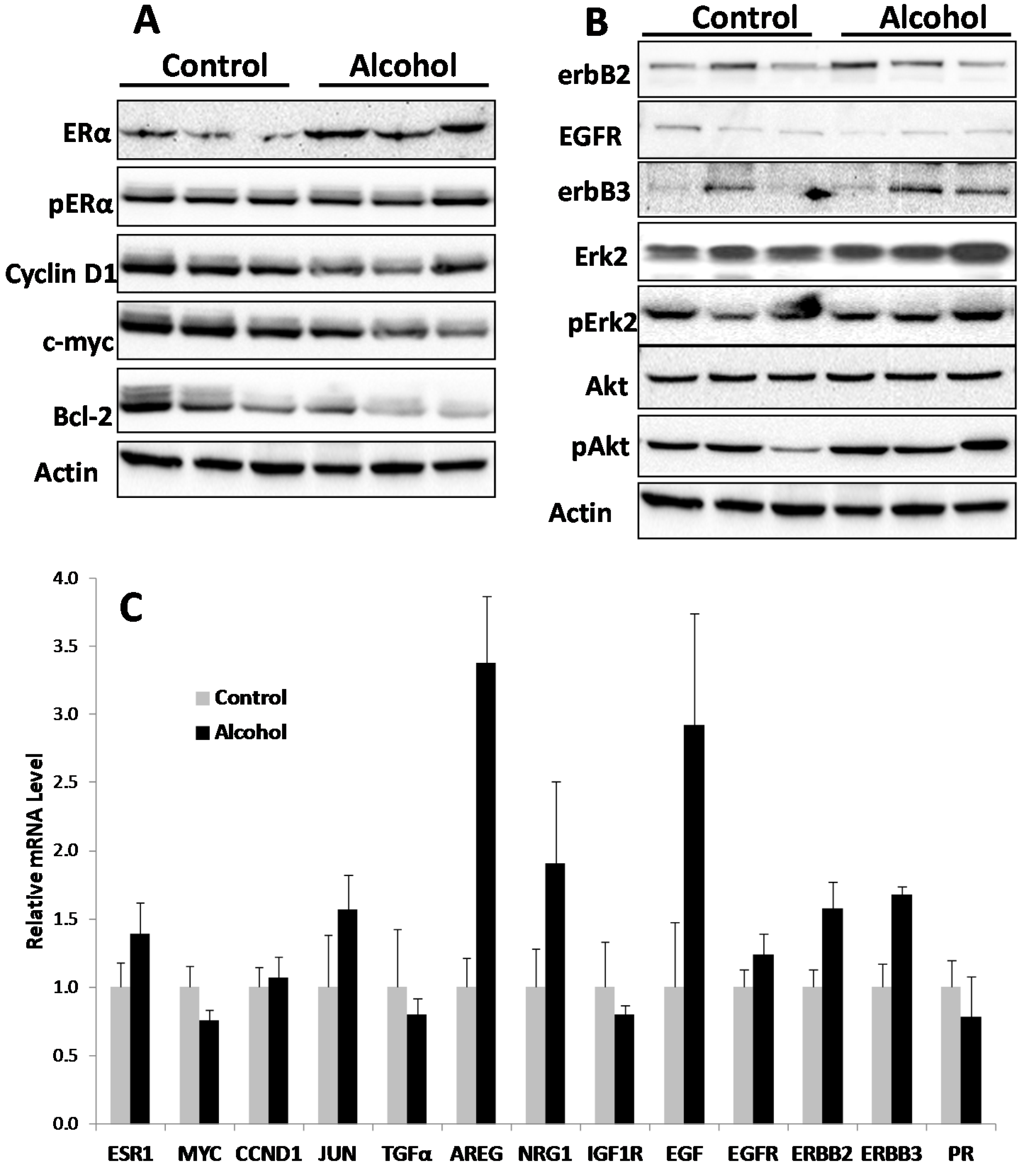
Cellular responses to these changes have not been extensively studied. In the present study, we focused on the regulation of the ER and erbB-2 pathways at both the protein and RNA levels in mammary tissues after different in utero treatments. Our results indicate that in utero exposure to low-dose alcohol induced significant upregulation of ERα expression and phosphorylation in mammary tissues at puberty. The molecular changes were more prominent in mammary tissues at puberty but less evident in 10-week-old animals. ER pathway activation in pubertal tissues was further supported by increased transcription/expression of classic ER target genes, including c-myc, cyclin D1, Bcl-2 and c-Jun, which are well established regulators of cell proliferation and survival. Activation of these potent molecules and the relevant pathways may partially explain the significant changes in mammary tissues. Interestingly, we noticed that although the ER pathway was generally activated, the mRNA levels of PR, a typical ER-responsive gene, were downregulated in mammary tissues after in utero alcohol exposure. This result differed from those of our studies on in utero exposure to genistein or bisphenol A (BPA), in which we observed increases in both ERα activation and PR mRNA levels in mammary tissues (unpublished data). The different effects of PR mRNA levels in this study might be related to a weaker estrogenic effect induced by in utero exposure to low-dose alcohol and the ability of this exposure to also induce changes in other PR regulators, which will be examined in future studies.
We examined molecular markers of the erbB family not just because erbB-2 is a predisposition factor of this transgenic model but also because regulation of the erbB family members plays a critical role in physiological mammary development and breast cancer pathogenesis [
19,
20]. We found that
in utero exposure to low-dose alcohol induced modest phosphorylation/activation of Akt and Erk but had mixed effects on erbB receptors and ligands. Our results showed that
in utero exposure to alcohol induced upregulation of erbB-3, Areg, NRG1 and TGFα transcription but downregulation of EGFR, erbB-2 and EGF at puberty. Among these proteins, Areg, NRG1, EGF and TGFα are the ligands for EGFR and/or erbB-3 [
33]. In the mammary tissues of 10-week-old mice, mRNA levels of Areg and EGF were significantly upregulated in the alcohol group. As erbB-2 is an orphan receptor and its activation is mainly regulated by its interaction with activated family members upon ligand binding, modulation of the expression of these ligands by
in utero exposure to alcohol would contribute to the modified signaling in the erbB-2 pathway and relevant phenotypic changes. For example, Areg and EGF specifically interact with the EGF receptor to promote the growth of normal epithelial cells [
33], which may partially explain the prolonged ductal tree extension. Taken together, the data suggest that regulation of erbB family signaling is a critical target of cellular changes induced by
in utero alcohol exposure. The underlying mechanisms of this effect and the impact of specific markers require further investigation. Moreover, to interpret the altered protein and mRNA markers, it is also possible that the shifts in signaling in the alcohol-treated animals reflect changes in the ratio of epithelial cells to stroma. This hypothesis will be tested by analyzing the relative composition of different mammary cell subtypes in future studies.
4. Experimental Section
4.1. Animals and Treatments
FVB/N-Tg/MMTV-erbB2 (MMTV-erbB-2) transgenic mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). All animal experiments were approved by the Institutional Animal Care and Use Committees (IACUC) of North Carolina Research Campus (NCRC). The animals were fed a phytoestrogen-free AIN-93G semipurified diet (Bio-Serv Co., Frenchtown, NJ, USA) ad libitum. To prepare pregnant mice, MMTV-erbB-2 breeding pairs were mated at 8 weeks of age. For alcohol treatment, pregnant mice were exposed to alcohol via gavage feeding of alcohol water (diluted from 200 proof, undenatured ethanol) twice a day between days 10 and 19 of gestation. The doses for each feeding were: 0.5 mL of 10% alcohol on gestation days 10–12; 0.5 mL 20% alcohol on gestation days 13–15; 0.6 mL 20% alcohol on gestation days 16–19. The estimated alcohol dosages were 3 g/kg/day between day 10 and day 12 and 6 g/kg/day between day 13 and day 19 of gestation. In parallel, pregnant mice in the control group were fed with water. The body weights at the end of gestation for control and alcohol treated mice were 32.95 ± 1.24 and 32.93 ± 1.54 g, respectively. We did not see weight gain among alcohol-fed dams, possibly owing to changes in food intake patterns associated with acute alcohol exposure. For each group, 15 pregnant dams were treated. Five from each group were used for blood collection by cardiac puncture on day 18 of gestation. The offspring from the rest of the dams were left with mothers to nurse. They were pooled after weaning and then randomly selected for each parameter.
To study the effect of in utero alcohol exposure on mammary development and tumor risk, female mice that had undergone in utero treatments were examined as follows. The newborns in each group were weighed each week for 4 weeks. Vaginal opening of the offspring was also examined daily starting from PND 20 to determine the vaginal opening dates. At 5 and 10 weeks of age, 10 offspring mice from each group at each point were euthanized for whole mount, protein and RNA analyses. An additional 20 offspring mice from each group were used for tumor development analysis. To this end, the mice were monitored twice a week, and the dates of tumor appearance and tumor volume were recorded. Per IACUC protocol, the animals were euthanized when tumor volume reached 1.8 cm3. The tumor latency was defined as the time to first tumor appearance by palpation. Tumor multiplicity was based on the number of tumors in each animal before euthanization. Kaplan–Meier curves were used to document tumor-free survival.
4.2. Blood Alcohol Concentration (BAC) Determination
To measure BAC, pregnant mice from the alcohol and control groups were exposed to alcohol or water as scheduled above. On Day 18 of gestation, blood was collected by tail clipping 30 min after a dose of 500 µL of 20% (v/v) ethanol solution or water via gavage. BAC was measured using an ethanol assay kit from Abcam (Cambridge, MA, USA) according to the manufacturer’s instructions. Briefly, blood samples from individual animals were diluted 1:10 in the assay buffer. The samples and the standard ethanol solutions were incubated with reaction mix containing an ethanol probe at 37 °C for 30 min in a 96-well plate. The plate was then read at 540 nm with a SynergyMx microplate reader. The BACs were calculated based on the standard curve and the dilution factor. The tests were based on 5 replicates from different animals.
4.3. Blood Estradiol Assay
Whole blood was collected by cardiac puncture on Day 18 in pregnant mice with in utero alcohol exposure as detailed above. Plasma was prepared by centrifugation at 3000 rpm at 4 °C for 20 min. Estradiol levels were measured using the estradiol enzyme immunoassay (EIA) kit from Cayman Chemical (Ann Arbor, MI, USA). The diluted sample and the standards AChE tracer and EIA antiserum were added to appropriate wells of a microtiter plate that was coated with anti-rabbit IgG. Following incubation at room temperature for 60 min, the wells were washed five times and developed with Ellman’s reagent for 60 min. Absorbance at 405 nm was measured using a SynergyMx microplate reader. Data based on 5 replicates from different animals are expressed as pg of estradiol per mL.
4.4. Whole Mount Analysis
Once the mice in specific groups reached the indicated end point, their inguinal (#4) mammary glands were collected and fixed with Carnoy’s fixative overnight, followed by re-hydration and carmine aluminum staining [
23]. The glands were then dehydrated and cleared with xylene. Prepared whole mounts were documented via digital photography for morphology analysis. For mammary glands around pubertal stages, the number of terminal end buds (TEBs) in each gland was counted. For whole mounts from older animals, mammary gland morphology was characterized by ductal extension beyond the lymph node and ductal density, as indicated by lateral bud numbers and branching.
4.5. Western Blot Analysis
Mammary tissues from the 4th and 5th pair of glands were collected, followed by snap freezing in a liquid nitrogen tank. Thawed tissues were homogenized at 4 °C in lysis buffer to extract total protein. The protein was quantified using a bicinchoninic acid (BCA) kit. Protein extracts were electrophoresed on a 10%–12% SDS-PAGE and transferred to nitrocellulose paper. The blots were incubated with TBS containing 0.1% Tween 20% and 5% powdered milk for 1 h and then incubated overnight with primary antibodies. Antibodies against ERα, c-myc, ERK2, Cyclin D1, Bcl-2, and β-actin were from Santa Cruz Biotechnology (Santa Cruz, CA, USA); antibodies against p-ERα, erbB-2, EGFR, erbB-3, p-Erk, p-Akt and Akt were from Cell Signaling Technology (Beverly, MA, USA). The membranes were washed and incubated with HRP-labeled anti-mouse or anti-rabbit secondary antibodies (Thermo Scientific, Waltham, MA, USA) for 1 h. Signals were visualized using Amersham enhanced chemiluminescence (ECL) reagents (GE Healthcare, Barrington, IL, USA) and captured with a FluorChem E imager from ProteinSimple (San Jose, CA, USA).
4.6. Quantitative Real Time PCR
Total RNA was isolated from the third pair of mammary glands using a Qiagen RNeasy mini kit with on-column DNAse digestion (Qiagen, Mississauga, ON, USA) per the manufacturer’s instructions. Equal amounts of RNA from each of 4 mice from the same group were pooled before cDNA was reverse transcribed using an iScript Reverse Transcriptase kit (Bio-Rad, Hercules, CA, USA). Real time PCR was performed with murine gene-specific primers using a Bio-Rad CFX96 Real Time PCR System (Bio-Rad). PCR amplification was carried out in a 20-µL reaction volume containing 50 ng of cDNA, 2 µM each of forward and reverse primers, and 10 µL Fast SYBR Green Master Mix (Bio-Rad). The PCR reactions were initiated with denaturation at 95 °C for 5 min, followed by 40 amplification cycles at 95 °C for 15 s and 60 °C for 1 min. Samples were run in triplicate, and data were normalized to beta actin before statistical analysis. The primers used in this study are listed in
Table 2.
Table 2.
List of primers used for qRT-PCR.
Table 2.
List of primers used for qRT-PCR.
| Genes | Forward | Reverse |
|---|
| ACTB | TTGCCGACAGGATGCAGAAGGA | AGGTGGACAGCGAGGCCAGGAT |
| AREG | ACCTGGAGGTGGTGACATGCA | TGCCGATGCCAATAGCTGCGA |
| CCND1 | GGGTGGGTTGGAAATGAAC | TCCTCTCCAAAATGCCAGAG |
| EGF | TTTTGCCTCAGAAGGAGTGG | GGCCACACTTGGCAGTATATC |
| EGFR | ATCCTCTGCAGGCTCAGAAA | GGCGTTGGAGGAAAAGAAAG |
| ERBB2 | GTCGCAACTTCATGTCGGTA | GATCATCATGGAGCTGGC |
| ERBB3 | GACCTTCCAGACTCCGTTTG | AAATGGCCTGCAGCTTACAC |
| IGF1R | GGCAGCACTCGTTGTTCTC | GTACAACTACCGCTGCTGGA |
| IGF2R | TGCACACTCTTCTTCTCCTGGCA | GCAGATGTTGATATAGAAGTCAGG |
| JUN | GGGACACAGCTTTCACCCTA | GAAAAGTAGCCCCCAACCTC |
| MYC | TGAAGTTCACGTTGAGGGG | AGAGCTCCTCGAGCTGTTTG |
| NRG1 | TTCATCACAACCCTGCACAT | GAACTTGGGTTGCTGTCCAT |
| PR | CACAGCGCTTCTACCAACTCACAA | TTGGGCAACTGGGCAGCAATAA |
4.7. Statistical Analysis
Kaplan–Meier analyses and the log-rank test were used for tumor-free survival analysis. Dates of vaginal opening were analyzed using non-parametric test. Student’s t-test was used for the other tests.