Abstract
We analysed the expression levels of 84 key genes involved in the regulated degradation of cellular protein by the ubiquitin-proteasome system in peripheral cells from patients with frontotemporal dementia (FTD) due to C9ORF72 and GRN mutations, as compared with sporadic FTD and age-matched controls. A SABiosciences PCR array was used to investigate the transcription profile in a discovery population consisting of six patients each in C9ORF72, GRN, sporadic FTD and age-matched control groups. A generalized down-regulation of gene expression compared with controls was observed in C9ORF72 expansion carriers and sporadic FTD patients. In particular, in both groups, four genes, UBE2I, UBE2Q1, UBE2E1 and UBE2N, were down-regulated at a statistically significant (p < 0.05) level. All of them encode for members of the E2 ubiquitin-conjugating enzyme family. In GRN mutation carriers, no statistically significant deregulation of ubiquitination pathway genes was observed, except for the UBE2Z gene, which displays E2 ubiquitin conjugating enzyme activity, and was found to be statistically significant up-regulated (p = 0.006). These preliminary results suggest that the proteasomal degradation pathway plays a role in the pathogenesis of FTD associated with TDP-43 pathology, although different proteins are altered in carriers of GRN mutations as compared with carriers of the C9ORF72 expansion.
1. Introduction
Frontotemporal lobar degeneration (FTLD) is a group of complex disorders resulting from the progressive deterioration of the frontal and anterior temporal lobes of the brain. The subcategories of the disease are defined by their dominant clinical symptom in patients; the behavioral variant frontotemporal dementia (FTD) that accounts for two-thirds of patients, and two language variants classified on their effects on fluency (Progressive non-fluent aphasia, PNFA) or semantic difficulties in communicative speech and understanding the semantic content of language (Semantic dementia, SD). In addition, FTD may overlap clinically with motor neuron disease (FTD-MND) [1]. Several genes have been identified as causative of autosomal dominant FTLD. In particular, microtubule associated protein tau (MAPT), progranulin (GRN) and chromosome 9 open reading frame (C9ORF)72 are considered the most important players of FTD, responsible for the majority of inherited cases. Mutations in GRN account for 5% to 11% of cases [2,3]. Upon autopsy, GRN mutation carriers exhibit cerebral atrophy and show a highly constituent pattern corresponding to FTLD-TAR DNA binding protein (TDP)-43 type A, which is characterized by numerous short dystrophic neurites and neuronal cytoplasmatic inclusions [4]. In late 2011, a repeated hexanucleotide in the first intron of C9ORF72 was found to be the most common genetic cause of amyotrophic lateral sclerosis (ALS) and FTD, with or without MND [5,6]. This expansion accounts for about 6% of cases [7], and is categorized histologically as a Type B pathology, with inclusion bodies in neurons and in glial cells that are TDP-43 positive [8]. However, Al-Sarraj and colleagues, described a subset of TDP-43 proteinopathy patients, carrying C9ORF72 expansion, who had unusual and abundant p62 positive, but TDP-43 negative inclusions in the cerebellum and hippocampus [9].
Efficient and rapid elimination of misfolded proteins is critical to the maintenance of cellular health. Under normal conditions, protein homeostasis is achieved through a molecular pathway called the ubiquitin-proteasome system (UPS) [10]. The UPS is a highly coordinated system, which regulates the degradation of a wide variety of proteins. Therefore, it has been supposed that dysfunction of the UPS is implicated in the pathogenesis of several neurodegenerative diseases, such as Huntington’s disease (HD) and Alzheimer’s disease (AD) [11]. Studies in animal models indicate that early impairment of the UPS could be considered the primary mediator of neurodegeneration, raising the possibility of proteostasis-based therapies to slow disease progression [12,13]. Therefore, the UPS represents, for mammalian cells, a major defense against misfolded proteins, particularly in post-mitotic neurons. In healthy conditions, proteins are marked for proteosomal degradation by covalent conjugation of ubiquitin (Ub), a highly conserved protein composed by 76 amino acids, in a three-step cascade. Firstly, the ubiquitin-activating enzyme (E1) activates Ub in an ATP-dependent reaction. Following activation, Ub is transferred in a second thioester linkage to one of several ubiquitn-conjugating enzymes (E2) [14]. A third class of enzyme, the ubiquitin ligases (E3s), mediates the attachment of poly-ubiquitin chains to specific substrate proteins. Poly-ubiquitinated proteins are recognized and subsequently degraded by the 26S proteasome [15]. Similar to other posttranslational modifications, the process of ubiquitination is reversible under the influence of specific de-ubiquitinating enzymes (DUBs) [16]. Both E2 and E3 proteins exist as large families and different combinations of E2s with different E3 proteins define the substrate specificity.
Another mechanism to remove misfolded protein is autophagy, the main cellular catabolic route for protein aggregates and damaged organelles. Autophagy plays a critical role in cytoprotection by preventing the accumulation of toxic proteins and acting in various aspects of immunity, including the elimination of invading microbes and its participation in antigen presentation. In the last decade, emerging evidence revealed that autophagy can distinguish and direct specific cargos to the lysosome.
Protein degradations performed by the UPS and autophagy were regarded for a long time as complementary but separate mechanisms [17]. However, on the basis of recent studies, there are overlaps between them. The way of degradation of a misfolded, redundant, or unneeded protein may be often governed by the momentary activity or capacity of these systems or, in some cases, determined by strict regulation. Moreover, the two pathways use common adaptors capable of directing ubiquitinylated target proteins to both. For example, recognition of ubiquitinylated proteins during autophagy is mediated by ubiquitin receptors interacting with ubiquitin noncovalently, via their ubiquitin-binding domains. Sequestosome 1 (p62/SQSTM1), the first protein reported to have such an adaptor function [18], was originally discovered as a scaffold in signaling pathways regulating cell growth and proliferation.
Recently, several rare mutations in the SQSTM1 gene, were reported in patients with FTLD and ALS [18,19,20]. Moreover, van der Zee et al., identified additional variants in SQSTM1 in FTLD patients [21].
Given the importance of UPS impairment in the pathogenesis of neurodegenerative diseases, in the present study we aimed at determining the contribution of ubiquitination pathway gene expression to the pathogenesis of FTLD. In particular, we analyzed the expression profile of 84 key genes involved in the UPS in peripheral cells from C9ORF72 and GRN mutation carriers, who are likely characterized by TDP-43 deposition in the brain, as compared with sporadic FTLD and age-matched controls.
2. Results
Gene expression profiles of 84 key genes involved in the regulated degradation of cellular proteins by the ubiquitin-proteasome system (Figure 1A) were performed in PBMCs from six each of: sporadic FTD patients, C9ORF72 expansion carriers, GRN Thr272fs (g.1977_1980delCACT) carriers, and age-matched healthy control groups.
A generalized down-regulation of the gene expression profile in C9ORF72 expansion carriers and sporadic bvFTD patients compared with controls was observed (Figure 1B,C). In particular, in both groups, four genes were found statistically significant down-regulated (mean ± SD versus controls): UBE2N (sporadic FTD patients: −1.54 ± 1.46 fold decrease, p = 0.014; C9ORF72 expansion carriers: −1.2 ± 1.24 fold decrease, p = 0.011), UBE2Q1 (sporadic FTD patients: −1.48 ± 1.32 fold decrease, p = 0.014; C9ORF72 expansion carriers: −1.13 ± 1.12 fold decrease, p = 0.0027), UBE2E1 (sporadic patients: −1.94 ± 1.27 fold decrease, p = 0.002; C9ORF72 expansion carriers: −1.69 ± 1.47 fold decrease, p = 0.007), UBE2I (sporadic FTD patients: −1.57 ± 1.32 fold decrease, p = 0.013; C9ORF72 expansion carriers: −1.42 ± 1.30 fold decrease, p = 0.011). All four genes encode for members of the E2 ubiquitin-conjugating enzyme family.
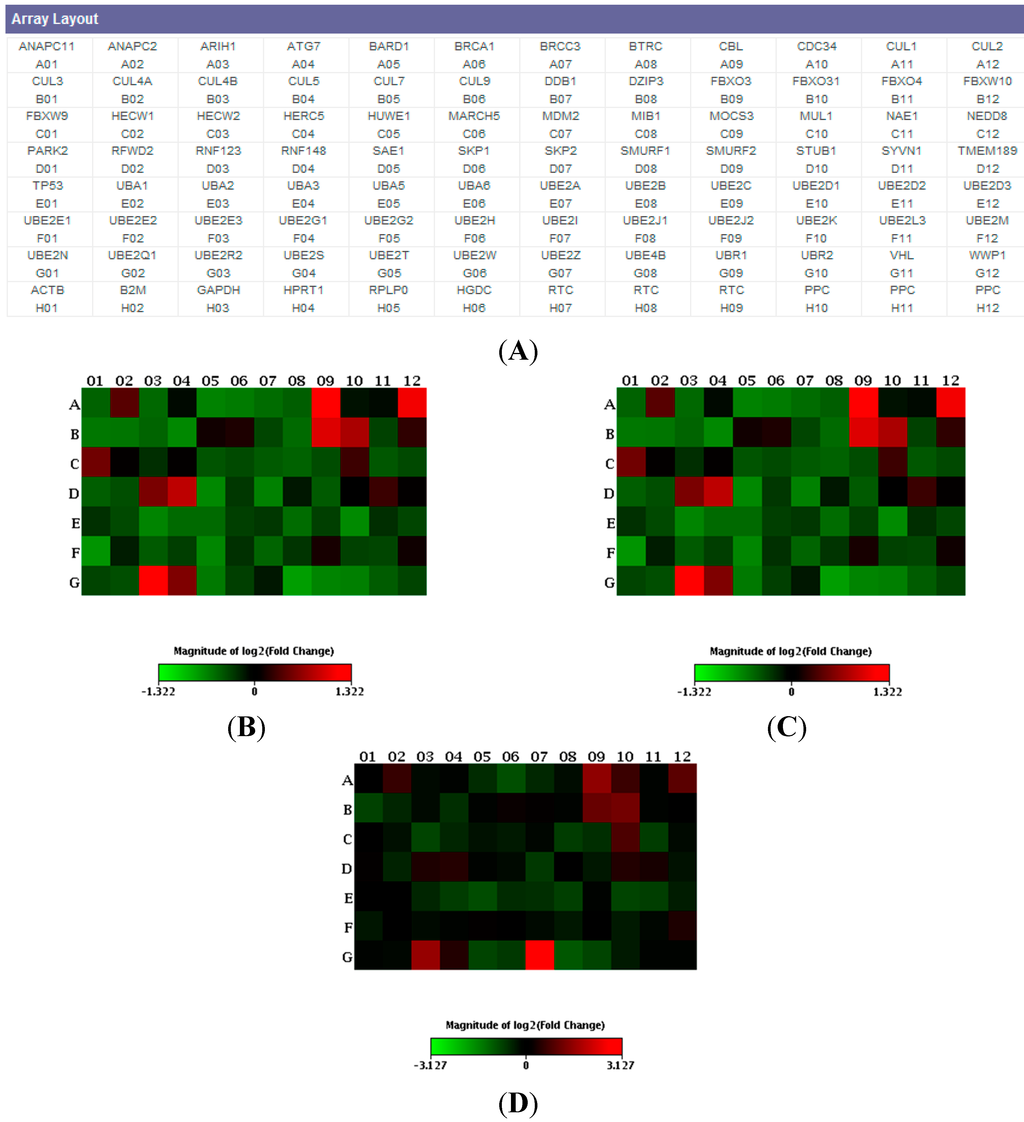
Figure 1.
(A) Layout of the ubiquitination (ubiquitylation) pathway PCR array (PAHS-079Z). Heat maps of patients versus controls; (B) C9ORF72; (C) sporadic; and (D) GRN. Data are expressed as fold change (fold difference) and each square represents a gene of the ubiquitination pathway. Green indicates down-regulation, red up-regulation.
In GRN mutation carriers, no statistically significant deregulation of ubiquitination pathway genes was observed, except for the UBE2Z gene, which was found to be statistically significant up-regulated (8.73 ± 1.06 fold increase over controls, p = 0.006, Figure 1D). Similarly to the previous four genes described, UBE2Z gene displays E2 ubiquitin conjugating enzyme activity.
In addition, despite that the significant threshold was not reached, the following genes were up-regulated in sporadic, C9ORF72, and GRN patients versus controls, independent of the presence of mutations: CBL (2.79 ± 1.3, 2.5 ± 1.8, 2.81 ± 0.8 fold increase, respectively, p > 0.05), FBXO3 (2.48 ± 1.4, 1.97 ± 1.08, 2.10 ± 0.8, respectively, p > 0.05) and UBE2R2 (2.81 ± 1.4, 2.35 ± 1.7, 2.98 ± 0.78, respectively, p > 0.05).
3. Discussion
Here, we report that genes involved in the regulated degradation of cellular proteins by the ubiquitin-proteasome system are deregulated in FTD. In particular, specific profiles have been found in carriers of GRN and C9ORF72 mutations, which are associated with TDP-43 pathology. We thus could hypothesize that different genetic defects (GRN or C9ORF72 mutations) converge, at some point during neurodegeneration, on the same pathway.
The control of protein synthesis and turnover is essential for the health of all cells. In neurons this equilibrium takes on the additional importance of supporting and regulating the highly dynamic connections between neurons, which are necessary for cognitive functions. The UPS is able to address this balance thanks to the combined activity of over 500 enzymes working together to regulate protein-protein interaction and eliminate unwanted proteins. Evidence continues to mount for the necessity of UPS involvement in the dynamic remodelling of synaptic structures following synaptic activity [22,23], but the full role of ubiquitination pertaining to synaptic structure remains incompletely understood [24]. Improper clearance of proteins is believed to be a causative or contributing factor in many neurodegenerative diseases, which are often characterized by the accumulation of aggregated proteins [25]. In particular, UPS dysfunction has been reported in the most common neurodegenerative diseases including AD, HD and Parkinson’s Disease (PD), but little is known about the role of ubiquitination in the pathogenesis of FTLD.
Our results reveal a generalized down-regulation of ubiquitination gene expression in sporadic patients and C9ORF72 expansion carriers. In particular, four genes UBE2I, UBE2Q1, UBE2E and UBE2N were down-regulated at statistically significant levels in both groups. On the contrary, no statistically significant deregulation of the ubiquitination gene pathway was observed in GRN mutation carriers, except for the UBE2Z gene, which was significantly up-regulated in GRN mutation carriers as compared with controls. Conversely, a few genes showed a trend towards an up-regulation all patients, independent of the presence of mutations, including CBL, FBXO3 and UBE2R2. However, to date, these genes are not very well characterized, despite being implicated in the ubiquitin ligase activity.
UBE2I, UBE2Q1, UBE2E and UBE2N genes encodes for members of the E2 ubiquitin-conjugating enzyme family, but little is known about the function of this enzyme. Recently, UBE2I protein was associated with early events occurring in astrocytes from animal models of amyotrophic lateral sclerosis [26]. Moreover, the expression levels of UBE2Q1 were found down regulated in rat brain following traumatic brain injury [27]. Hans et al., demonstrated that UBE2E protein binds TDP-43 directly, and identified a new potential modifier of TDP-43 neurotoxiticy [28]. Taken together these findings show that a modulation of ubiquitination enzymes might become useful in future preclinical studies, but the complete mechanism of TDP-43 turnover remains to be identified.
In addition, it is interesting to note that the UBE2Z gene is located on chromosome 17q21, quite close to the GRN gene position and is widely expressed in human tissues [29]. According to Hapmap data, they are not however in the same linkage disequilibrium block, suggesting that mutations in GRN gene should not influence UBE2Z genomic structure and transcription.
Regarding the population studied, cases were homogeneous in terms of clinical presentation and phenotype and age at onset. Nevertheless, we acknowledge that our cohort is quite small and no neuropathological confirmation is available. This point is important especially for sporadic patients, because we cannot predict the pathology (TDP-43 versus tau). Therefore a replication in a larger and pathologically confirmed population is advisable. On the other hand, although preliminary, data we obtained from samples deriving from patients may help to better understand processes occurring in vivo, which may hopefully result in the identification of potential therapeutic targets.
Lastly, the UPS is not alone in its handling of unwanted proteins. The autophagy system is the other major mechanism by which protein clearance is achieved and it may be especially important given the p62 pathology in C9ORF72 cases. Given that, this kind of analysis should be conducted also for autophagy gene expression pathway.
4. Materials and Methods
4.1. Patients and Controls
Eighteen patients with FTD (six each of GRN mutation carriers, C9ORF72 expansion carriers and the sporadic groups) were recruited at the Alzheimer Unit of the Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, University of Milan (Milan, Italy). All patients underwent a standard battery of examinations, including medical history, physical and neurological examination, screening laboratory tests, neurocognitive evaluation and imaging. Cognitive dysfunctions were assessed by the clinical dementia rating (CDR), the mini mental state examination (MMSE), the frontal assessment battery (FAB), the Wisconsin Card Sorting Test (WCST), and the Tower of London test. The presence of significant vascular brain damage was excluded (Hachinski Ischemic Score < 4). The diagnosis of FTD was made according to current consensus criteria [30] and subsequent revisions [31,32].
The control group consisted of six non-demented volunteers matched for ethnic background and age, without memory and psycho-behavioural dysfunctions (MMSE ≥ 28). Informed consent to participate in this study was given by all subjects or their caregivers. Characteristics of patients and controls are summarized in Table 1.

Table 1.
Characteristics of frontotemporal dementia (FTD) patients and controls.
| Characteristics | Sporadic | C9ORF72 | GRN | Controls |
|---|---|---|---|---|
| Number of patients | 6 | 6 | 6 | 6 |
| Gender (M:F) | 4:2 | 5:1 | 4:2 | 3:3 |
| Mean age (years ± SEM) | 66.3 ± 8.6 | 63.9 ± 8.1 | 62 ± 9.4 | 65 ± 7.3 |
| Mean Age at onset (years ± SEM) | 64.5 ± 0.44 | 61 ± 0.36 | 59 ± 0.98 | – |
SEM = Standard error of mean.
4.2. Screening of GRN and C9ORF72 Mutations
High molecular weight DNA was isolated from whole blood using a Flexigene Kit (Qiagen, Hildren, Gemany). GRN sequencing was performed by direct sequencing, as previously described [33]. C9ORF72 genotyping was carried out by repeat-primed polymerase chain reaction and sequencing [5]. This method allows detection of 30 to 50 repeats; According to current literature [34], a characteristic stutter amplification pattern (>40 repeats) on the electropherogram is considered evidence of a pathogenic repeat expansion.
4.3. Total mRNA Isolation from Peripheral Blood Mononuclear Cells (PBMC)
For each subject, 14 mL of blood have been collected in two BD VacutainerR CPTTM (1 mL NC, 2 mL Ficoll) as previously described [35]. From each tube, PBMCs were separated by gradient centrifugation and total RNA extracted with the single step acid phenol method, using Trizol (Invitrogen, Carlsbad, CA, USA). RNA purity was measured by optical density and only samples with an OD 260/280 ratio ranging from 1.8 to 2 and an OD 260/230 of 1.8 or greater were used.
4.4. Screening of Ubiquitination Pathways by PCR Array
RNA was retrotranscribed with RT2 First Strand Kit (SABiosciences, Frederick, MD, USA), according to the instruction of the manufacturer. For real time PCR experiments, the ubiquitination (ubiquitylation) pathway PCR array (PAHS-079Z, Figure 1) was used and runs were performed in an Applied BioSystems StepOne Plus system (Foster City, CA, USA). The array profiles the expression of 84 key genes involved in the regulated degradation of cellular proteins by the ubiquitin-proteasome system. These arrays included also five housekeeping genes (ACTB, B2M, G3PDH, HPRT1 and RPLP0) for the proper normalization of the data, mRNA reverse transcription control and a positive PCR control (Figure 1A).
4.5. Statistical Analysis
The SABiosciences PCR Array data analysis was based on ΔΔCt method with normalization of the raw data to housekeeping genes (using the software available at http://www.sabiosciences.com/pcarraydataanalysis.php). p Values were calculated based on a Student’s t-test of the replicate 2−ΔCt) values for each gene in the control and FTD groups. Best hits were chosen based on statistical significance (p < 0.05). Haploview 4.2 software was used to test for linkage disequilibrium (LD) blocks.
5. Conclusions
In conclusion, knowledge of the UPS components that are involved in FTD pathogenesis will be helpful for the identification of potential therapeutic targets, which aim to limit, at early stages, the accumulation of misfolded proteins without disturbance of this major proteolytic pathway.
Acknowledgments
This work has been carried out thanks to funds from the Italian Ministry of Health and the Monzino Foundation.
Author Contributions
Maria Serpente: work plan, data analysis and writing; Chiara Fenoglio: work plan and data analysis; Sara M. G. Cioffi and Rossana Bonsi technical assistance; Andrea Arighi, Giorgio G. Fumagalli and Laura Ghezzi: sample collection, diagnosis and follow up; Elio Scarpini and Daniela Galimberti: writing and supervision.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Seltman, R.E.; Matthews, B.R. Frontotemporal lobar degeneration: Epidemiology, pathology, diagnosis and management. CNS Drugs 2012, 26, 841–870. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.; Mackenzie, I.R.; Pickering-Brown, S.M.; Gass, J.; Rademakers, R.; Lindholm, C.; Snowden, J.; Adamson, J.; Sadovnick, A.D.; Rollinson, S.; et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 2006, 442, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, I.R.; Neumann, M.; Bigio, E.H.; Cairns, N.J.; Alafuzoff, I.; Kril, J.; Kovacs, G.G.; Ghetti, B.; Halliday, G.; Holm, I.E.; et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: An update. Acta Neuropathol. 2010, 119, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, I.R.; Munoz, D.G.; Kusaka, H.; Yokota, O.; Ishihara, K.; Roeber, S.; Kretzschmar, H.A.; Cairns, N.J.; Neumann, M. Distinct pathological subtypes of FTLD-FUS. Acta Neuropathol. 2011, 121, 207–218. [Google Scholar] [CrossRef] [PubMed]
- DeJesus-Hernandez, M.; Mackenzie, I.R.; Boeve, B.F.; Boxer, A.L.; Baker, M.; Rutherford, N.J.; Nicholson, A.M.; Finch, N.A.; Flynn, H.; Adamson, J. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 2011, 72, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Renton, A.E.; Majounie, E.; Waite, A.; Simón-Sánchez, J.; Rollinson, S.; Gibbs, J.R.; Schymick, J.C.; Laaksovirta, H.; van Swieten, J.C.; Myllykangas, L.; et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 2011, 72, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, D.; Fenoglio, C.; Serpente, M.; Villa, C.; Bonsi, R.; Arighi, A.; Fumagalli, G.G.; del Bo, R.; Bruni, A.C.; Anfossi, M.; et al. Autosomal dominant frontotemporal lobar degeneration due to the C9ORF72 hexanucleotide repeat expansion: Late-onset psychotic clinical presentation. Biol. Psychiatry 2013, 74, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, J.T.; Sun, F.R.; Ou, J.R.; Qu, S.B.; Tan, L. The clinical and pathological phenotypes of frontotemporal dementia with C9ORF72 mutations. J. Neurol. Sci. 2013, 335, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Al-Sarraj, S.; King, A.; Troakes, C.; Smith, B.; Maekawa, S.; Bodi, I.; Rogelj, B.; Al-Chalabi, A.; Hortobágyi, T.; Shaw, C.E. p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta Neuropathol. 2011, 122, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Hartl, F.U.; Bracher, A.; Hayer-Hartl, M. Molecular chaperones in protein folding and proteostasis. Nature 2011, 475, 324–332. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, C.; Tabrizi, S.J. The ubiquitin-proteasome system in neurodegeneration. Antioxid. Redox. Signal. 2014, 21, 2302–2321. [Google Scholar] [CrossRef] [PubMed]
- Tomaru, U.; Takahashi, S.; Ishizu, A.; Miyatake, Y.; Gohda, A.; Suzuki, S.; Ono, A.; Ohara, J.; Baba, T.; Murata, S.; et al. Decreased proteasomal activity causes age-related phenotypes and promotes the development of metabolic abnormalities. Am. J. Pathol. 2012, 180, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Vilchez, D.; Morantte, I.; Liu, Z.; Douglas, P.M.; Merkwirth, C.; Rodrigues, A.P.; Manning, G.; Dillin, A. RPN-6 determines C. elegans longevity under proteotoxic stress conditions. Nature 2012, 489, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Hershko, A. Ubiquitin: Roles in protein modification and breakdown. Cell 1983, 34, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Glickman, M.H.; Ciechanover, A. The ubiquitin-proteasome proteolytic pathway: Destruction for the sake of construction. Physiol. Rev. 2002, 82, 373–428. [Google Scholar] [PubMed]
- Hochstrasser, M. Ubiquitin, proteasomes, and the regulation of intracellular protein degradation. Curr. Opin. Cell Biol. 1995, 7, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Korolchuk, V.I.; Menzies, F.M.; Rubinsztein, D.C. Mechanisms of cross-talk between the ubiquitin-proteasome and autophagy-lysosome systems. FEBS Lett. 2010, 584, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Pankiv, S.; Clausen, T.H.; Lamark, T.; Brech, A.; Bruun, J.-A.; Outzen, H.; Øvervatn, A.; Bjørkøy, G.; Johansen, T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 2007, 282, 24131–24145. [Google Scholar] [CrossRef] [PubMed]
- Rubino, E.; Rainero, I.; Chiò, A.; Rogaeva, E.; Galimberti, D.; Fenoglio, P.; Grinberg, Y.; Isaia, G.; Calvo, A.; Gentile, S.; et al. SQSTM1 mutations in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Neurology 2012, 79, 1556–1562. [Google Scholar] [CrossRef] [PubMed]
- Le Ber, I.; Camuzat, A.; Guerreiro, R.; Bouya-Ahmed, K.; Bras, J.; Nicolas, G.; Gabelle, A.; Didic, M.; de Septenville, A.; Millecamps, S.; et al. SQSTM1 mutations in French patients with frontotemporal dementia or frontotemporal dementia with amyotrophic lateral sclerosis. JAMA Neurol. 2013, 70, 1403–1410. [Google Scholar] [PubMed]
- Van der Zee, J.; van Langenhove, T.; Kovacs, G.G.; Dillen, L.; Deschamps, W.; Engelborghs, S.; Matěj, R.; Vandenbulcke, M.; Sieben, A.; Dermaut, B.; et al. Rare mutations in SQSTM1 modify susceptibility to frontotemporal lobar degeneration. Acta Neuropathol. 2014, 128, 397–410. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, G.P.; Alves, C.J.; Chadi, G. Early gene expression changes in spinal cord from SOD1(G93A) Amyotrophic Lateral Sclerosis animal model. Front. Cell. Neurosci. 2013, 7, 216. [Google Scholar] [PubMed]
- Hanus, C.; Schuman, E.M. Proteostasis in complex dendrites. Nat. Rev. Neurosci. 2013, 14, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Figueiredo-Pereira, M.E. Ubiquitin/proteasome pathway impairment in neurodegeneration: Therapeutic implications. Apoptosis 2010, 15, 1292–1311. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Chen, J.; Hu, B.; Zou, H.; Li, A.; Guo, A.; Jiang, J. Down-regulation of UBE2Q1 is associated with neuronal apoptosis in rat brain cortex following traumatic brain injury. J. Neurosci. Res. 2014, 92, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zhao, F.; Zheng, M.; Fei, X.; Chen, X.; Huang, S.; Xie, Y.; Mao, Y. Cloning and characterization of a gene encoding the human putative ubiquitin conjugating enzyme E2Z (UBE2Z). Mol. Biol. Rep. 2007, 34, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Hung, A.Y.; Sung, C.C.; Brito, I.L.; Sheng, M. Degradation of postsynaptic scaffold GKAP and regulation of dendritic spine morphology by the TRIM3 ubiquitin ligase in rat hippocampal neurons. PLoS One 2010, 5, e9842. [Google Scholar] [CrossRef] [PubMed]
- Hans, F.; Fiesel, F.C.; Strong, J.C.; Jäckel, S.; Rasse, T.M.; Geisler, S.; Springer, W.; Schulz, J.B.; Voigt, A.; Kahle, P.J. UBE2E ubiquitin-conjugating enzymes and ubiquitin isopeptidase Y regulate TDP-43 protein ubiquitination. J. Biol. Chem. 2014, 289, 19164–19179. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.K.; Hung, K.W.; Fu, W.Y.; Shen, C.; Chen, Y.; Xia, J.; Lai, K.O.; Ip, N.Y. APC (Cdh1) mediates EphA4-dependent down-regulation of AMPA receptors in homeostatic plasticity. Nat. Neurosci. 2011, 14, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Neary, D.; Snowden, J.S.; Gustafson, L.; Passant, U.; Stuss, D.; Black, S.; Freedman, M.; Kertesz, A.; Robert, P.H.; Albert, M.; et al. Frontotemporal lobar degeneration. A consensus on clinical diagnostic criteria. Neurology 1998, 51, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.M.; Albert, M.S.; Grossman, M.; Miller, B.; Dickson, D.; Trojanowski, J.Q. Clinical and pathological diagnosis of frontotemporal dementia: Report of the work group on frontotemporal dementia and Pick’s disease. Arch. Neurol. 2001, 58, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; van Swieten, J.C.; Seelaar, H.; Dopper, E.G.; Onyike, C.U.; et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011, 134, 2456–2477. [Google Scholar] [CrossRef] [PubMed]
- Pietroboni, A.M.; Fumagalli, G.G.; Ghezzi, L.; Fenoglio, C.; Cortini, F.; Serpente, M.; Cantoni, C.; Rotondo, E.; Corti, P.; Carecchio, M.; et al. Phenotypic heterogeneity of the GRN Asp22fs mutation in a large Italian kindred. J. Alzheimers Dis. 2011, 24, 253–259. [Google Scholar] [PubMed]
- Dobson-Stone, C.; Hallupp, M.; Bartley, L.; Shepherd, C.E.; Halliday, G.M.; Schofield, P.R.; Hodges, J.R.; Kwok, J.B. C9ORF72 repeat expansion in clinical and neuropathologic frontotemporal dementia cohorts. Neurology 2012, 79, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Serpente, M.; Fenoglio, C.; Villa, C.; Cortini, F.; Cantoni, C.; Ridolfi, E.; Clerici, F.; Marcone, A.; Benussi, L.; Ghidoni, R.; et al. Role of OLR1 and its regulating hsa-miR369–3p in Alzheimer’s disease: Genetics and expression analysis. J. Alzheimers Dis. 2011, 26, 787–793. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).