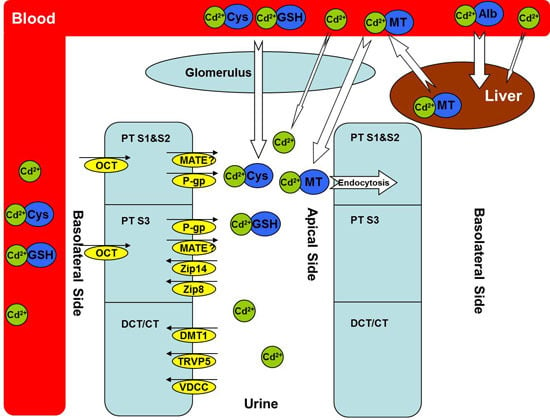
Cadmium Transporters in the Kidney and Cadmium-Induced Nephrotoxicity
Abstract
:1. Introduction
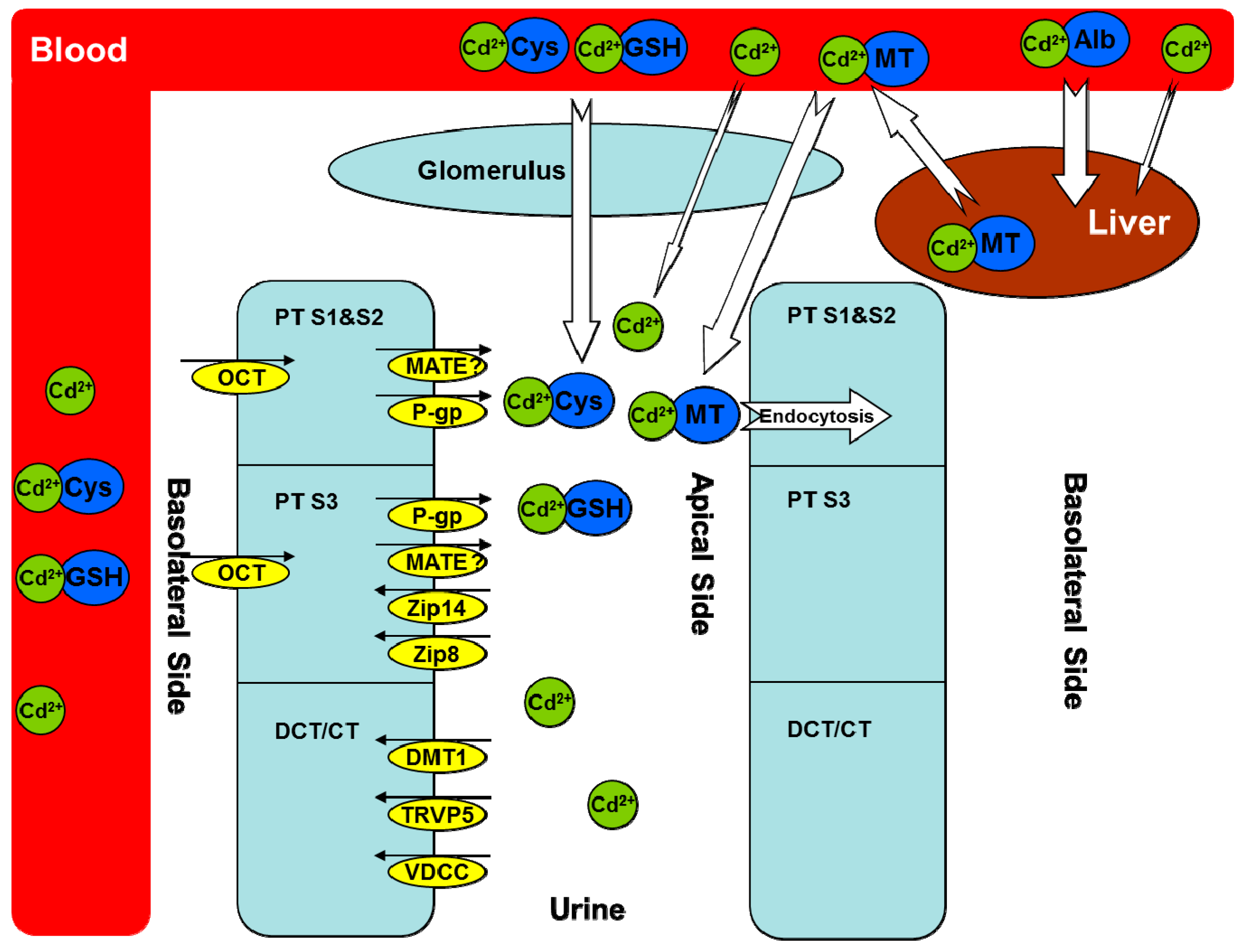
2. Metallothioneins (MTs)
2.1. Evidence as a Cadmium Transporter
2.2. Role in Cadmium-Induced Nephrotoxicity
3. Zinc Transporters
3.1. Evidence as a Cadmium Transporter
3.2. Role in Cadmium-Induced Nephrotoxicity
4. Calcium Transporters
4.1. Evidence as a Cadmium Transporter
4.2. Role in Cadmium-Induced Nephrotoxicity
5. Divalent Metal-Ion Transporter-1 (DMT1)
5.1. Evidence as a Cadmium Transporter
5.2. Role in Cadmium-Induced Nephrotoxicity
6. Organic Cation Transporters (OCTs) and Multidrug and Toxin Extrusion Proteins (MATEs)
6.1. Evidence as a Cadmium Transporter
6.2. Role in Cadmium-Induced Nephrotoxicity
7. Other Transport Mechanisms and Transporters
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, J.; Qu, W.; Kadiiska, M.B. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Kumar, A.; Lal, A.; Pant, M. Cellular mechanisms of cadmium-induced toxicity: A review. Int. J. Environ. Health Res. 2014, 24, 378–399. [Google Scholar] [CrossRef] [PubMed]
- Thevenod, F.; Lee, W.K. Cadmium and cellular signaling cascades: Interactions between cell death and survival pathways. Arch. Toxicol. 2013, 87, 1743–1786. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shu, Y.; University of Maryland at Baltimore, Baltimore City, MD. Unpublished work. 2015.
- Klaassen, D.C.; Liu, J.; Choudhuri, S. Metallothionein: An intracellular protein to protect against cadmium toxicity. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 267–294. [Google Scholar] [CrossRef] [PubMed]
- Kagi, J.H.; Valee, B.L. Metallothionein: A cadmium- and zinc-containing protein from equine renal cortex. J. Biol. Chem. 1960, 235, 3460–3465. [Google Scholar] [PubMed]
- Dudley, E.R.; Gammal, L.M.; Klaassen, C.D. Cadmium-induced hepatic and renal injury in chronically exposed rats: Likely role of hepatic cadmium-metallothionein in nephrotoxicity. Toxicol. Appl. Pharmacol. 1985, 77, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Zalups, R.K.; Ahmad, S. Molecular handling of cadmium in transporting epithelia. Toxicol. Appl. Pharmacol. 2003, 186, 163–188. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.M.; Zhu, L.F.; Zhong, R.; Grant, D.; Goyer, R.A.; Cherian, M.G. Nephrotoxicity in rats following liver transplantation from cadmium-exposed rats. Toxicol. Appl. Pharmacol. 1993, 123, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Habeebu, S.M.; Waalkes, M.P.; Klaassen, C.D. Metallothionein-I/II null mice are sensitive to chronic oral cadmium-induced nephrotoxicity. Toxicol. Sci. 2000, 57, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Dorian, C.; Gattone, V.H., 2nd; Klaassen, C.D. Accumulation and degradation of the protein moiety of cadmium-metallothionein (CdMT) in the mouse kidney. Toxicol. Appl. Pharmacol. 1992, 117, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Habeebu, S.S.; Klaassen, C.D. Susceptibility of MT-null mice to chronic CdCl2-induced nephrotoxicity indicates that renal injury is not mediated by the CdMT complex. Toxicol. Sci. 1998, 46, 197–203. [Google Scholar] [PubMed]
- Jenkitkasemwong, S.; Wang, C.Y.; Mackenzie, B.; Knutson, M.D. Physiologic implications of metal-ion transport by ZIP14 and ZIP8. Biometals 2012, 25, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Fujishiro, H.; Yano, Y.; Takada, Y.; Tanihara, M.; Himeno, S. Roles of ZIP8, ZIP14, and DMT1 in transport of cadmium and manganese in mouse kidney proximal tubule cells. Metallomics 2012, 4, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Boulukos, K.E.; Poggi, M.C.; Pognonec, P. Long-term extracellular signal-related kinase activation following cadmium intoxication is negatively regulated by a protein kinase C-dependent pathway affecting cadmium transport. FEBS J. 2009, 276, 1667–1679. [Google Scholar] [CrossRef] [PubMed]
- Fujishiro, H.; Doi, M.; Enomoto, S.; Himeno, S. High sensitivity of RBL-2H3 cells to cadmium and manganese: An implication of the role of ZIP8. Metallomics 2011, 3, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Fujishiro, H.; Ohashi, T.; Takuma, M.; Himeno, S. Suppression of ZIP8 expression is a common feature of cadmium-resistant and manganese-resistant RBL-2H3 cells. Metallomics 2013, 5, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Barbier, O.; Jacquillet, G.; Tauc, M.; Poujeol, P.; Cougnon, M. Acute study of interaction among cadmium, calcium, and zinc transport along the rat nephron in vivo. Am. J. Physiol. Renal. Physiol. 2004, 287, F1067–F1075. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Sadovic, S.; Shaikh, Z.A. Nephrotoxicity of cadmium-metallothionein: Protection by zinc and role of glutathione. Toxicol. Appl. Pharmacol. 1998, 151, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Agirdir, B.V.; Bilgen, I.; Dinc, O.; Ozcaglar, H.U.; Fisenk, F.; Turhan, M.; Oner, G. Effect of zinc ion on cadmium-induced auditory changes. Biol. Trace Elem. Res. 2002, 88, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Jacquillet, G.; Barbier, O.; Cougnon, M.; Tauc, M.; Namorado, M.C.; Martin, D.; Reyes, J.L.; Poujeol, P. Zinc protects renal function during cadmium intoxication in the rat. Am. J. Physiol. Renal. Physiol. 2006, 290, F127–F137. [Google Scholar] [CrossRef] [PubMed]
- Rentschler, G.; Kippler, M.; Axmon, A.; Raqib, R.; Skerfving, S.; Vahter, M.; Broberg, K. Cadmium concentrations in human blood and urine are associated with polymorphisms in zinc transporter genes. Metallomics 2014, 6, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Ho, W.C.; Caffrey, J.L.; Sonawane, B. Low serum zinc is associated with elevated risk of cadmium nephrotoxicity. Environ. Res. 2014, 134C, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zalups, R.K.; Barfuss, D.W. Potential mechanisms involved in the absorptive transport of cadmium in isolated perfused rabbit renal proximal tubules. Toxicol. Lett. 2010, 193, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.; Montalbetti, N.; Franz, M.C.; Graeter, S.; Simonin, A.; Hediger, M.A. Human TRPV5 and TRPV6: Key players in cadmium and zinc toxicity. Cell Calcium 2013, 54, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.; Danko, T.; Bergeron, M.J.; Balazs, B.; Suzuki, Y.; Zsembery, A.; Hediger, M.A. Heavy metal cations permeate the TRPV6 epithelial cation channel. Cell Calcium 2011, 49, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C. Role of calcium channels in heavy metal toxicity. ISRN Toxicol. 2013, 2013, 184360. [Google Scholar] [CrossRef] [PubMed]
- Bannon, D.I.; Abounader, R.; Lees, P.S.; Bressler, J.P. Effect of DMT1 knockdown on iron, cadmium, and lead uptake in Caco-2 cells. Am. J. Physiol. Cell Physiol. 2003, 284, C44–C50. [Google Scholar] [CrossRef] [PubMed]
- Fujishiro, H.; Kubota, K.; Inoue, D.; Inoue, A.; Yanagiya, T.; Enomoto, S.; Himeno, S. Cross-resistance of cadmium-resistant cells to manganese is associated with reduced accumulation of both cadmium and manganese. Toxicology 2011, 280, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Illing, A.C.; Shawki, A.; Cunningham, C.L.; Mackenzie, B. Substrate profile and metal-ion selectivity of human divalent metal-ion transporter-1. J. Biol. Chem. 2012, 287, 30485–30496. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, H.; Katsuta, O.; Toyota, N.; Tsuchitani, M.; Umemura, T.; Marumo, F. Chronic cadmium exposure-induced renal anemia in ovariectomized rats. Toxicol. Appl. Pharmacol. 1996, 137, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Kim, K.Y.; Choi, B.S.; Youn, P.; Ryu, D.Y.; Klaassen, C.D.; Park, J.D. Regulation of metal transporters by dietary iron, and the relationship between body iron levels and cadmium uptake. Arch. Toxicol. 2007, 81, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, K.M.; Stocker, S.L.; Wittwer, M.B.; Xu, L.; Giacomini, K.M. Renal transporters in drug development. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 503–529. [Google Scholar] [CrossRef] [PubMed]
- Soodvilai, S.; Nantavishit, J.; Muanprasat, C.; Chatsudthipong, V. Renal organic cation transporters mediated cadmium-induced nephrotoxicity. Toxicol. Lett. 2011, 204, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Zalups, R.K. Evidence for basolateral uptake of cadmium in the kidneys of rats. Toxicol. Appl. Pharmacol. 2000, 164, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, A.Z.; Northup, J.B.; Vestergaard, P. Dependence of cadmium-metallothionein nephrotoxicity on glutathione. J. Toxicol. Environ. Health. A 1999, 57, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Kimura, O.; Endo, T.; Hotta, Y.; Sakata, M. Effects of P-glycoprotein inhibitors on transepithelial transport of cadmium in cultured renal epithelial cells, LLC-PK1 and LLC-GA5-COL 150. Toxicology 2005, 208, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.K.; Torchalski, B.; Kohistani, N.; Thevenod, F. ABCB1 protects kidney proximal tubule cells against cadmium-induced apoptosis: Roles of cadmium and ceramide transport. Toxicol. Sci. 2011, 121, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Fareh, M.; Poggi, M.C.; Boulukos, K.E.; Pognonec, P. Manganese is highly effective in protecting cells from cadmium intoxication. Biochem. Biophys. Res. Commun. 2006, 351, 294–299. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Shu, Y. Cadmium Transporters in the Kidney and Cadmium-Induced Nephrotoxicity. Int. J. Mol. Sci. 2015, 16, 1484-1494. https://doi.org/10.3390/ijms16011484
Yang H, Shu Y. Cadmium Transporters in the Kidney and Cadmium-Induced Nephrotoxicity. International Journal of Molecular Sciences. 2015; 16(1):1484-1494. https://doi.org/10.3390/ijms16011484
Chicago/Turabian StyleYang, Hong, and Yan Shu. 2015. "Cadmium Transporters in the Kidney and Cadmium-Induced Nephrotoxicity" International Journal of Molecular Sciences 16, no. 1: 1484-1494. https://doi.org/10.3390/ijms16011484
APA StyleYang, H., & Shu, Y. (2015). Cadmium Transporters in the Kidney and Cadmium-Induced Nephrotoxicity. International Journal of Molecular Sciences, 16(1), 1484-1494. https://doi.org/10.3390/ijms16011484