Insecticide-Mediated Up-Regulation of Cytochrome P450 Genes in the Red Flour Beetle (Tribolium castaneum)
Abstract
:1. Introduction
| T. castaneum CYP Genes | Most Similar CYP Genes Found in Other Insect Species by BLASTP Search | ||||
|---|---|---|---|---|---|
| Species | CYP Genes | Identity (%) a | Overexpression Related to Insecticide Resistance b | Up-Regulation Mediated by Insecticides and Other Chemicals | |
| CYP12H1 | M. domestica | CYP12A1 | 37 | Pyrethroids [11] | Pyrethroids [17] |
| D. melanogaster | CYP12D1 | 37 | DDT [18] | pyrethrum [19] | |
| D. melanogaster | CYP12A4 | 39 | Lufenuron [20] | - | |
| A. gambiae | CYP12F1 | 36 | DDT [12] | - | |
| A. aegypti | CYP12F8 | 40 | - | Fluoranthene [21,22] | |
| CYP4G7 | M. domestica | CYP4G2 | 48 | - | Permethrin [23] |
| B. germanica | CYP4G19 | 51 | Pyrethroids [24] | - | |
| C. tentans | CYP4G33 | 54 | - | Atrazine [25] | |
| B. mori | CYP4G25 | 52 | - | Diazinon, permethrin [26] | |
| A. aegypti | CYP4G36 | 51 | - | Imidacloprid [27] | |
| CYP4BR3 | A. gambiae | CYP4H15 | 39 | DDT [12] | - |
| A. aegypti | CYP4H28 | 38 | - | Permethrin [28] | |
| C. pallens | CYP4H21 | 37 | Deltamethrin [29] | - | |
| C. quinquefasciatus | CYP4H34 | 38 | Permethrin [14] | - | |
| D. melanogaster | CYP4E2 | 41 | - | Phenobarbital, caffeine [30] | |
| D. melanogaster | CYP4E3 | 41 | - | Phenobarbital, caffeine [30] | |
| CYP4Q4 | M. sexta | CYP4M1 | 44 | Alkaloids, nicotine [31] | - |
| B. mori | CYP4M5 | 43 | - | Dichlorvos, deltamethrin [32] | |
| H. armigera | CYP4M6 | 43 | Deltamethrin [33] | - | |
| D. virgifera virgifera | CYP4AJ1 | 45 | Parathion, carbaryl [34] | - | |
| CYP6BK11 | M. domestica | CYP6A36 | 49 | Pyrethroids [35] | - |
| D. melanogaster | CYP6A8 | 46 | DDT, malathion [36] | Phenobarbital [30,37] | |
| A. gambiae | CYP6P3 | 43 | Permethrin [38] | - | |
| A. gambiae | CYP6M2 | 43 | Permethrin [39] | - | |
| A. aegypti | CYP6M11 | 41 | Deltamethrin [40] | Permethrin [21] | |
| P. xylostella | CYP6BG1 | 39 | Cypermethrin [41] | Permethrin [5] | |
| CYP345A1 | D. melanogaster | CYP6G1 | 43 | DDT, imidacloprid [42] | DDT, caffeine [37] |
| M. domestica | CYP6D3 | 38 | Pyrethroids [43] | Phenobarbital [44] | |
| C. quinquefasciatus | CYP6F1 | 37 | Permethrin [45] | - | |
| A. aegypti | CYP6AL1 | 37 | Fluoranthene [21] | - | |
| H. zea | CYP6B8 | 36 | Cypermethrin [46] | Chlorogenic acid [47] | |
| CYP9D4 | C. quinquefasciatus | CY9M10 | 37 | Permethrin [48] | - |
| H. armigera | CYP9A12 | 46 | Pyrethroids [49] | - | |
| H. armigera | CYP9A14 | 43 | Pyrethroids [50] | - | |
| A. aegypti | CYP9J27 | 41 | Pyrethroids [13] | - | |
| A. aegypti | CYP9J32 | 44 | Pyrethroids [13] | - | |
| CYP9Z5 | A. mellifera | CYP9Q1 | 38 | Acaricides [51] | - |
| B. mori | CYP9A19 | 45 | - | - | |
| B. mori | CYP9A20 | 45 | - | Dichlorvos, deltamethrin [32] | |
| D. melanogaster | CYP9F2 | 41 | Pyrethrum [19] | - | |
2. Results and Discussion
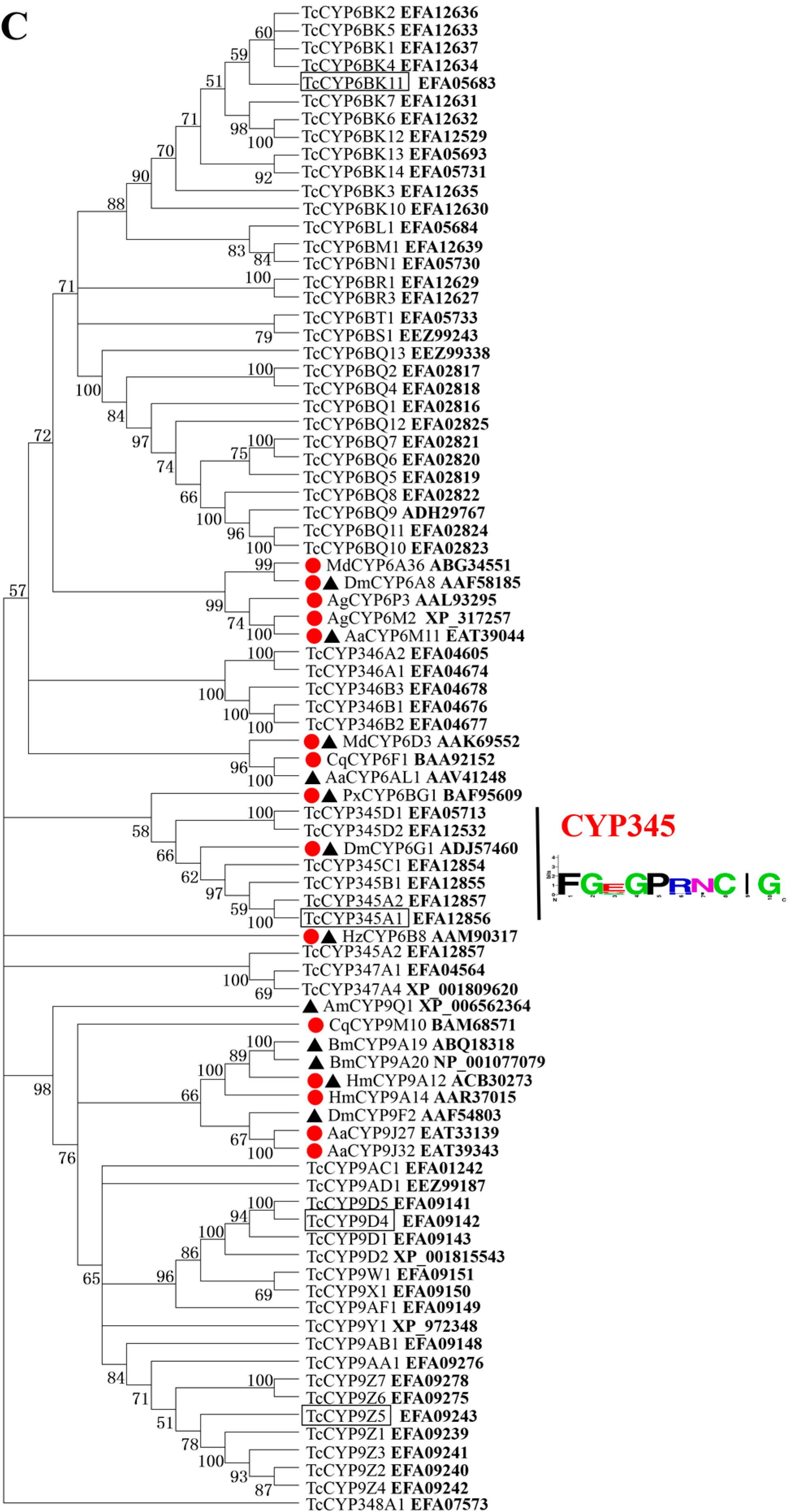
2.1. Phylogenetic Analysis of Deduced Amino Acid Sequences of T. castaneum CYP Genes
2.2. Selection of CYP Genes for Studying Insecticide-Mediated Up-Regulation

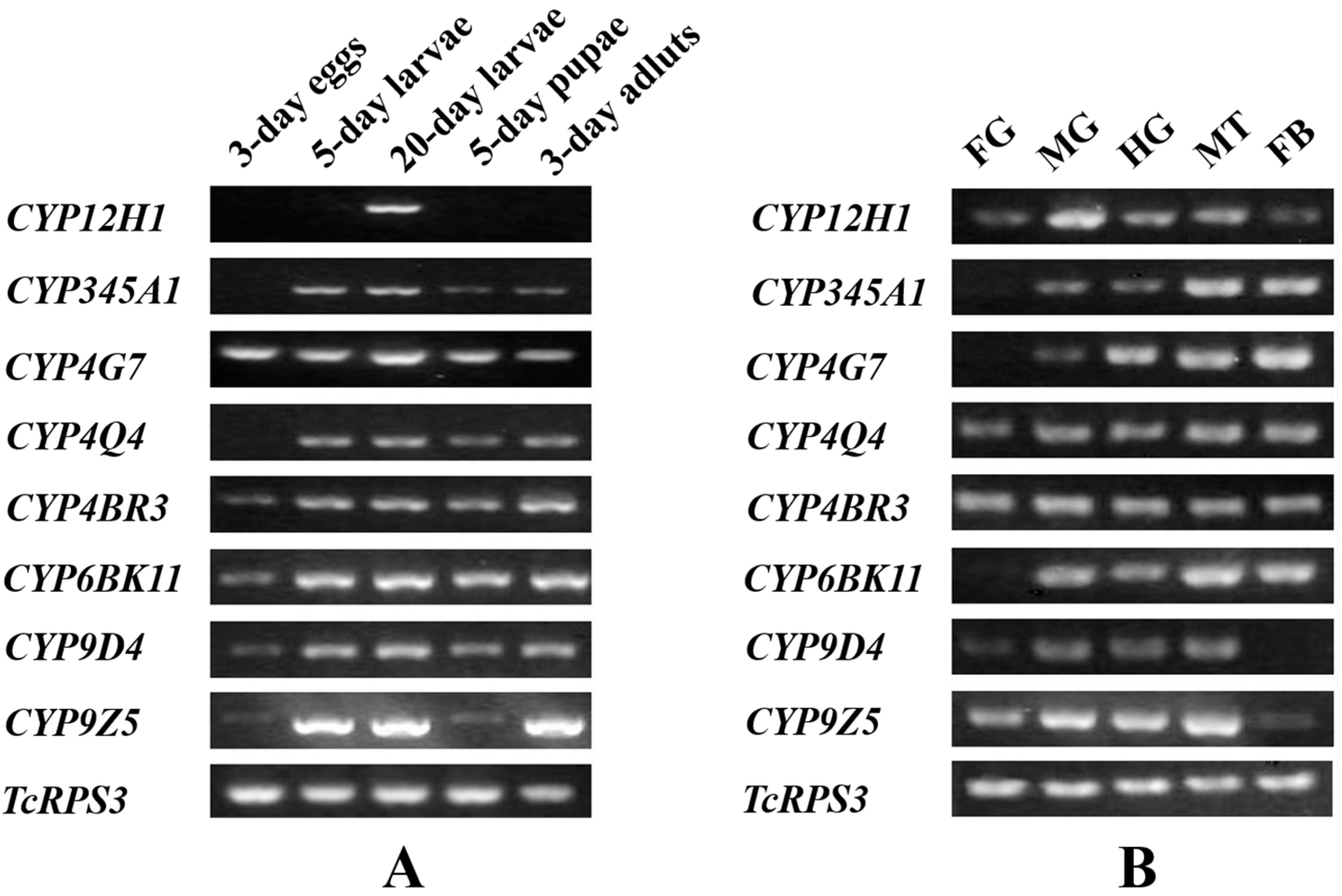
2.3. Stage and Tissue Dependent Expression Patterns of Eight CYP Genes
2.4. Selection of Insecticide Concentrations to Mediate Up-Regulation of CYP Genes
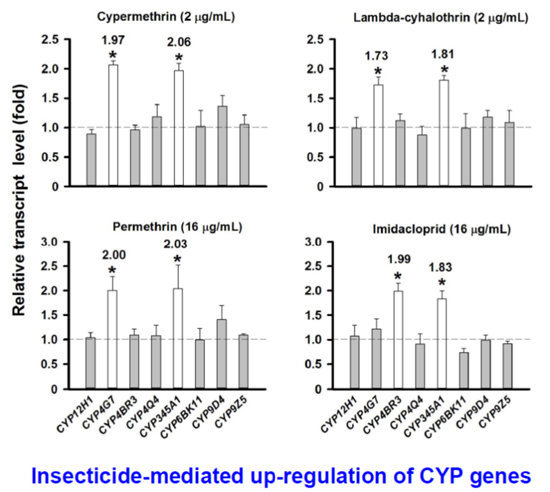
2.5. Evaluation of CYP Gene Up-Regulation by Insecticides
| Insecticides | LC20, μg/mL (95% CI) | LC50, μg/mL (95% CI) | Slope a | Intercept a | χ2 | p b |
|---|---|---|---|---|---|---|
| Cypermethrin | 2.27 (1.6–3.0) | 7.77 (6.5–9.4) | 3.66 ± 0.67 | 1.94 ± 0.94 | 5.13 | 1 |
| Lambda-cyhalothrin | 1.76 (1.6–3.3) | 4.24 (3.6–5.0) | 3.49 ± 0.40 | 2.98 ± 0.32 | 7.16 | 0.85 |
| Permethrin | 23.73 (15.3–32) | 77.46 (63.6–94.9) | 3.51 ± 0.80 | 1.63 ± 1.25 | 41.86 | 0.08 |
| Imidacloprid | 14.25 (12.0–16.5) | 48.65 (44.9–53.4) | 1.80 ± 0.49 | 2.02 ± 0.84 | 28.48 | 0.15 |
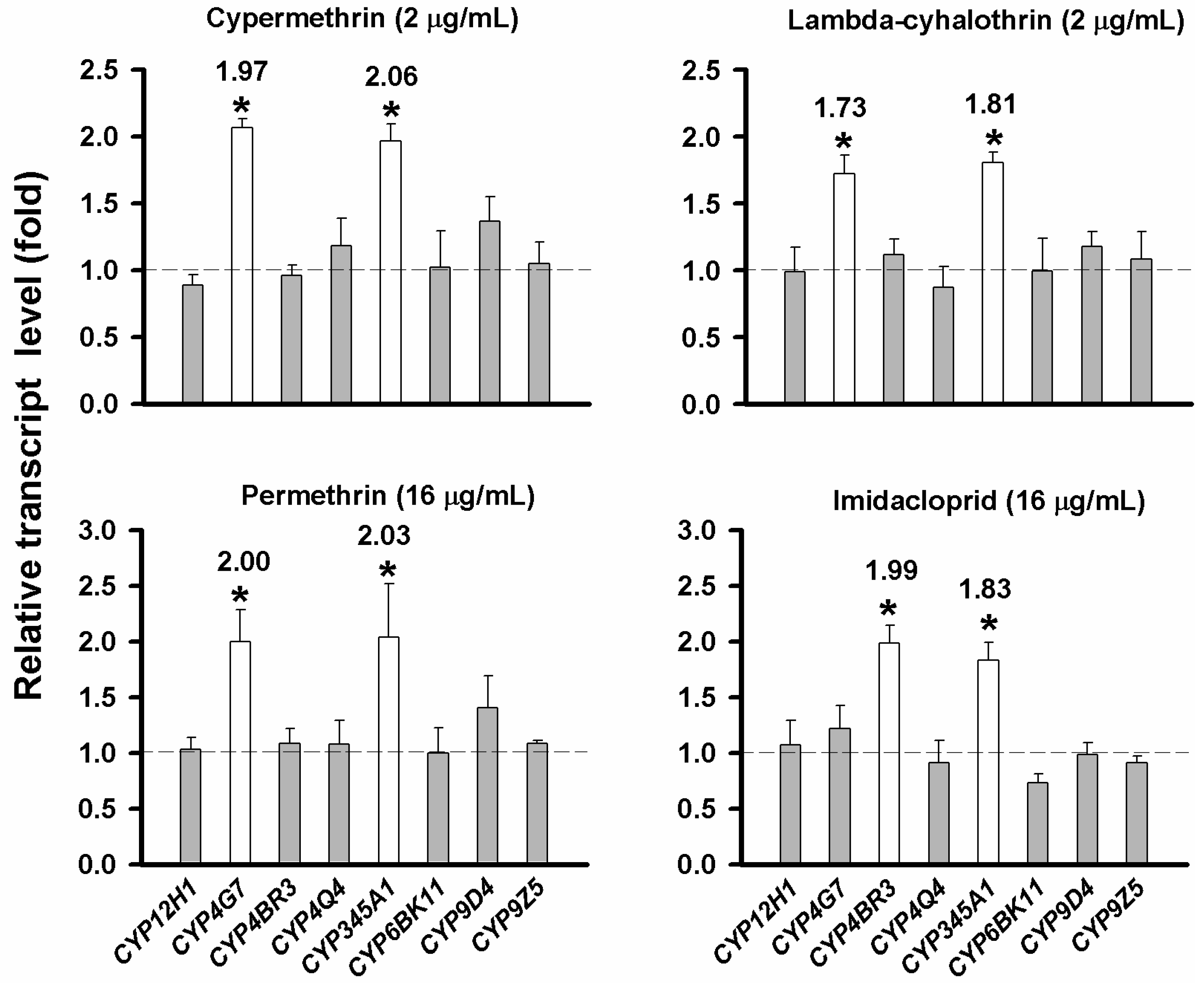
2.6. Concentration- and Time-Dependent Effect on CYP Up-Regulation
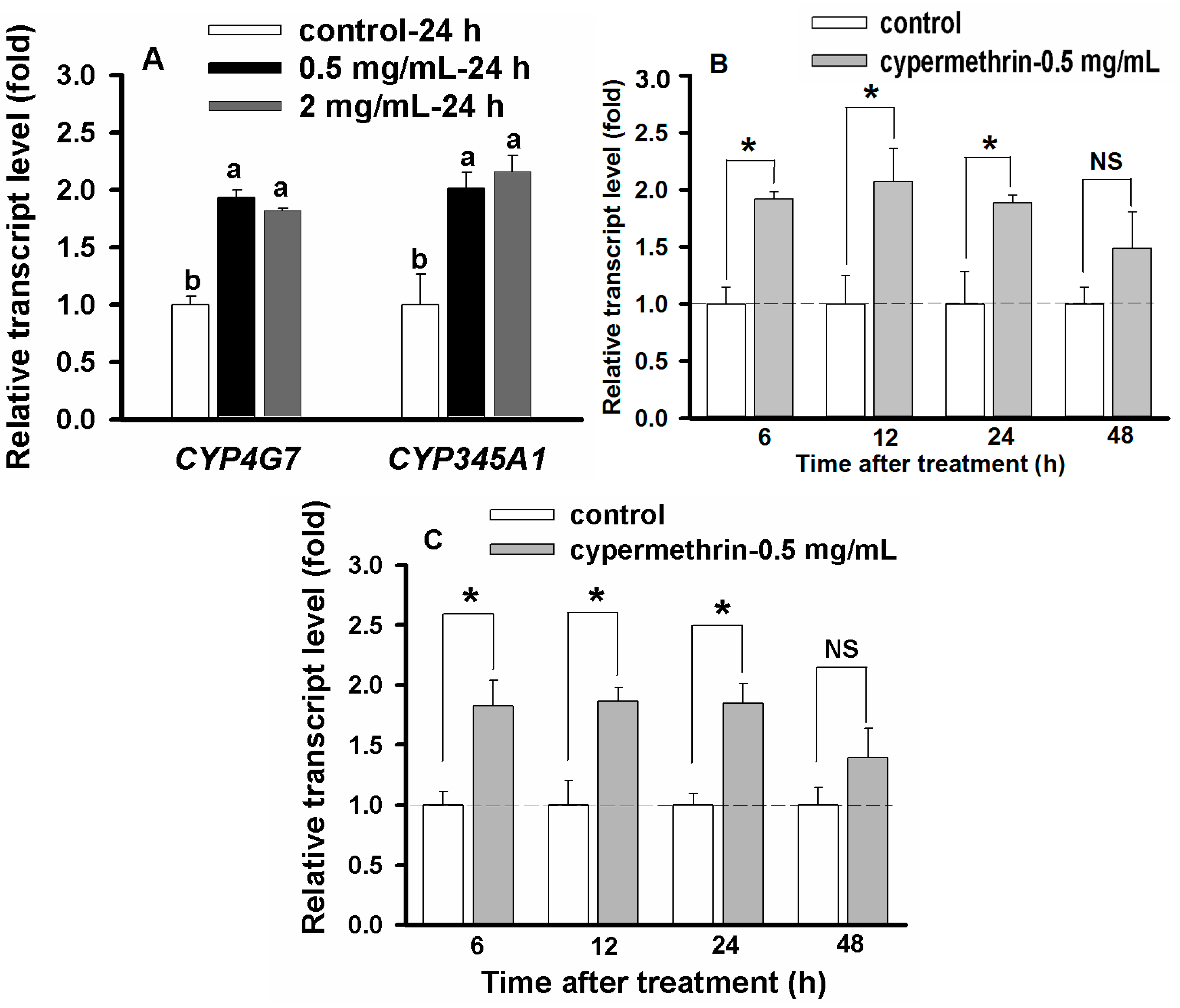
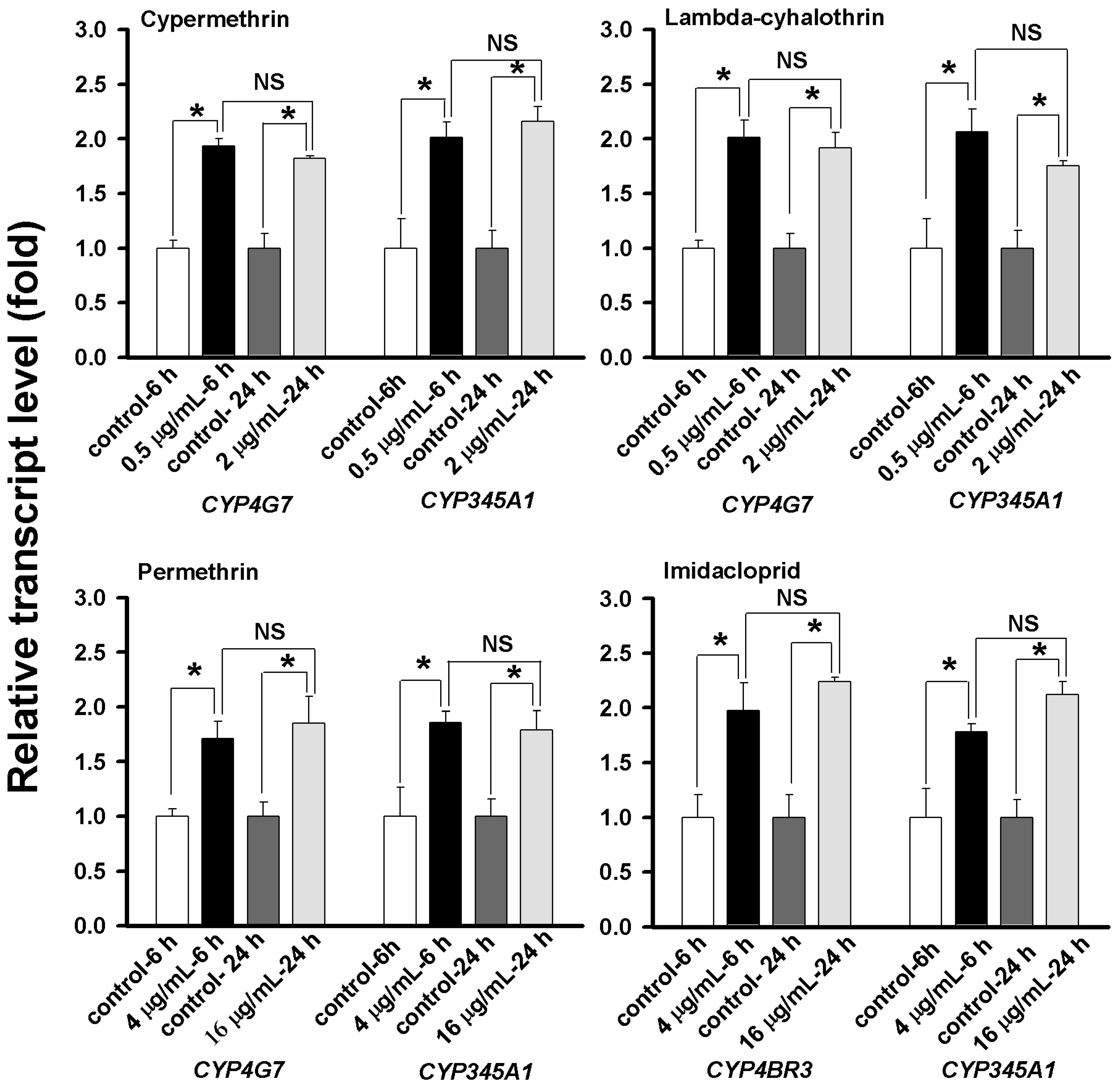
2.7. Discussion
3. Experimental Section
3.1. Insect Culture
3.2. Total RNA Isolation and First Strand cDNA Synthesis
3.3. Phylogenetic Tree Construction
3.4. Selection of Representative CYP Genes
3.5. Reverse Transcription Quantitative PCR (RT-qPCR) Analyses
| Primers | Sequence (5'–3') | Tm (°C) | Product Length (bp) |
|---|---|---|---|
| TcCYP12H1-F | AACCGCAAAAACTGATACGG | 60.0 | 299 |
| TcCYP12H1-R | ACCGGTCGTGTCTATTCCTG | 60.0 | |
| TcCYP4G7-F | CGCTGCCAACAGAGACATTA | 60.0 | 207 |
| TcCYP4G7-F | AATGACCCTGAAACCGTCAG | 60.0 | |
| TcCYP4BR3-F | CATCGGTTGTACCCTCCTGT | 59.9 | 168 |
| TcCYP4BR3-R | GAATCGGTCAGGGTCAAAGA | 59.8 | |
| TcCYP4Q4-F | TGGTTCCAATCACCCAATTT | 60.0 | 203 |
| TcCYP4Q4-R | TTTTTGCTCTTTGCGACCTT | 59.5 | |
| TcCYP345A1-F | TTTTTCGATTTTCGGTGGAG | 60.0 | 120 |
| TcCYP345A1-R | TTCGCGAAGGAAGTTGCTAT | 60.0 | |
| TcCYP6BK11-F | GTCAATTTGCGGAAACAGGT | 60.1 | 167 |
| TcCYP6BK11-R | CTACGTCCGTAAACCCGAAA | 60.3 | |
| TcCYP9D4-F | GTGGCACAACTAGCTCCACA | 59.9 | 172 |
| TcCYP9D4-R | GTTTTCCTTTACGGGCTTCC | 60.0 | |
| TcCYP9Z5-F | AGTCATGCAAAACTGCAACG | 59.9 | 250 |
| TcCYP9Z5-R | GTCCGGATTGGGGAAGTATT | 60.0 | |
| TcRPS3-F | CCGTCGTATTCGTGAATTGACTT | 59.3 | 143 |
| TcRPS3-R | TCTAAGAGACTCTGCTTGTGCAATG | 60.8 |
3.6. Stage and Tissue-Dependent Expression Patterns of CYP Genes
3.7. Insecticide Bioassay
3.8. Evaluation of Up-Regulation of CYP Genes Mediated by Insecticides
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Werck-Reichhart, D.; Feyereisen, R. Cytochromes P450: A success story. Genome Biol. 2000, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Terriere, L.C. Induction of detoxication enzymes in insects. Annu. Rev. Entomol. 1984, 29, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Londono, D.K.; Siqueira, H.A.; Wang, H.; Sarath, G.; Lydy, M.J.; Siegfried, B.D. Cloning and expression of an atrazine inducible cytochrome P450, CYP4G33, from Chironomus tentans (Diptera: Chironomidae). Pestic. Biochem. Physiol. 2007, 89, 104–110. [Google Scholar] [CrossRef]
- Scott, J.G. Cytochrome P450 and insecticide resistance. Insect Biochem. Mol. Biol. 1999, 29, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Bautista, M.A.M.; Tanaka, T.; Miyata, T. Identification of permethrin-inducible cytochrome P450s from the diamondback moth, Plutella xylostella (L.) and the possibility of involvement in permethrin resistance. Pestic. Biochem. Physiol. 2007, 87, 85–93. [Google Scholar] [CrossRef]
- Feyereisen, R. Insect cytochrome P450. Compr. Mol. Insect Sci. 2005, 4, 1–77. [Google Scholar]
- Urlacher, V.B.; Girhard, M. Cytochrome P450 monooxygenases: An update on perspectives for synthetic application. Trends Biotechnol. 2012, 30, 26–36. [Google Scholar] [CrossRef]
- Feyereisen, R. Insect CYP Genes and P450 Enzymes. In Insect Molecular Biology and Biochemistry; Lawrence, I.G., Ed.; Elsevier: Oxford, UK, 2011; pp. 236–316. [Google Scholar]
- Guo, Y.Q.; Zhang, J.Z.; Yang, M.L.; Yan, L.Z.; Zhu, K.Y.; Guo, Y.P.; Ma, E.B. Comparative analysis of cytochrome P450-like genes from Locusta migratoria manilensis: Expression profiling and response to insecticide exposure. Insect Sci. 2012, 19, 75–85. [Google Scholar] [CrossRef]
- Giraudo, M.; Unnithan, G.C.; le Goff, G.; Feyereisen, R. Regulation of cytochrome P450 expression in Drosophila: Genomic insights. Pestic. Biochem. Physiol. 2010, 97, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Le Goff, G.; Hilliou, F.; Siegfried, B.D.; Boundy, S.; Wajnberg, E.; Sofer, L.; Audant, P.; Feyereisen, R. Xenobiotic response in Drosophila melanogaster: Sex dependence of P450 and GST gene induction. Insect Biochem. Mol. Biol. 2006, 36, 674–682. [Google Scholar] [CrossRef] [PubMed]
- David, J.P.; Strode, C.; Vontas, J.; Nikou, D.; Vaughan, A.; Pignatelli, P.M.; Louis, C.; Hemingway, J.; Ranson, H. The Anopheles gambiae detoxification chip: A highly specific microarray to study metabolic-based insecticide resistance in malaria vectors. Proc. Natl. Acad. Sci. USA 2005, 102, 4080–4084. [Google Scholar] [CrossRef] [PubMed]
- Strode, C.; Wondji, C.S.; David, J.P.; Hawkes, N.J.; Lumjuan, N.; Nelson, D.R.; Drane, D.R.; Karunaratne, S.; Hemingway, J.; Black, W.C., IV. Genomic analysis of detoxification genes in the mosquito Aedes aegypti. Insect Biochem. Mol. Biol. 2008, 38, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Komagata, O.; Kasai, S.; Tomita, T. Overexpression of cytochrome P450 genes in pyrethroid-resistant Culex quinquefasciatus. Insect Biochem. Mol. Biol. 2010, 40, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Parthasarathy, R.; Bai, H.; Woithe, K.; Kaussmann, M.; Nauen, R.; Harrison, D.A.; Palli, S.R. A brain-specific cytochrome P450 responsible for the majority of deltamethrin resistance in the QTC279 strain of Tribolium castaneum. Proc. Natl. Acad. Sci. USA 2010, 107, 8557–8562. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Moural, T.W.; Shah, K.; Palli, S.R. Integrated analysis of cytochrome P450 gene superfamily in the red flour beetle, Tribolium castaneum. BMC Genomics 2013, 14, 174. [Google Scholar] [CrossRef] [PubMed]
- Guzov, V.M.; Unnithan, G.C.; Chernogolov, A.A.; Feyereisen, R. CYP12A1, a mitochondrial cytochrome P450 from the house fly. Arch. Biochem. Biophys. 1998, 359, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Scharf, M.; Pedra, J.; Holmes, G.; Dean, A.; Kreitman, M.; Pittendrigh, B.R. Differential expression and induction of two Drosophila cytochrome P450 genes near the Rst(2)DDT locus. Insect Mol. Biol. 2002, 11, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.; Scott, I.; Sims, S.; Trudeau, V.; Arnason, J. The effect of a synergistic concentration of a Piper nigrum extract used in conjunction with pyrethrum upon gene expression in Drosophila melanogaster. Insect Mol. Biol. 2006, 15, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Bogwitz, M.R.; Chung, H.; Magoc, L.; Rigby, S.; Wong, W.; O’Keefe, M.; McKenzie, J.A.; Batterham, P.; Daborn, P.J. CYP12A4 confers lufenuron resistance in a natural population of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2005, 102, 12807–12812. [Google Scholar] [CrossRef] [PubMed]
- Poupardin, R.; Riaz, M.; Vontas, J.; David, J.; Reynaud, S. Transcription profiling of eleven cytochrome P450s potentially involved in xenobiotic metabolism in the mosquito Aedes aegypti. Insect Mol. Biol. 2010, 19, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Poupardin, R.; Reynaud, S.; Strode, C.; Ranson, H.; Vontas, J.; David, J.P. Cross-induction of detoxification genes by environmental xenobiotics and insecticides in the mosquito Aedes aegypti: Impact on larval tolerance to chemical insecticides. Insect Biochem. Mol. Biol. 2008, 38, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Li, T.; Zhang, L.; Liu, N. Co-up-regulation of three P450 genes in response to permethrin exposure in permethrin resistant house flies, Musca domestica. BMC Physiol. 2008, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Pridgeon, J.W.; Zhang, L.; Liu, N. Overexpression of CYP4G19 associated with a pyrethroid-resistant strain of the German cockroach, Blattella germanica (L.). Gene 2003, 314, 157–163. [Google Scholar] [CrossRef]
- Londono, D.K.; Siegfried, B.D.; Lydy, M.J. Atrazine induction of a family 4 cytochrome P450 gene in Chironomus tentans (Diptera: Chironomidae). Chemosphere 2004, 56, 701–706. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ichinose, H.; Aso, Y.; Fujii, H. Expression analysis of cytochrome P450s in the silkmoth, Bombyx mori. Pestic. Biochem. Physiol. 2010, 97, 1–6. [Google Scholar] [CrossRef]
- Riaz, M.A.; Poupardin, R.; Reynaud, S.; Strode, C.; Ranson, H.; David, J.P. Impact of glyphosate and benzo[a]pyrene on the tolerance of mosquito larvae to chemical insecticides: Role of detoxification genes in response to xenobiotics. Aquat. Toxicol. 2009, 93, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Saavedra-Rodriguez, K.; Suarez, A.F.; Salas, I.F.; Strode, C.; Ranson, H.; Hemingway, J.; Black, I. Transcription of detoxification genes after permethrin selection in the mosquito Aedes aegypti. Insect Mol. Biol. 2012, 21, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Dong, H.Q.; Tian, H.S.; Ma, L.; Li, X.L.; Wu, G.L.; Zhu, C.L. Cytochrome P450 genes expressed in the deltamethrin-susceptible and-resistant strains of Culex pipiens pallens. Pestic. Biochem. Physiol. 2003, 75, 19–26. [Google Scholar] [CrossRef]
- Misra, J.R.; Horner, M.A.; Lam, G.; Thummel, C.S. Transcriptional regulation of xenobiotic detoxification in Drosophila. Genes Dev. 2011, 25, 1796–1806. [Google Scholar] [CrossRef] [PubMed]
- Snyder, M.J.; Stevens, J.L.; Andersen, J.F.; Feyereisen, R. Expression of cytochrome P450 genes of the CYP4 family in midgut and fat body of the tobacco hornworm, Manduca sexta. Arch. Biochem. Biophys. 1995, 321, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.D.; Zhao, S.S.; Gao, R.N.; Wang, R.X.; Zhang, T.; Ding, H.; Li, B.; Lu, C.D.; Shen, W.D.; Wei, Z.G. Transcription profiling of eight cytochrome P450s potentially involved in xenobiotic metabolism in the silkworm, Bombyx mori. Pestic. Biochem. Physiol. 2011, 100, 251–255. [Google Scholar] [CrossRef]
- Brun-Barale, A.; Héma, O.; Martin, T.; Suraporn, S.; Audant, P.; Sezutsu, H.; Feyereisen, R. Multiple P450 genes overexpressed in deltamethrin-resistant strains of Helicoverpa armigera. Pest Manag. Sci. 2010, 66, 900–909. [Google Scholar] [PubMed]
- Scharf, M.; Parimi, S.; Meinke, L.J.; Chandler, L.; Siegfried, B.D. Expression and induction of three family 4 cytochrome P450 (CYP4) genes identified from insecticide-resistant and susceptible western corn rootworms, Diabrotica virgifera virgifera. Insect Mol. Biol. 2001, 10, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Feng, J.N.; Zhang, L.; Liu, N. Characterization of two novel cytochrome P450 genes in insecticide-resistant house-flies. Insect Mol. Biol. 2008, 17, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Waters, L.C.; Zelhof, A.C.; Shaw, B.J.; Chang, L.Y. Possible involvement of the long terminal repeat of transposable element 17.6 in regulating expression of an insecticide resistance-associated P450 gene in Drosophila. Proc. Natl. Acad. Sci. USA 1992, 89, 4855–4859. [Google Scholar] [CrossRef] [PubMed]
- Morra, R.; Kuruganti, S.; Lam, V.; Lucchesi, J.; Ganguly, R. Functional analysis of the cis-acting elements responsible for the induction of the CYP6A8 and CYP6G1 genes of Drosophila melanogaster by DDT, phenobarbital and caffeine. Insect Mol. Biol. 2010, 19, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Mueller, P.; Chouaibou, M.; Pignatelli, P.; Etang, J.; Walker, E.D.; Donnelly, M.J.; Simard, F.; Ranson, H. Pyrethroid tolerance is associated with elevated expression of antioxidants and agricultural practice in Anopheles arabiensis sampled from an area of cotton fields in Northern Cameroon. Mol. Ecol. 2008, 17, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Donnelly, M.J.; Ranson, H. Transcription profiling of a recently colonised pyrethroid resistant Anopheles gambiae strain from Ghana. BMC Genomics 2007, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Marcombe, S.; Mathieu, R.B.; Pocquet, N.; Riaz, M.A.; Poupardin, R.; Sélior, S.; Darriet, F.; Reynaud, S.; Yébakima, A.; Corbel, V. Insecticide resistance in the dengue vector Aedes aegypti from Martinique: Distribution, mechanisms and relations with environmental factors. PLoS One 2012, 7, e30989. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Clark, J.M.; Lee, S.H. Cross-strain comparison of cypermethrin-induced cytochrome P450 transcription under different induction conditions in diamondback moth. Pestic. Biochem. Physiol. 2010, 96, 43–50. [Google Scholar] [CrossRef]
- Festucci-Buselli, R.; Carvalho-Dias, A.; Oliveira-Andrade, D.; Caixeta-Nunes, C.; Li, H.M.; Stuart, J.; Muir, W.; Scharf, M.; Pittendrigh, B. Expression of CYP6G1 and CYP12D1 in DDT resistant and susceptible strains of Drosophila melanogaster. Insect Mol. Biol. 2005, 14, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, E.; Yamakawa, M.; Shono, T.; Kono, Y. Molecular cloning, nucleotide sequences and gene expression of new cytochrome P450s (CYP6A24, CYP6D3v2) from the pyrethroid resistant housefly, Musca domestica L: Diptera: Muscidae. Appl. Entomol. Zool. 2001, 36, 225–229. [Google Scholar] [CrossRef]
- Kasai, S.; Scott, J.G. Expression and regulation of CYP6D3 in the house fly, Musca domestica (L.). Insect Biochem. Mol. Biol. 2001, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kasai, S.; Weerashinghe, I.S.; Shono, T.; Yamakawa, M. Molecular cloning, nucleotide sequence and gene expression of a cytochrome P450 (CYP6F1) from the pyrethroid-resistant mosquito, Culex quinquefasciatus Say. Insect Biochem. Mol. Biol. 2000, 30, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, B.W.; Longnecker, M.T.; Pietrantonio, P.V. Transcriptional overexpression of CYP6B8/CYP6B28 and CYP6B9 is a mechanism associated with cypermethrin survivorship in field-collected Helicoverpa zea (Lepidoptera: Noctuidae) moths. Pest Manag. Sci. 2011, 67, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Jasmonate and salicylate induce expression of herbivore cytochrome P450 genes. Nature 2002, 419, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Hardstone, M.; Komagata, O.; Kasai, S.; Tomita, T.; Scott, J. Use of isogenic strains indicates CYP9M10 is linked to permethrin resistance in Culex pipiens quinquefasciatus. Insect Mol. Biol. 2010, 19, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ma, C.; Li, M.; Sheng, C.; Liu, H.; Qiu, X. CYP9A12 and CYP9A17 in the cotton bollworm, Helicoverpa armigera: Sequence similarity, expression profile and xenobiotic response. Pest Manag. Sci. 2010, 66, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, S.; Wu, S.; Yue, L.; Wu, Y. Constitutive overexpression of multiple cytochrome P450 genes associated with pyrethroid resistance in Helicoverpa armigera. J. Econ. Entomol. 2006, 99, 1784–1789. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Schuler, M.A.; Berenbaum, M.R. CYP9Q-mediated detoxification of acaricides in the honey bee (Apis mellifera). Proc. Natl. Acad. Sci. USA 2011, 108, 12657–12662. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Sztal, T.; Pasricha, S.; Sridhar, M.; Batterham, P.; Daborn, P.J. Characterization of Drosophila melanogaster cytochrome P450 genes. Proc. Natl. Acad. Sci. USA 2009, 106, 5731–5736. [Google Scholar] [CrossRef] [PubMed]
- Chahine, S.; O’Donnell, M.J. Interactions between detoxification mechanisms and excretion in Malpighian tubules of Drosophila melanogaster. J. Exp. Biol. 2011, 214, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Mittapalli, O.; Bai, X.; Mamidala, P.; Rajarapu, S.P.; Bonello, P.; Herms, D.A. Tissue-specific transcriptomics of the exotic invasive insect pest emerald ash borer (Agrilus planipennis). PLoS One 2010, 5, e13708. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, L.; Chung, H.; Lumb, C.; Robin, C.; Batterham, P.; Daborn, P.J. A comparison of Drosophila melanogaster detoxification gene induction responses for six insecticides, caffeine and phenobarbital. Insect Biochem. Mol. Biol. 2006, 36, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Z.; Zou, C.; Cao, C. Transcription profiling of 12 asian gypsy moth (Lymantria dispar) cytochrome p450 genes in response to insecticides. Arch. Insect Biochem. Physiol. 2014, 85, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Liang, X.; Gao, X.; Yao, J.; Zhu, K.Y. The lethal giant larvae gene in Tribolium castaneum: Molecular properties and roles in larval and pupal development as revealed by RNA interference. Int. J. Mol. Sci. 2014, 15, 6880–6896. [Google Scholar] [CrossRef] [PubMed]
- Togawa, T.; Dunn, W.A.; Emmons, A.C.; Nagao, J.; Willis, J.H. Developmental expression patterns of cuticular protein genes with the R&R Consensus from Anopheles gambiae. Insect Biochem. Mol. Biol. 2008, 38, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Xiao, D.; He, Y.; Yao, J.; Zhu, G.; Zhu, K.Y. Insecticide-Mediated Up-Regulation of Cytochrome P450 Genes in the Red Flour Beetle (Tribolium castaneum). Int. J. Mol. Sci. 2015, 16, 2078-2098. https://doi.org/10.3390/ijms16012078
Liang X, Xiao D, He Y, Yao J, Zhu G, Zhu KY. Insecticide-Mediated Up-Regulation of Cytochrome P450 Genes in the Red Flour Beetle (Tribolium castaneum). International Journal of Molecular Sciences. 2015; 16(1):2078-2098. https://doi.org/10.3390/ijms16012078
Chicago/Turabian StyleLiang, Xiao, Da Xiao, Yanping He, Jianxiu Yao, Guonian Zhu, and Kun Yan Zhu. 2015. "Insecticide-Mediated Up-Regulation of Cytochrome P450 Genes in the Red Flour Beetle (Tribolium castaneum)" International Journal of Molecular Sciences 16, no. 1: 2078-2098. https://doi.org/10.3390/ijms16012078
APA StyleLiang, X., Xiao, D., He, Y., Yao, J., Zhu, G., & Zhu, K. Y. (2015). Insecticide-Mediated Up-Regulation of Cytochrome P450 Genes in the Red Flour Beetle (Tribolium castaneum). International Journal of Molecular Sciences, 16(1), 2078-2098. https://doi.org/10.3390/ijms16012078