Analysis of Phenolic Compounds and Antioxidant Abilities of Extracts from Germinating Vitis californica Seeds Submitted to Cold Stress Conditions and Recovery after the Stress
Abstract
:1. Introduction
2. Results and Discussion
2.1. Content of Phenolic Compounds
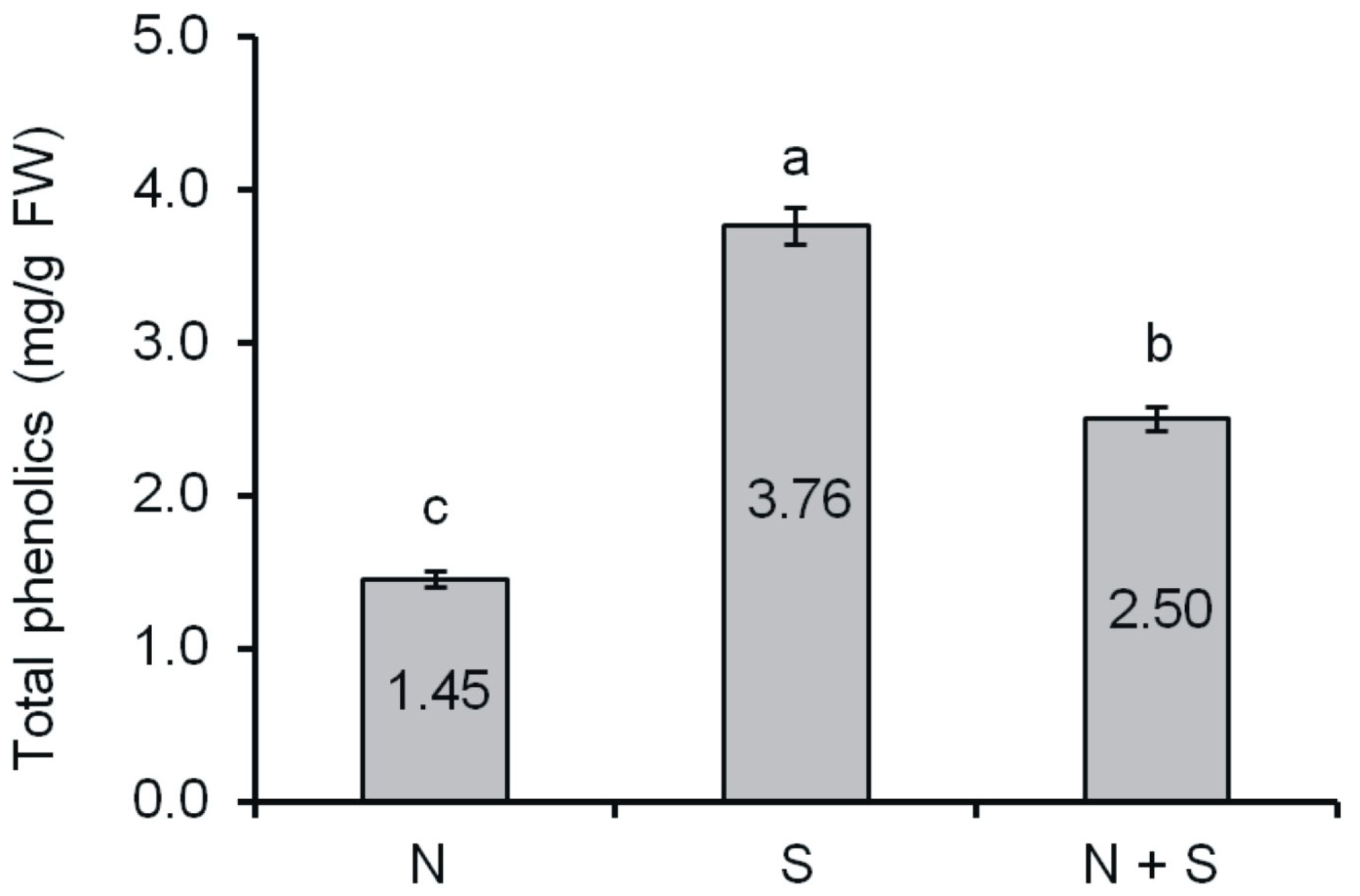
| Group | Absorbance at 500 nm/mg Extract | Absorbance at 510 nm/mg Extract |
|---|---|---|
| NS | 0.147 ± 0.005b | 0.153 ± 0.014c |
| S | 0.282 ± 0.008a | 0.384 ± 0.010a |
| S + R | 0.134 ± 0.003c | 0.212 ± 0.013b |
| Group | Catechin | Epicatecin | Catechin + Epicatechin |
|---|---|---|---|
| NS | 20.9 ± 0.5b | 32.0 ± 0.8b | 52.9 ± 1.3b |
| S | 23.6 ± 0.6a | 39.4 ± 1.0a | 63.0 ± 1.6a |
| S + R | 15.5 ± 0.5c | 25.3 ± 0.6c | 40.8 ± 1.1c |
| Species | Form of Phenolic Acids | Gallic Acid | Caffeic Acid | p-Coumaric Acid | Ferulic Acid |
|---|---|---|---|---|---|
| NS | Free | 5.62 ± 0.01c | 1.11 ± 0.01a | 0.59 ± 0.01a | 0.17 ± 0.01 |
| Esterified | 31.56 ± 0.01c | 5.51 ± 0.01c | 3.67 ± 0.01b | 0.75 ± 0.01a | |
| Glucosided | 5.22 ± 0.03c | - | - | - | |
| Total | 142.40 ± 0.04b | 6.62 ± 0.02c | 4.26 ± 0.02b | 0.92 ± 0.02a | |
| S | Free | 15.22 ± 0.38a | 0.36 ± 0.01b | 0.08 ± 0.01b | trace |
| Esterified | 173.81 ± 4.34a | 6.69 ± 0.18b | 2.51 ± 0.01c | 0.56 ± 0.01c | |
| Glucosided | 14.97 ± 0.037b | - | - | - | |
| Total | 204.00 ± 5.10a | 7.06 ± 0.19b | 2.59 ± 0.01c | 0.56 ± 0.01c | |
| S + R | Free | 12.43 ± 0.31b | 0.13 ± 0.01c | 0.03 ± 0.01c | trace |
| Esterified | 167.62 ± 4.19b | 17.17 ± 0.50a | 5.38 ± 0.01a | 0.62 ±0.01b | |
| Glucosided | 16.06 ± 0.40a | - | - | - | |
| Total | 196.11 ± 4.90a | 20.13 ± 0.43a | 5.41 ± 0.01a | 0.62 ±0.01b |
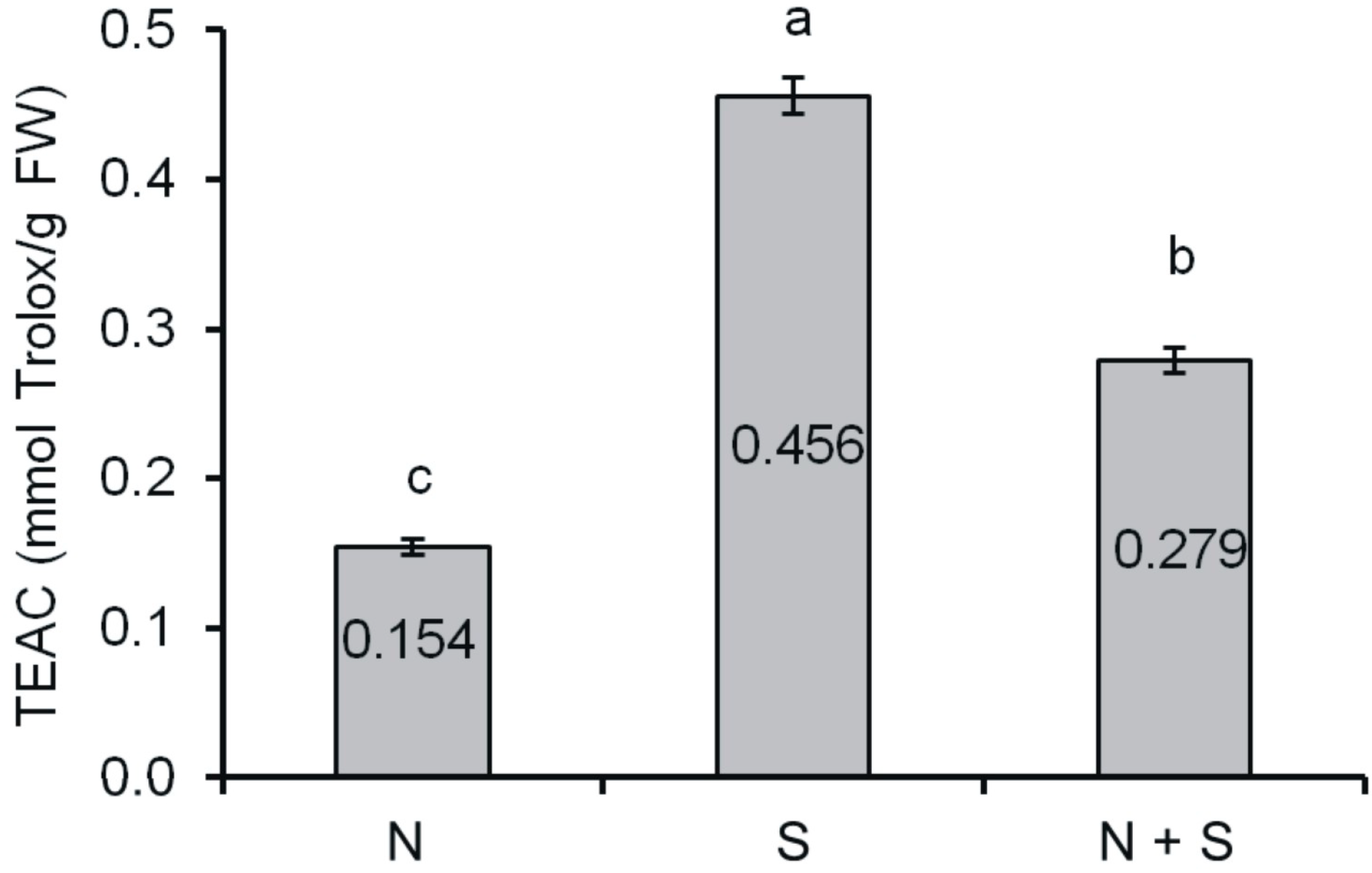
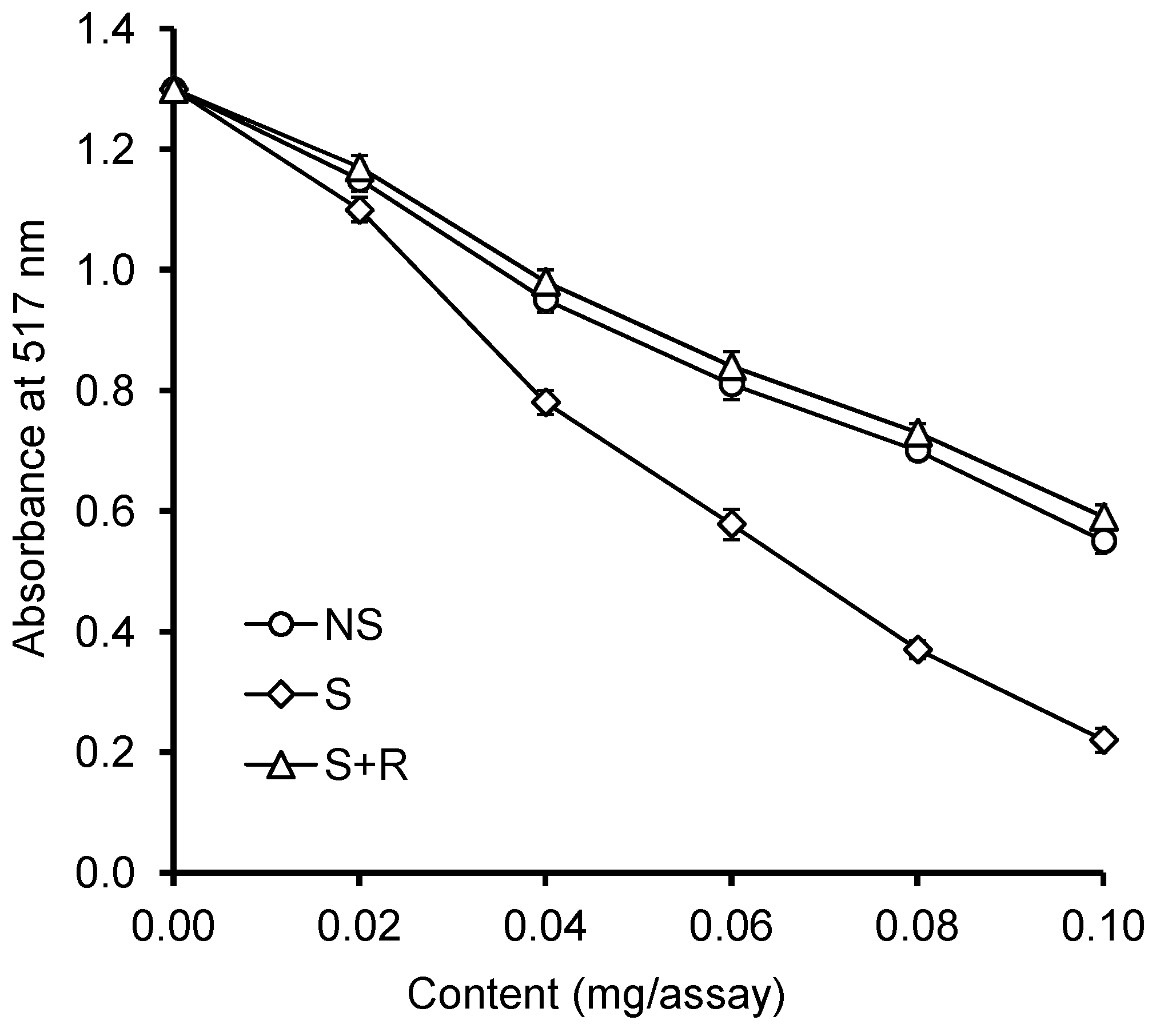
2.2. Antioxidant Activity

3. Experimental Section
3.1. Chemicals
3.2. Plant Material
3.3. Stratification and Germination under Normal and Chill Stress Conditions
3.4. Extract Preparation
3.5. Content of Total Phenolics
3.6. Condensed Tannins
3.7. Trolox Equivalent Antioxidant Capacity
3.8. Reducing Power
3.9. Scavenging of the 2,2'-Diphenyl-1-picrylhydrazyl (DPPH) Radical
3.10. High Performance Liquid Chromatography with Photodiode Array Detection (HPLC-PAD) Analysis of Catechins
3.11. HPLC-PAD Analysis of Phenolic Acids
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Kmieć, B.; Drynda, R.; Wołoszyńska, M. Molekular basis of plant response to low temperature. Biotechnologia 2005, 3, 184–200. [Google Scholar]
- Bartosz, G. Oxidative stress in plants. Acta Physiol. Plant 1997, 19, 47–64. [Google Scholar] [CrossRef]
- Mittova, V.; Volokita, M.; Guy, M.; Tal, M. Activities of SOD and the ascorbate-glutathione cycle enzymes in subcellular compartments in leaves and roots of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii. Physiol. Plant 2000, 110, 45–54. [Google Scholar] [CrossRef]
- GwoŹdź, E.A. Molecular basis of plant response to environmental stress. In New Trends in Molecular Biology and Genetic Engineering, and Medicine; Barciszewski, J., Łatowski, K., Twardowski, T., Eds.; Sorus: Poznań, Poland, 1996; pp. 469–492. [Google Scholar]
- Michalak, A. Phenolic compound and their antioxidant activity in plants growing under heavy metal stress. Polish J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Amarowicz, R.; Weidner, S. Biological activity of grapevine phenolic compounds. In Grapevine Molecular Physiology and Biochemistry, 2nd ed.; Roubelakis-Angelakis, K.A., Ed.; Springer Science + Business Media B.V.: Dordrecht, The Netherlands, 2009; pp. 389–405. [Google Scholar]
- Heo, H.J.; Kim, Y.J.; Chung, D.; Kim, D.O. Antioxidant capacities of individual and combined phenolics in a model system. Food Chem. 2007, 104, 87–92. [Google Scholar] [CrossRef]
- Oh, M.-M.; Carey, E.E.; Rajashekar, C.B. Environmental stresses induce health-promoting phytochemicals in lettuce. Plant Physiol. Biochem. 2009, 47, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.; Bernal, A.; Pomar, F.; Merino, F. Induction of shikimate dehydrogenase and peroxidase in pepper (Capsicum annum L.) seedlings in response to copper stress and its relation to lignifications. Plant Sci. 2001, 161, 179–186. [Google Scholar]
- Escribano-Bailón, T.; Gutierrez-Fernandez, Y.; Rivas-Gonzolo, J.; Santos-Buelga, C. Charakterization of procyanidins of Vitis vinifera v. Tinta Del Pais grape seeds. J. Agric. Food Chem. 1992, 40, 1794–1799. [Google Scholar]
- Nunez, V.; Gomez-Cordoves, C.; Bartolome, B.; Hong, Y.; Mitchel, A.E. Non-galloylated and galloylated proanthocyanidin oligomers in grape seeds from Vitis vinifera L. cv. Graciano, Tempranillo and Cabernet Sauvignon. J. Sci. Agric. 2006, 86, 915–921. [Google Scholar]
- Saint-Cricq de Gaulejac, N.; Augustin, M.; Vivas, N.; Glories, Y. A biochemical approach to the evolution of procyanidins in grape seeds during the ripening of red grapes (Vitis vinifera L. cv. Merlot Noir). J. Wine Res. 1997, 8, 159–167. [Google Scholar]
- Pekić, B.; Kovać, V.; Alonso, E.; Revilla, E. Study of extraction of proanthocyanidins from grape seeds. Food Chem. 1998, 61, 201–206. [Google Scholar]
- Kennedy, J.A.; Troup, G.J.; Pilbrow, J.R.; Hutton, D.R.; Hewitt, D.; Hunter, C.R.; Ristic, R.; Iland, P.G.; Jones, G.P. Development of seed polyphenols in berries from Vitis vinifera L. cv. Shiraz. Aust. J. Grape Wine Res. 2000, 6, 244–254. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Singh, R.P.; Sakariach, K.K. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 2001, 73, 285–290. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Selvi, T.; Sakariah, K.K. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extract. Food Res. Int. 2003, 36, 117–122. [Google Scholar] [CrossRef]
- Shafiee, M.; Carbonneau, M.A.; Urban, N.; Descomps, B.; Leger, C.L. Grape and grape seed extract capacities at protecting LDL against oxidation generated by Cu2+, AAPH or SIN-1 and decreasing superoxide THP-1 cell production. A comparison to other extracts or compounds. Free Radic. Res. 2003, 37, 573–584. [Google Scholar] [PubMed]
- Wróbel, M.; Karamać, M.; Amarowicz, R.; Frączek, E.; Weidner, S. Metabolism of phenolic compounds in Vitis riparia seeds during stratification and during germination under optimal and low temperature stress conditions. Acta Physiol. Plant 2005, 27, 313–320. [Google Scholar]
- Da Silva, J.M.R.; Darmon, N.; Fernandez, Y.; Mitjavila, S. Oxygen free radical scavenger capacity in aqueous models of different procyanidins from grape seeds. J. Agric. Food Chem. 1991, 39, 1549–1552. [Google Scholar]
- Saito, M.; Hosoyama, H.; Ariga, T.; Kataoka, S.; Yamaji, N. Anticulcer activity of grape seed extract and procyanidins. J. Agric. Food Chem. 1998, 46, 1460–1464. [Google Scholar] [CrossRef]
- Weidner, S.; Brosowska-Arendt, W.; Szczechura, W.; Karamać, M.; Kosińska, A.; Amarowicz, R. Effect of osmotic stress and post-stress recovery on the content of phenolics and properties of antioxidants in germinating seeds of grapevine Vitis californica. Acta Soc. Bot. Pol. 2011, 80, 11–19. [Google Scholar] [CrossRef]
- Weidner, S.; Frączek, E.; Amarowicz, R.; Abe, S. Alternations in phenolic acids content in developing rye grains in normal environment and during enforced dehydratation. Acta Physiol. Plant 2001, 23, 475–482. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylopropanoid metabolism. Plant Cell. 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Solecka, D.; Boundet, A.M.; Kacperska, A. Phenylopropanoid and anthocyanin changes in low-temperature treated winter oilseed rape leaves. Plant Physiol. Biochem. 1999, 37, 491–496. [Google Scholar] [CrossRef]
- Janas, K.M.; Cvikrova, M.; Pałągiewicz, A.; Eder, J. Alternations in phenylpropanoid content in soybean roots during low temperature acclimation. Plant Physiol. Biochem. 2000, 38, 587–593. [Google Scholar] [CrossRef]
- Paul, H.L. (Ed.) The role of phenolic compounds in plants stress response. In Low Temperature Stress Physiology in Crops; Paul, H.L. (Ed.) CRC Press: Boca Raton, FL, USA, 1989; pp. 67–79.
- Vaughan, D.; Ord, B.G. Extraction of potential allelochemicals and their effect on root morphology and nutrient contents. In Plant Root Growth; Atkinson, D., Ed.; An Ecological Perspective, Blackwell Sci Pub.: Oxford, UK, 1991; pp. 399–421. [Google Scholar]
- Yen, G.-C.; Chen, H.-Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Kosińska, A.; Karamać, M. Antioxidant capacity of roasted health-promoting products. Pol. J. Food Nutr. Sci. 2006, 56, 80–84. [Google Scholar]
- Amarowicz, R.; Karamać, M.; Weidner, S.; Abe, S.; Shahidi, F. Antioxidant activity of wheat caryopses and embryos extracts. J. Food Lipids 2002, 9, 201–210. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B.; Rahimi-Moghaddam, P.; Barl, B.; Weil, J.L. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004, 84, 551–562. [Google Scholar] [CrossRef]
- Price, N.J.; van Scoyoc, S.; Butler, L.G. A critical evaluation of the vanillic reactions an assay for tannin in sorghum grain. J. Agric. Food Chem. 1978, 26, 1214–1218. [Google Scholar] [CrossRef]
- Hagerman, A.; Butler, L. Protein precipitation method for quantitative determination of tannins. J. Agric. Food Chem. 1978, 26, 809–811. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on products of browning reaction—Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1978, 44, 307–315. [Google Scholar]
- Weidner, S.; Amarowicz, R.; Karamać, M.; Frączek, E. Changes in endogenous phenolic acids during development of Secale cereale caryopses and after dehydration treatment of unripe rye grains. Plant Physiol. Biochem. 2000, 38, 595–602. [Google Scholar] [CrossRef]
- Weidner, S.; Krupa, U.; Amarowicz, R.; Karamać, M.; Abe, S. Phenolic compounds in embryos of triticale caryopses at different stages of development and maturation in normal environment and after dehydration treatment. Euphytica 2002, 126, 115–122. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Weidner, S.; Chrzanowski, S.; Karamać, M.; Król, A.; Badowiec, A.; Mostek, A.; Amarowicz, R. Analysis of Phenolic Compounds and Antioxidant Abilities of Extracts from Germinating Vitis californica Seeds Submitted to Cold Stress Conditions and Recovery after the Stress. Int. J. Mol. Sci. 2014, 15, 16211-16225. https://doi.org/10.3390/ijms150916211
Weidner S, Chrzanowski S, Karamać M, Król A, Badowiec A, Mostek A, Amarowicz R. Analysis of Phenolic Compounds and Antioxidant Abilities of Extracts from Germinating Vitis californica Seeds Submitted to Cold Stress Conditions and Recovery after the Stress. International Journal of Molecular Sciences. 2014; 15(9):16211-16225. https://doi.org/10.3390/ijms150916211
Chicago/Turabian StyleWeidner, Stanisław, Sebastian Chrzanowski, Magdalena Karamać, Angelika Król, Anna Badowiec, Agnieszka Mostek, and Ryszard Amarowicz. 2014. "Analysis of Phenolic Compounds and Antioxidant Abilities of Extracts from Germinating Vitis californica Seeds Submitted to Cold Stress Conditions and Recovery after the Stress" International Journal of Molecular Sciences 15, no. 9: 16211-16225. https://doi.org/10.3390/ijms150916211
APA StyleWeidner, S., Chrzanowski, S., Karamać, M., Król, A., Badowiec, A., Mostek, A., & Amarowicz, R. (2014). Analysis of Phenolic Compounds and Antioxidant Abilities of Extracts from Germinating Vitis californica Seeds Submitted to Cold Stress Conditions and Recovery after the Stress. International Journal of Molecular Sciences, 15(9), 16211-16225. https://doi.org/10.3390/ijms150916211






