Prophylactic Protective Effects and Its Potential Mechanisms of IcarisideII on Streptozotocin Induced Spermatogenic Dysfunction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Blood Glucose Level, Body Weight and Reproductive Organ Weight
| Parameter | Control | Vehicle | ICAII0.5 | ICAII1.5 | ICAII4.5 |
|---|---|---|---|---|---|
| Blood glucose (mg·dL−1) | 106.85 ± 15.81 | 423.66 ± 13.73 * | 421.32 ± 15.87 * | 421.08 ± 15.67 * | 419.26 ± 17.85 * |
| Initial body weight (g) | 247.33 ± 9.48 | 246.83 ± 8.13 | 247.67 ± 12.83 | 248.17 ± 8.57 | 247.33 ± 11.29 |
| Final body weight (g) | 453.83 ± 10.26 | 345.83 ± 10.57 * | 347.33 ± 12.32 * | 344.33 ± 9.79 * | 346.17 ± 14.27 * |
| Testis weight (g) | 3.23 ± 0.13 | 2.08 ± 0.08 * | 2.10 ± 0.06 * | 2.07 ± 0.12 * | 2.10 ± 0.09 * |
| Epididymis weight (g) | 1.46 ± 0.11 | 1.13 ± 0.15 * | 1.11 ± 0.11 * | 1.11 ± 0.09 * | 1.16 ± 0.20 * |
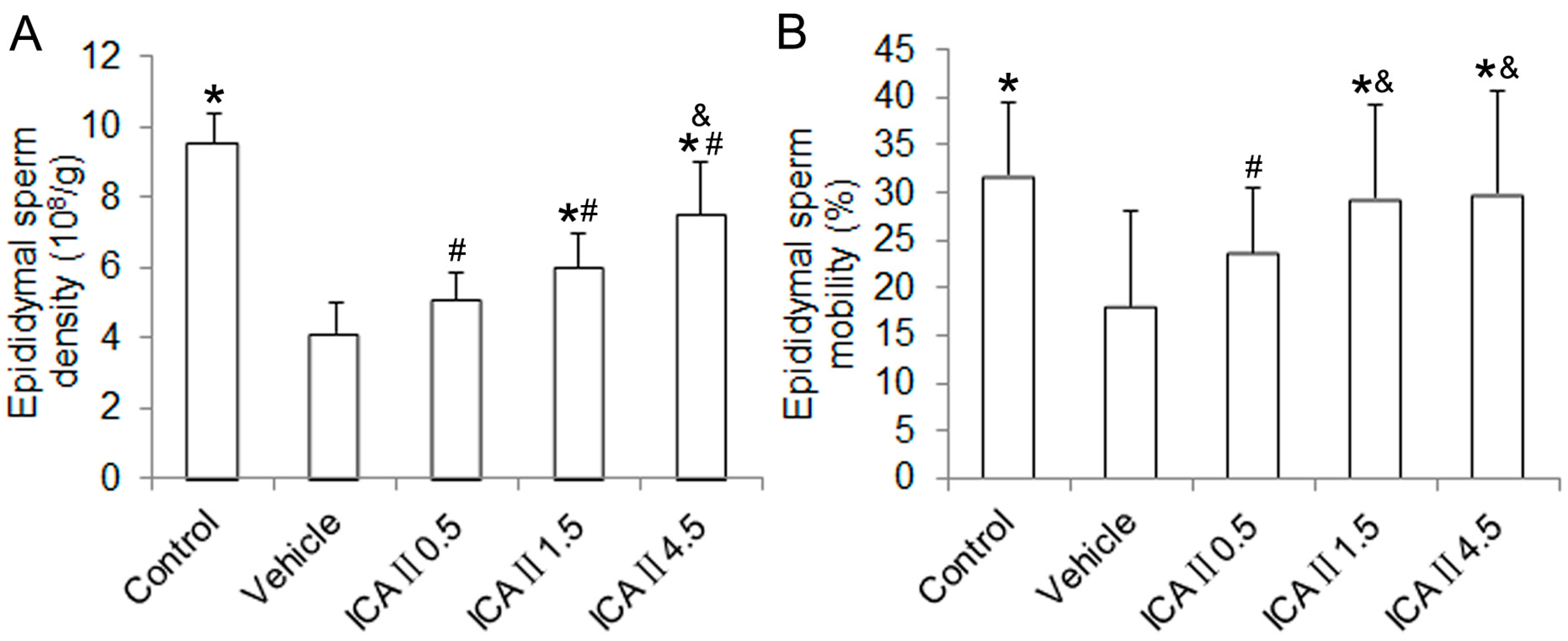
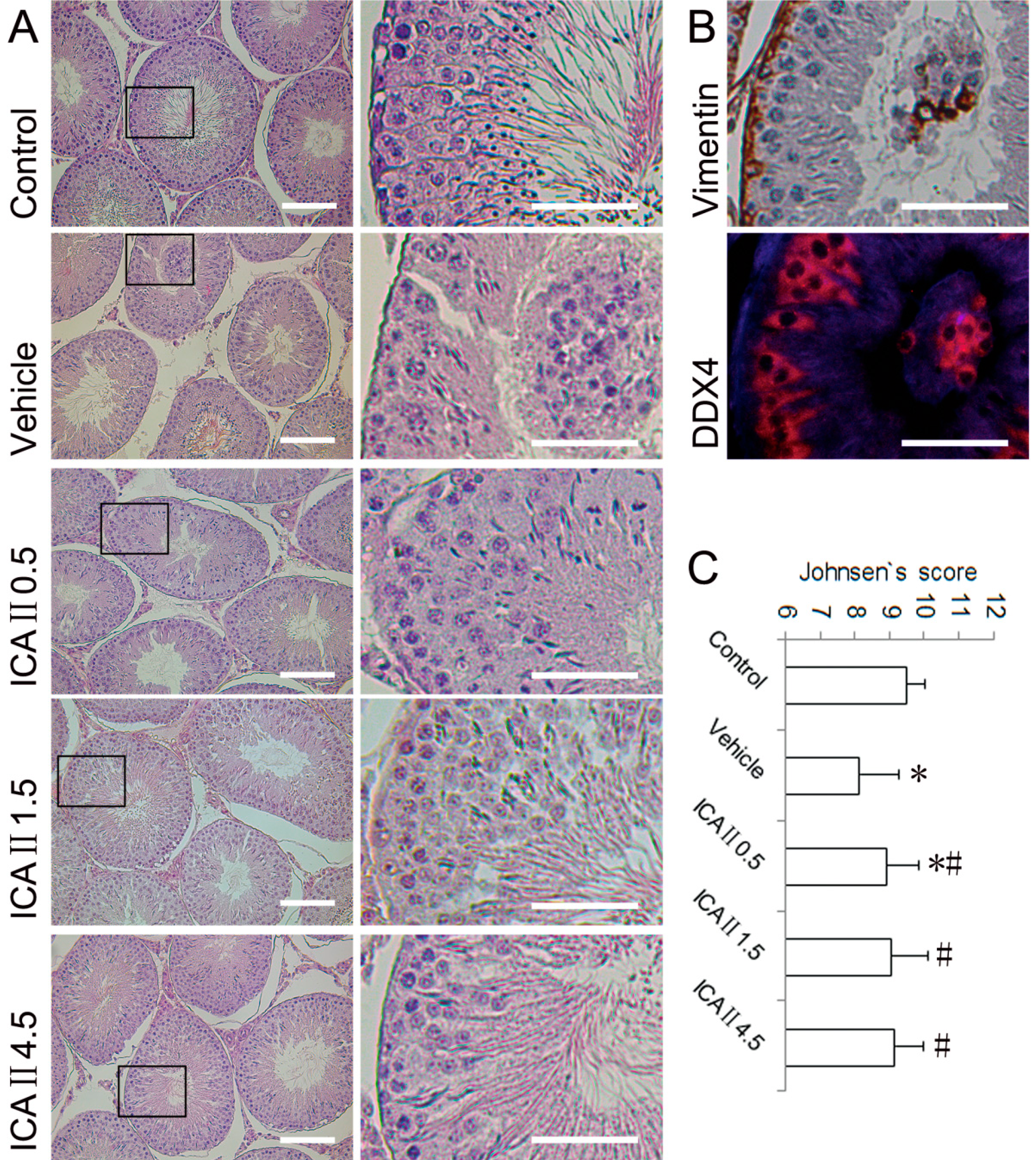
2.1.2. Histopathological Changes
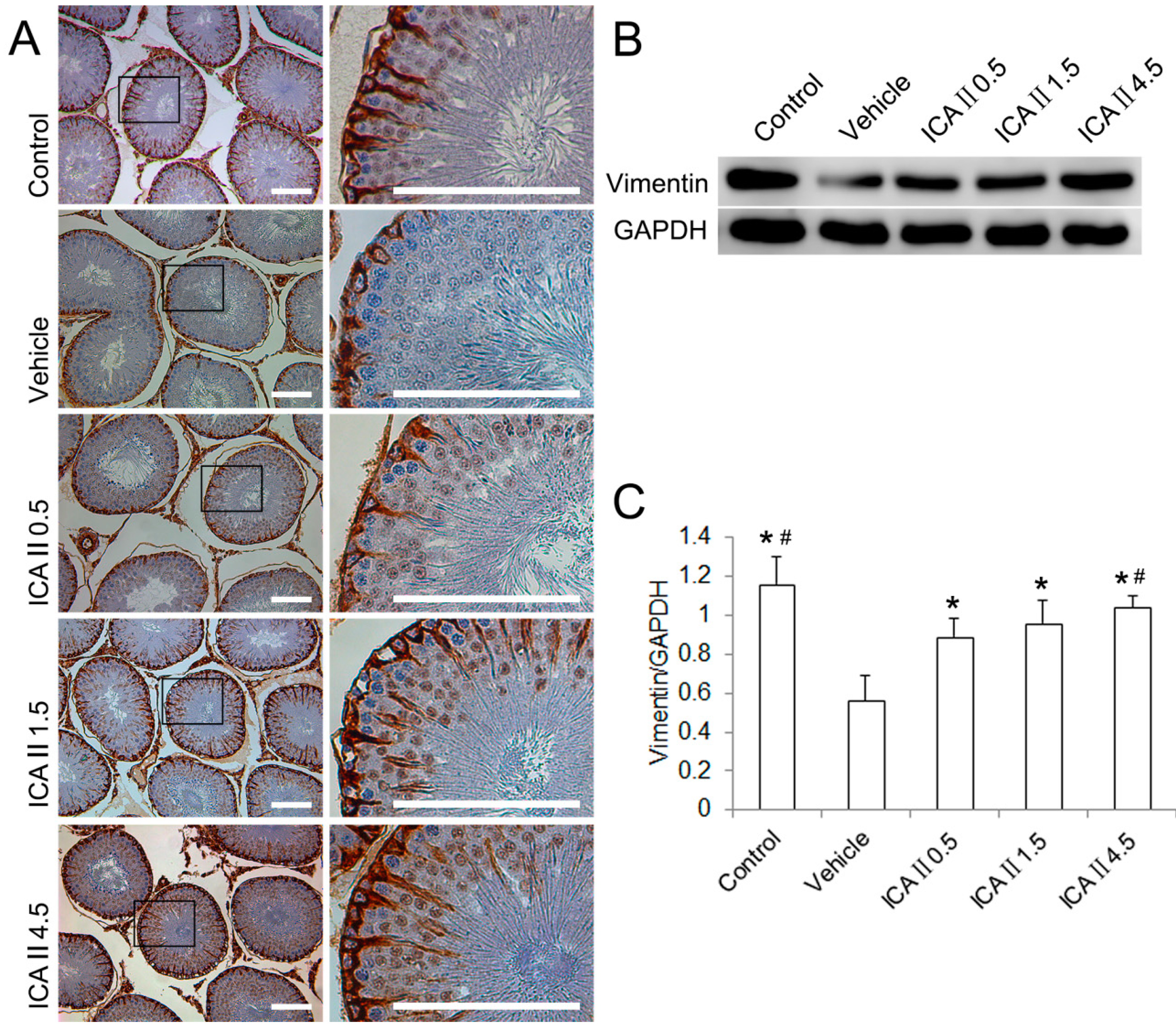
2.1.3. Disruption of Sertoli Cell Vimentin Filaments
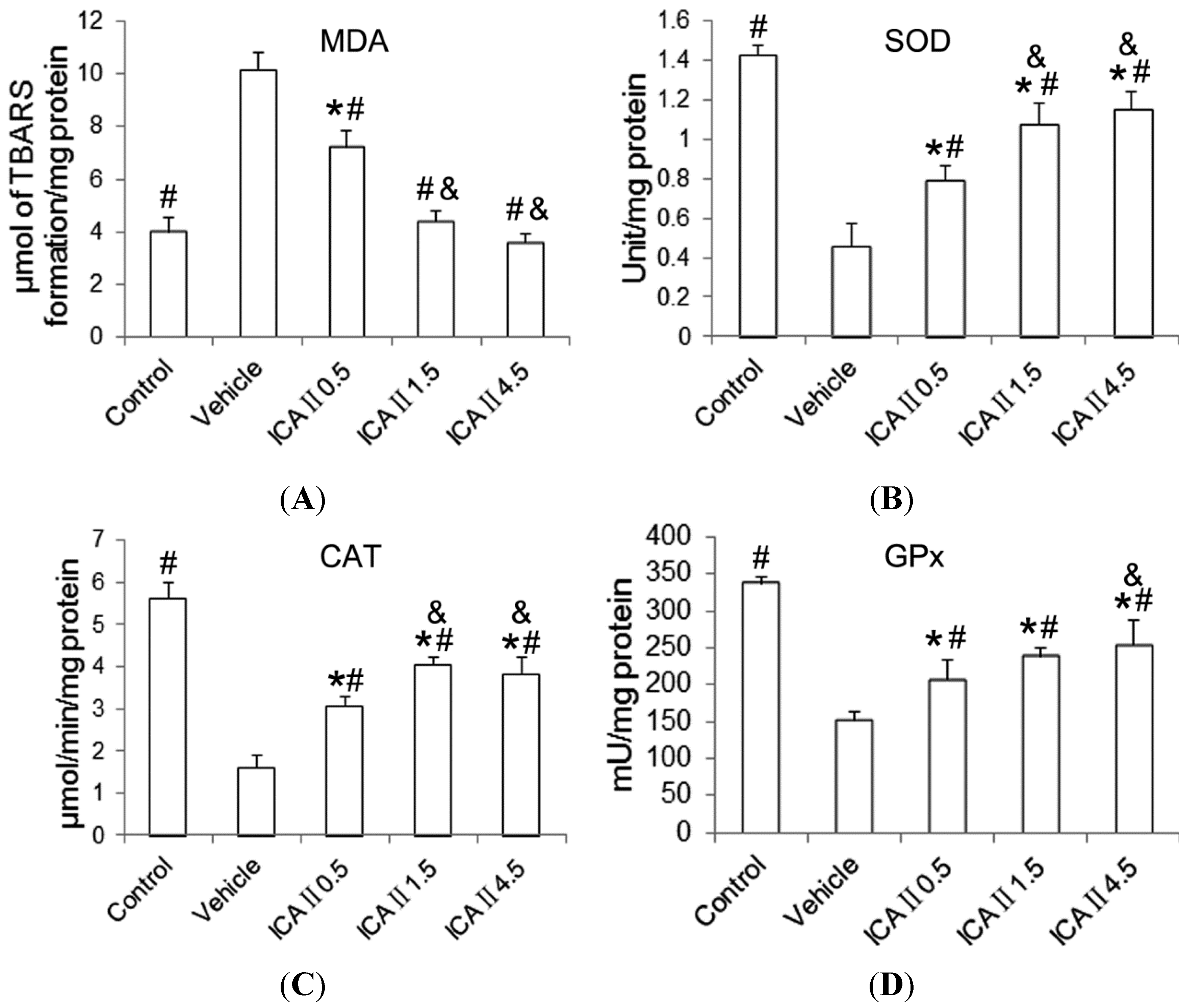
2.1.4. Lipid Peroxidation and Antioxidant Enzyme Activity
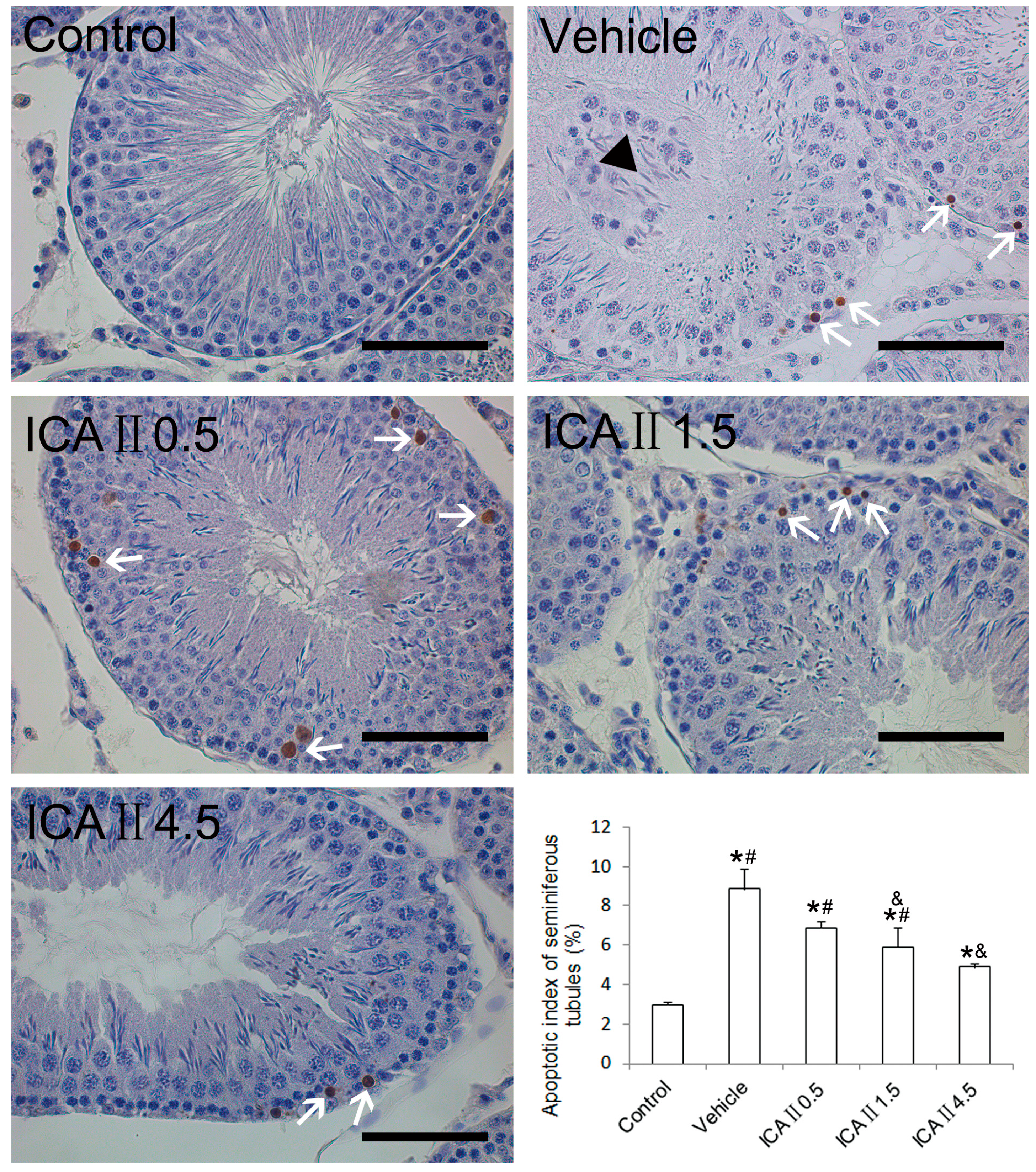
2.1.5. Analysis of Apoptotic Index (AI)
2.2. Discussion
3. Experimental Section
3.1. Animals
3.2. Plasma Glucose, Body and Reproductive Organ Weights
3.3. Evaluation of Epididymal Sperm Density and Epididymal Sperm Motility
3.4. Measurement of Lipid Peroxidation and Antioxidant Enzyme Assays
3.5. HE Staining, IHC and TUNEL
3.6. Western Blotting
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C. Diabetes, oxidative stress and therapeutic strategies. Biochim. Biophys. Acta 2014, 9, 2709–2729. [Google Scholar]
- Haidara, M.A.; Yassin, H.Z.; Rateb, M.; Ammar, H.; Zorkani, M.A. Role of oxidative stress in development of cardiovascular complications in diabetes mellitus. Curr. Vasc. Pharmacol. 2006, 4, 215–227. [Google Scholar] [CrossRef]
- Sandireddy, R.; Yerra, V.G.; Areti, A.; Komirishetty, P.; Kumar, A. Neuroinflammation and oxidative stress in diabetic neuropathy: Futuristic strategies based on These targets. Int. J. Endocrinol. 2014, 2014. [Google Scholar] [CrossRef]
- Bhattacharya, S.M.; Ghosh, M.; Nandi, N. Diabetes mellitus and abnormalities in semen analysis. J. Obstet. Gynaecol. Res. 2014, 40, 167–171. [Google Scholar] [CrossRef]
- Alves, M.G.; Martins, A.D.; Rato, L.; Moreira, P.I.; Socorro, S.; Oliveira, P.F. Molecular mechanisms beyond glucose transport in diabetes-related male infertility. Biochim. Biophys. Acta 2013, 1832, 626–635. [Google Scholar]
- Shrilatha, B. Muralidhara Early oxidative stress in testis and epididymal sperm in streptozotocin-induced diabetic mice: Its progression and genotoxic consequences. Reprod. Toxicol. 2007, 23, 578–587. [Google Scholar] [CrossRef]
- Khaki, A.; Fathiazad, F.; Nouri, M.; Khaki, A.; Maleki, N.A.; Khamnei, H.J.; Ahmadi, P. Beneficial effects of quercetin on sperm parameters in streptozotocin-induced diabetic male rats. Phytother. Res. 2010, 24, 1285–1291. [Google Scholar] [CrossRef]
- Wu, J.; Xu, H.; Wong, P.F.; Xia, S.; Xu, J.; Dong, J. Icaritin attenuates cigarette smoke-mediated oxidative stress in human lung epithelial cells via activation of PI3K-AKT and Nrf2 signaling. Food Chem. Toxicol. 2014, 64, 307–313. [Google Scholar] [CrossRef]
- Zheng, M.; Qu, L.; Lou, Y. Effects of icariin combined with Panax notoginseng saponins on ischemia reperfusion-induced cognitive impairments related with oxidative stress and CA1 of hippocampal neurons in rat. Phytother. Res. 2008, 22, 597–604. [Google Scholar] [CrossRef]
- Liu, T.; Xin, H.; Li, W.R.; Zhou, F.; Li, G.Y.; Gong, Y.Q.; Gao, Z.Z.; Qin, X.C.; Cui, W.S.; Shindel, A.W.; et al. Effects of icariin on improving erectile function in streptozotocin-induced diabetic rats. J. Sex. Med. 2011, 8, 2761–2772. [Google Scholar] [CrossRef]
- Wong, S.P.; Shen, P.; Lee, L.; Li, J.; Yong, E.L. Pharmacokinetics of prenylflavonoids and correlations with the dynamics of estrogen action in sera following ingestion of a standardized Epimedium extract. J. Pharm. Biomed. Anal. 2009, 50, 216–223. [Google Scholar] [CrossRef]
- Cai, W.J.; Huang, J.H.; Zhang, S.Q.; Wu, B.; Kapahi, P.; Zhang, X.M.; Shen, Z.Y. Icariin and its derivative icariside II extend healthspan via insulin/IGF-1 pathway in C. elegans. PLoS One 2011, 6, e28835. [Google Scholar] [CrossRef]
- Zhou, F.; Xin, H.; Liu, T.; Li, G.Y.; Gao, Z.Z.; Liu, J.; Li, W.R.; Cui, W.S.; Bai, G.Y.; Park, N.C.; et al. Effects of icariside II on improving erectile function in rats with streptozotocin-induced diabetes. J. Androl. 2012, 33, 832–844. [Google Scholar] [CrossRef]
- De Young, L.; Yu, D.; Bateman, R.M.; Brock, G.B. Oxidative stress and antioxidant therapy: Their impact in diabetes-associated erectile dysfunction. J. Androl. 2004, 25, 830–836. [Google Scholar]
- Matough, F.A.; Budin, S.B.; Hamid, Z.A.; Alwahaibi, N.; Mohamed, J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ. Med. J. 2012, 12, 5–18. [Google Scholar] [CrossRef]
- Ghlissi, Z.; Atheymen, R.; Boujbiha, M.A.; Sahnoun, Z.; Makni Ayedi, F.; Zeghal, K.; El Feki, A.; Hakim, A. Antioxidant and androgenic effects of dietary ginger on reproductive function of male diabetic rats. Int. J. Food Sci. Nutr. 2013, 64, 974–978. [Google Scholar] [CrossRef]
- Kyathanahalli, C.N.; Manjunath, M.J. Oral supplementation of standardized extract of Withania somnifera protects against diabetes-induced testicular oxidative impairments in prepubertal rats. Protoplasma 2014, 251, 1021–1029. [Google Scholar] [CrossRef]
- Morgan, N.G.; Cable, H.C.; Newcombe, N.R.; Williams, G.T. Treatment of cultured pancreatic B-cells with streptozotocin induces cell death by apoptosis. Biosci. Rep. 1994, 14, 243–250. [Google Scholar] [CrossRef]
- Bedoya, F.J.; Solano, F.; Lucas, M. N-monomethyl-arginine and nicotinamide prevent streptozotocin-induced double strand DNA break formation in pancreatic rat islets. Experientia 1996, 52, 344–347. [Google Scholar] [CrossRef]
- Tindall, D.J.; Rowley, D.R.; Murthy, L.; Lipshultz, L.I.; Chang, C.H. Structure and biochemistry of the Sertoli cell. Int. Rev. Cytol. 1985, 94, 127–149. [Google Scholar] [CrossRef]
- Alves, M.G.; Martins, A.D.; Cavaco, J.E.; Socorro, S.; Oliveira, P.F. Diabetes, insulin-mediated glucose metabolism and Sertoli/blood-testis barrier function. Tissue Barriers 2013, 1, e23992. [Google Scholar] [CrossRef]
- Erkekoglu, P.; Zeybek, N.D.; Giray, B.; Asan, E.; Hincal, F. The effects of di(2-ethylhexyl)phthalate exposure and selenium nutrition on Sertoli cell vimentin structure and germ-cell apoptosis in rat testis. Arch. Environ. Contam. Toxicol. 2012, 62, 539–547. [Google Scholar] [CrossRef]
- Tay, T.W.; Andriana, B.B.; Ishii, M.; Tsunekawa, N.; Kanai, Y.; Kurohmaru, M. Disappearance of vimentin in Sertoli cells: A mono(2-ethylhexyl) phthalate effect. Int. J. Toxicol. 2007, 26, 289–295. [Google Scholar] [CrossRef]
- Xu, Y.; Lei, H.; Guan, R.; Gao, Z.; Li, H.; Wang, L.; Song, W.; Gao, B.; Xin, Z. Studies on the mechanism of testicular dysfunction in the early stage of a streptozotocin induced diabetic rat model. Biochem. Biophys. Res. Commun. 2014, 1, 87–92. [Google Scholar]
- Johnsen, S.G. Testicular biopsy score count—A method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones 1970, 1, 2–25. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xu, Y.; Lei, H.; Guan, R.; Gao, Z.; Li, H.; Wang, L.; Hui, Y.; Zhou, F.; Xin, Z. Prophylactic Protective Effects and Its Potential Mechanisms of IcarisideII on Streptozotocin Induced Spermatogenic Dysfunction. Int. J. Mol. Sci. 2014, 15, 16100-16113. https://doi.org/10.3390/ijms150916100
Xu Y, Lei H, Guan R, Gao Z, Li H, Wang L, Hui Y, Zhou F, Xin Z. Prophylactic Protective Effects and Its Potential Mechanisms of IcarisideII on Streptozotocin Induced Spermatogenic Dysfunction. International Journal of Molecular Sciences. 2014; 15(9):16100-16113. https://doi.org/10.3390/ijms150916100
Chicago/Turabian StyleXu, Yongde, Hongen Lei, Ruili Guan, Zhezhu Gao, Huixi Li, Lin Wang, Yu Hui, Feng Zhou, and Zhongcheng Xin. 2014. "Prophylactic Protective Effects and Its Potential Mechanisms of IcarisideII on Streptozotocin Induced Spermatogenic Dysfunction" International Journal of Molecular Sciences 15, no. 9: 16100-16113. https://doi.org/10.3390/ijms150916100
APA StyleXu, Y., Lei, H., Guan, R., Gao, Z., Li, H., Wang, L., Hui, Y., Zhou, F., & Xin, Z. (2014). Prophylactic Protective Effects and Its Potential Mechanisms of IcarisideII on Streptozotocin Induced Spermatogenic Dysfunction. International Journal of Molecular Sciences, 15(9), 16100-16113. https://doi.org/10.3390/ijms150916100




