Norcembranoidal Diterpenes from the Cultured-Type Octocoral Sinularia numerosa
Abstract
:1. Introduction
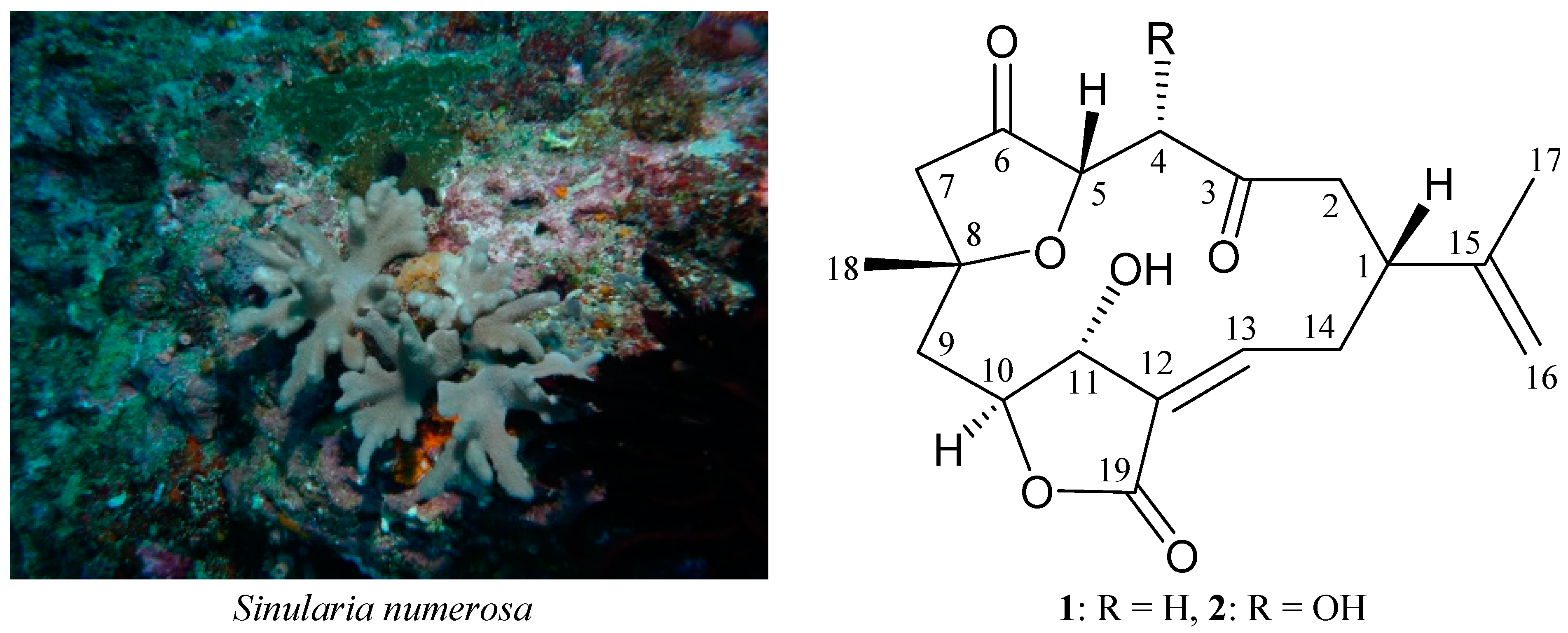
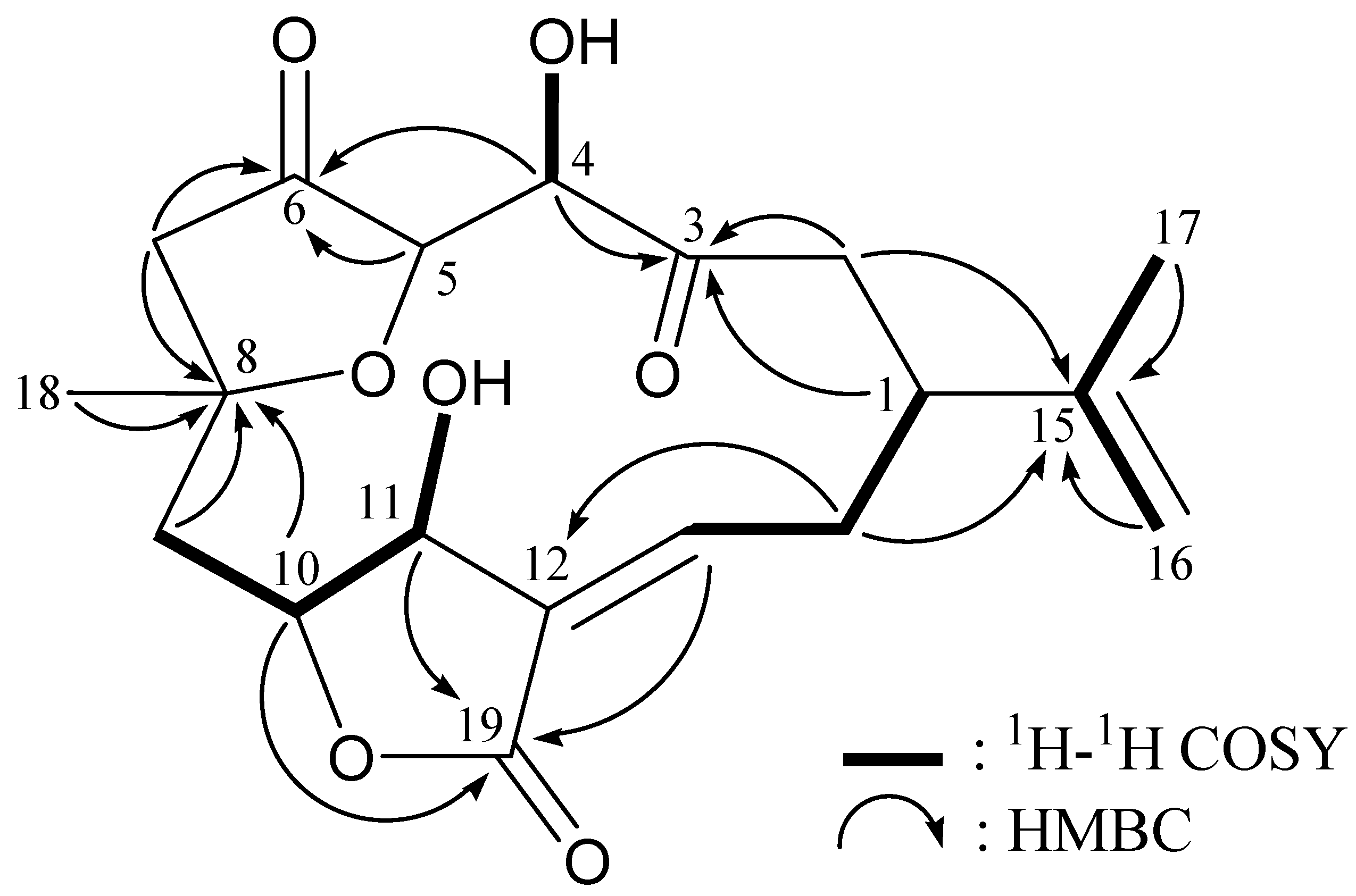
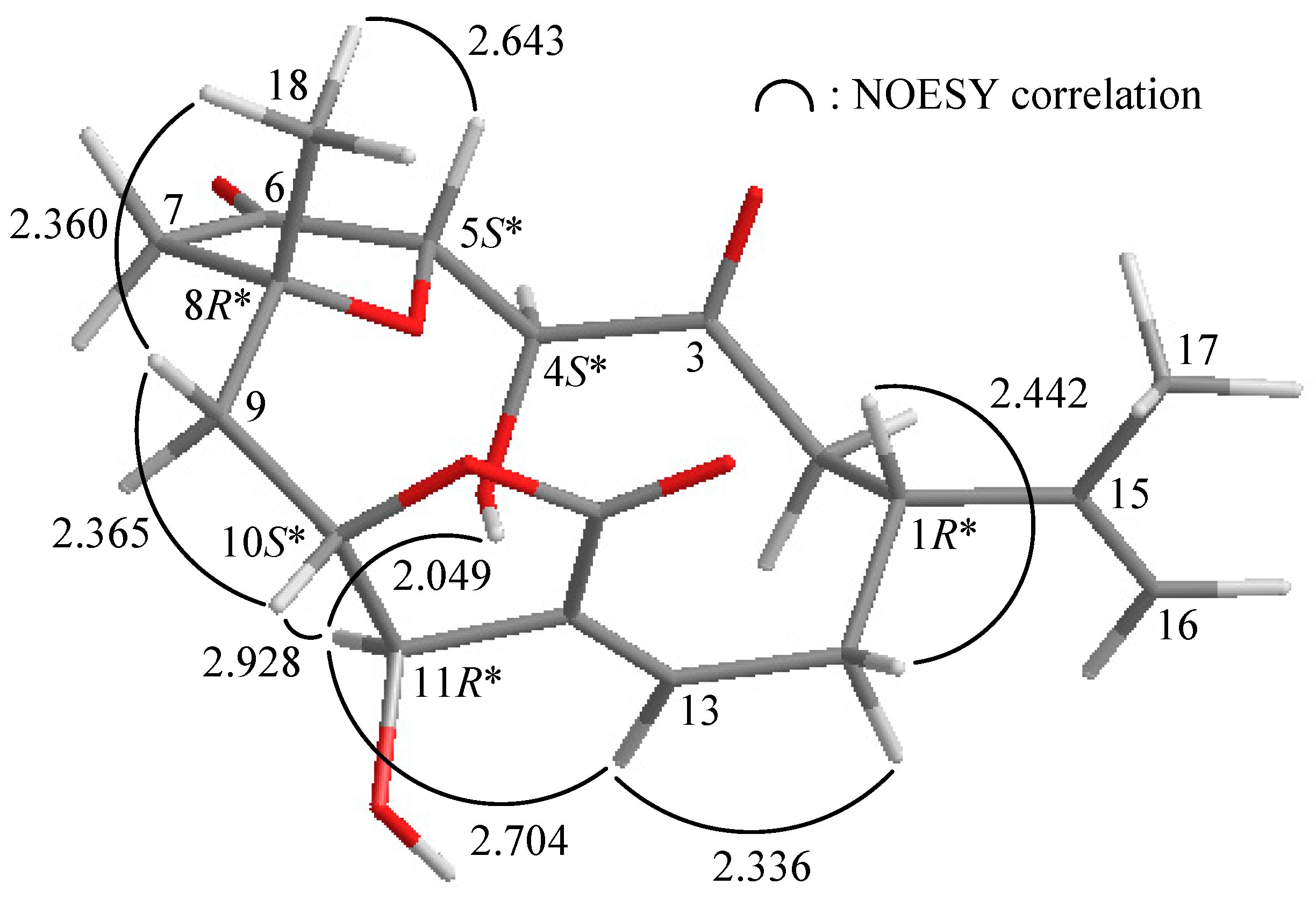
2. Results and Discussion
| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 2.80 m | 44.4, CH | H-14α | C-3, -14 |
| 2 | 2.67–2.78 m | 41.7, CH2 | n.o. c | C-1, -3, -14, -15 |
| 3 | 208.7, C | |||
| 4 | 4.34 dd (4.8, 3.6) a | 79.1, CH | OH-4 | C-3, -6 |
| 5 | 4.33 br d (3.6) a | 76.6, CH | n.o. | C-6 |
| 6 | 211.5, C | |||
| 7α | 2.31 d (16.8) | 52.6, CH2 | H-7β | C-6, -8, -9, -18 |
| β | 2.44 d (16.8) | H-7α | C-6, -8, -9, -18 | |
| 8 | 80.2, C | |||
| 9α | 2.10 br d (14.8) | 42.5, CH2 | H-9β | C-7, -8, -18 |
| β | 2.52 dd (14.8, 8.0) | H-9α, H-10 | C-8, -10, -11, -18 | |
| 10 | 4.67 dd (8.0, 0.8) | 83.3, CH | H-9β, H-11 | C-8, -11, -19 |
| 11 | 4.49 s | 76.2, CH | H-10, OH-11 | C-9, -10, -13, -19 |
| 12 | 130.9, C | |||
| 13 | 6.63 ddd (10.4, 6.8, 0.8) | 147.2 CH | H2-14 | C-11, -19 |
| 14α | 2.41 ddd (13.6, 6.8, 6.8) | 31.2, CH2 | H-1, H-13, H-14β | C-1, -2, -12, -13, -15 |
| β | 3.64 ddd (13.6, 10.4, 3.6) b | H-13, H-14α | C-2 | |
| 15 | 147.0, C | |||
| 16a | 4.87 d (0.8) | 110.4, CH2 | H-16b, H3-17 | C-1, -15, -17 |
| b | 4.89 d (0.8) | H-16a, H3-17 | C-1, -15, -17 | |
| 17 | 1.79 s | 20.8, CH3 | H2-16 | C-1, -15, -16 |
| 18 | 1.42 s | 25.6, CH3 | C-7, -8, -9 | |
| 19 | 169.2, C | |||
| OH-4 | 3.66 d (4.8) b | H-4 | n.o. | |
| OH-11 | 2.01 br s | H-11 | n.o. |
| Compounds | Cell Lines IC50 (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| CCRF-CEM | HL-60 | K-562 | U-937 | DLD-1 | LNCaP | MCF7 | |
| 1 | 11.07 | 11.11 | NA | NA | NA | NA | NA |
| 2 | 4.21 | 10.38 | 18.07 | 10.08 | NA | 15.33 | NA |
| Doxorubicin a | 0.01 | 0.01 | 0.35 | 0.05 | 0.49 | 0.10 | 0.16 |
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Molecular Mechasnics Calculations
3.5. MTT Antiproliferative Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, W.-T.; Li, Y.; Guo, Y.-W. Terpenoids of Sinularia soft corals: Chemistry and bioactivity. Acta Pharm. Sin. B 2012, 2, 227–237. [Google Scholar] [CrossRef]
- Rocha, J.; Peixe, L.; Gomes, N.C.M.; Calado, R. Cnidarians as a source of new marine bioactive compounds—An overview of the last decade and future steps for bioprospecting. Mar. Drugs 2011, 9, 1860–1886. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.-F. Reef environment and coral fauna of Southern Taiwan. Atoll Res. Bull. 1991, 354, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Yu, X.; Zeng, L.; Mak, T.C.W. Novel polyhydroxylated sterols from the soft coral Sinularia numerosa. J. Nat. Prod. 1989, 52, 934–940. [Google Scholar] [CrossRef]
- Tillekeratne, L.M.V.; Liyanage, G.K.; Ratnasooriya, W.D.; Ksebati, M.B.; Schmitz, F.J. A new spermatostatic glycoside from the soft coral Sinularia crispa. J. Nat. Prod. 1989, 52, 1143–1145. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.-J.; Yang, Y.-C.; Wang, S.-K.; Duh, C.-Y. Numerosol A–D, new cembranoid diterpenes from the soft coral Sinularia numerosa. Mar. Drugs 2014, 12, 3371–3380. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Fenical, W.; Zheng, Q.-T.; Clardy, J. Norcembrene diterpenoids from Pacific soft-corals of the genus Sinularia (Alcyonacea: Octocorallia). Tetrahedron 1985, 41, 4303–4308. [Google Scholar] [CrossRef]
- Qin, M.-L.; Li, X.-M.; Wang, B.-G. Chemical constituents of sesquiterpenes in soft coral Sinularia numerosa. Oceanol. Limnol. Sin. 2009, 40, 540–544. [Google Scholar]
- Yamashita, T.; Nakao, Y.; Matsunaga, S.; Oikawa, T.; Imahara, Y.; Fusetani, N. A new antiangiogenic C24 oxylipin from the soft coral Sinularia numerosa. Bioorg. Med. Chem. 2009, 17, 2181–2184. [Google Scholar] [CrossRef] [PubMed]
- Bowden, B.F.; Coll, J.C.; Mitchell, S.J.; Mulder, J.; Stokie, G.J. Studies of Australian soft corals. IX A novel nor-diterpene from the soft coral Sinularia leptoclados. Aust. J. Chem. 1978, 31, 2049–2056. [Google Scholar]
- Turner, K.E.; Bowden, B.F.; Stokie, G.J.; Howard, C.J. (4R*,8S*,11R*,13S*,14R*)-8,11-epoxy-14-hydroxy-11-methyl-4-(1-methylvinyl)-6,9-dioxocyclotetradec-1-ene-1,13-carbolactone. Acta Cryst. 1979, B35, 1283–1284. [Google Scholar] [CrossRef]
- Sheu, J.-H.; Ahmed, A.F.; Shiue, R.-T.; Dai, C.-F.; Kuo, Y.-H. Scabrolides A–D, four new norditerpenoids isolated from the soft coral Sinularia scabra. J. Nat. Prod. 2002, 65, 1904–1908. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.F.; Shiue, R.-T.; Wang, G.-H.; Dai, C.-F.; Kuo, Y.-H.; Sheu, J.-H. Five novel norcembranoids from Sinularia leptoclados and S. parva. Tetrahedron 2003, 59, 7337–7344. [Google Scholar] [CrossRef]
- Tseng, Y.-J.; Ahmed, A.F.; Dai, C.-F.; Chiang, M.Y.; Sheu, J.-H. Sinulochmodins A–C, three novel terpenoids from the soft coral Sinularia lochmodes. Org. Lett. 2005, 7, 3813–3816. [Google Scholar] [CrossRef] [PubMed]
- Kamel, H.N.; Fronczek, F.R.; Khalifa, S.I.; Slattery, M. Microbial transformation of 5-episinuleptolide. Chem. Pharm. Bull. 2007, 55, 537–540. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Allinger, N.L. Conformational analysis. 130. MM2. A hydrocarbon force field utilizing V1 and V2 torsional terms. J. Am. Chem. Soc. 1977, 99, 8127–8134. [Google Scholar]
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar] [PubMed]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar] [PubMed]
- Lu, M.-C.; Hwang, S.-L.; Chang, F.-R.; Chen, Y.-H.; Chang, T.-T.; Hung, C.-S.; Wang, C.-L.; Chu, Y.-H.; Pan, S.-H.; Wu, Y.-C. Immunostimulatory effect of Antrodia camphorata extract on functional maturation of dendritic cells. Food Chem. 2009, 113, 1049–1057. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-F.; Yin, C.-T.; Cheng, C.-H.; Lu, M.-C.; Fang, L.-S.; Wang, W.-H.; Wen, Z.-H.; Chen, J.-J.; Wu, Y.-C.; Sung, P.-J. Norcembranoidal Diterpenes from the Cultured-Type Octocoral Sinularia numerosa. Int. J. Mol. Sci. 2015, 16, 3298-3306. https://doi.org/10.3390/ijms16023298
Chen W-F, Yin C-T, Cheng C-H, Lu M-C, Fang L-S, Wang W-H, Wen Z-H, Chen J-J, Wu Y-C, Sung P-J. Norcembranoidal Diterpenes from the Cultured-Type Octocoral Sinularia numerosa. International Journal of Molecular Sciences. 2015; 16(2):3298-3306. https://doi.org/10.3390/ijms16023298
Chicago/Turabian StyleChen, Wu-Fu, Chen-Ting Yin, Ching-Hsiao Cheng, Mei-Chin Lu, Lee-Shing Fang, Wei-Hsien Wang, Zhi-Hong Wen, Jih-Jung Chen, Yang-Chang Wu, and Ping-Jyun Sung. 2015. "Norcembranoidal Diterpenes from the Cultured-Type Octocoral Sinularia numerosa" International Journal of Molecular Sciences 16, no. 2: 3298-3306. https://doi.org/10.3390/ijms16023298
APA StyleChen, W.-F., Yin, C.-T., Cheng, C.-H., Lu, M.-C., Fang, L.-S., Wang, W.-H., Wen, Z.-H., Chen, J.-J., Wu, Y.-C., & Sung, P.-J. (2015). Norcembranoidal Diterpenes from the Cultured-Type Octocoral Sinularia numerosa. International Journal of Molecular Sciences, 16(2), 3298-3306. https://doi.org/10.3390/ijms16023298









