Leptin Induces Oncostatin M Production in Osteoblasts by Downregulating miR-93 through the Akt Signaling Pathway
Abstract
:1. Introduction
2. Results
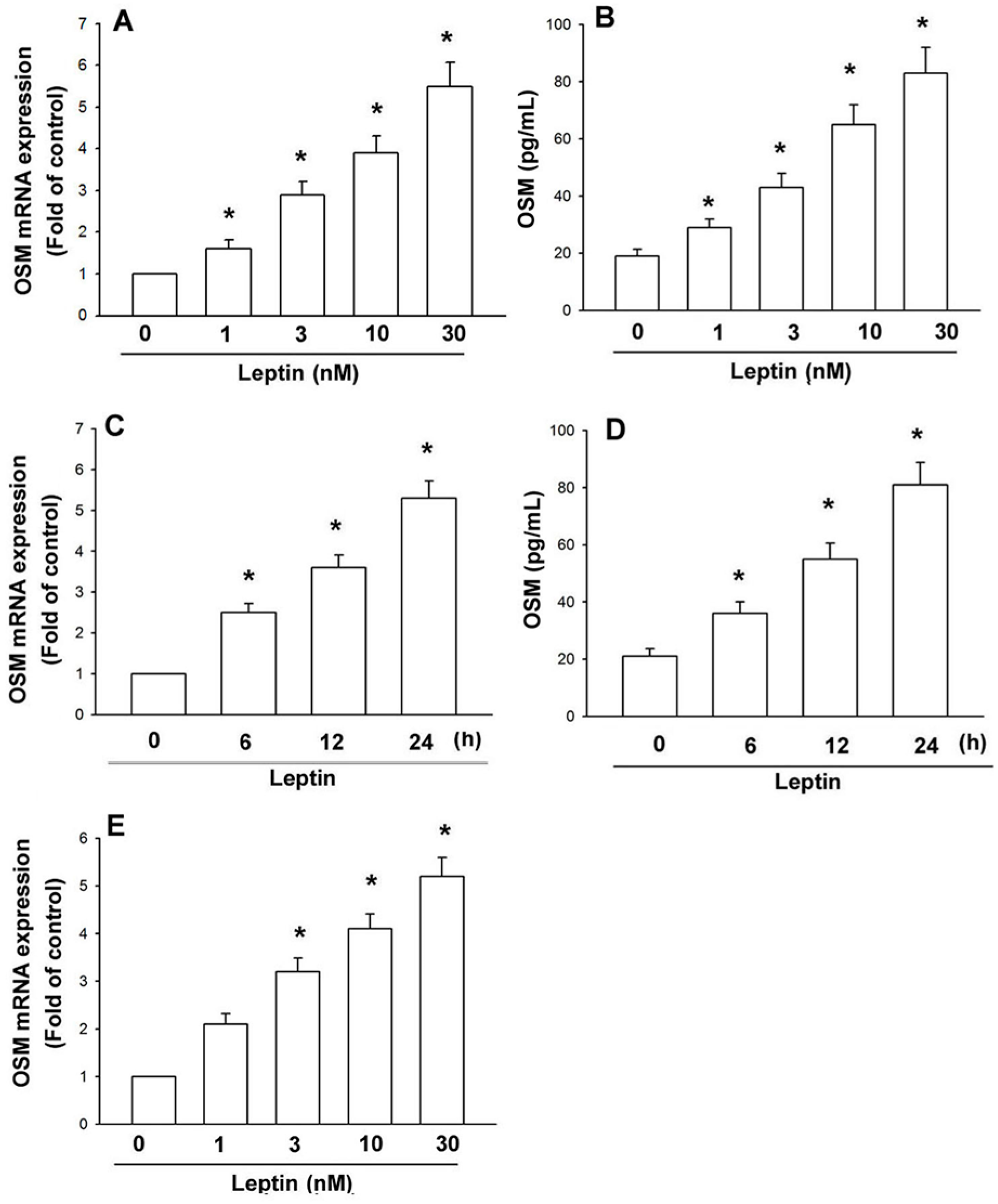
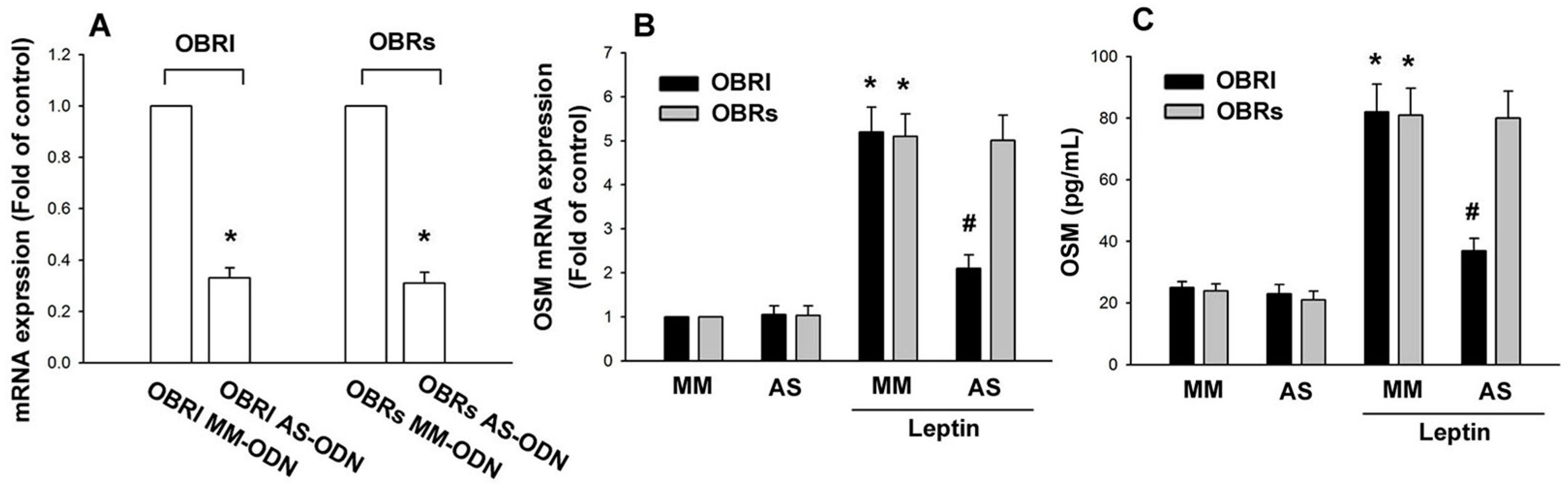
2.1. Leptin Induces OSM Expression in Human Osteoblasts through the OBRl Receptor
2.2. Leptin Increases OSM Production in Osteoblasts by Inhibition of miR-93 Expression
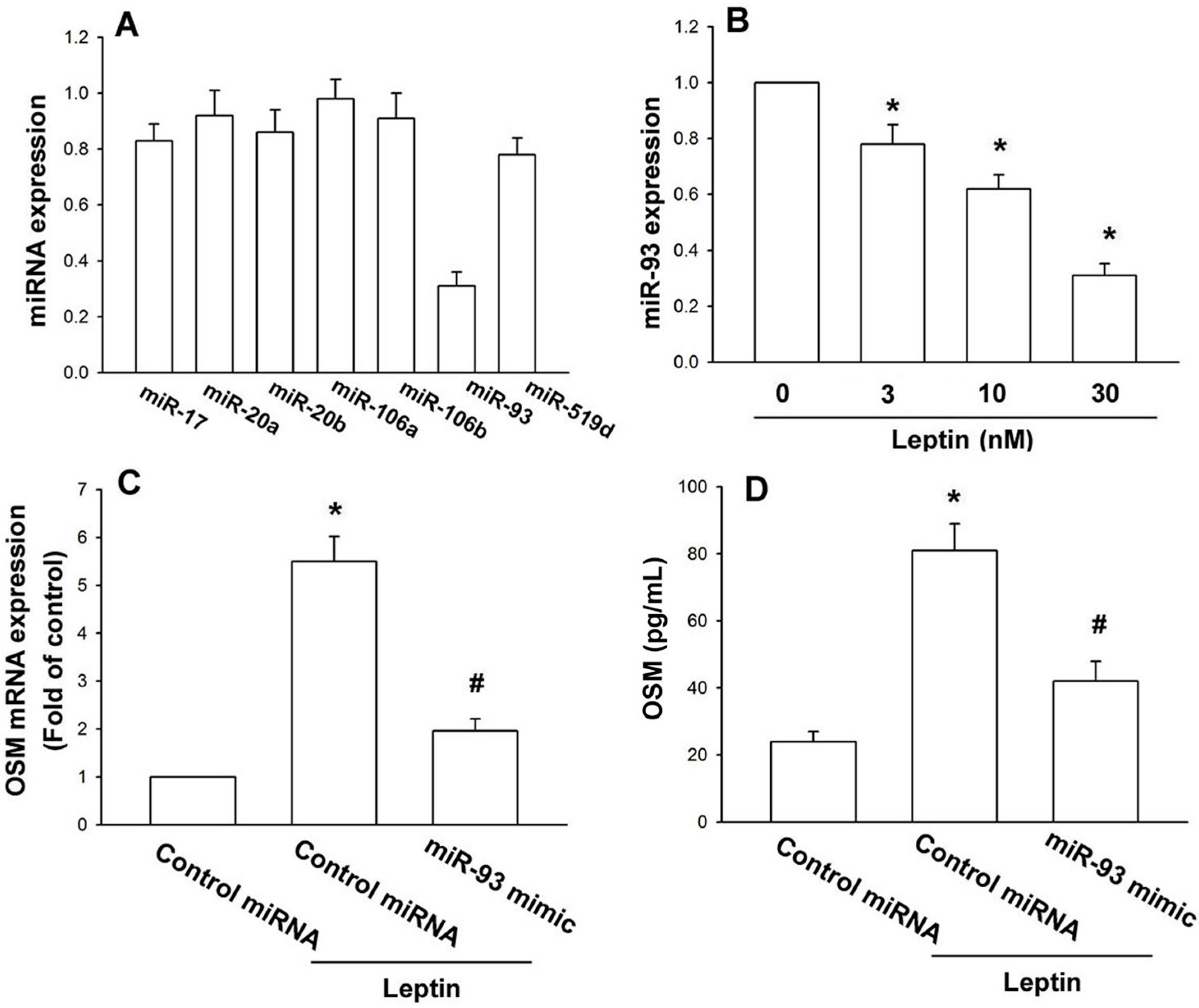
2.3. Leptin Increases OSM Expression through the Akt Signaling Pathway
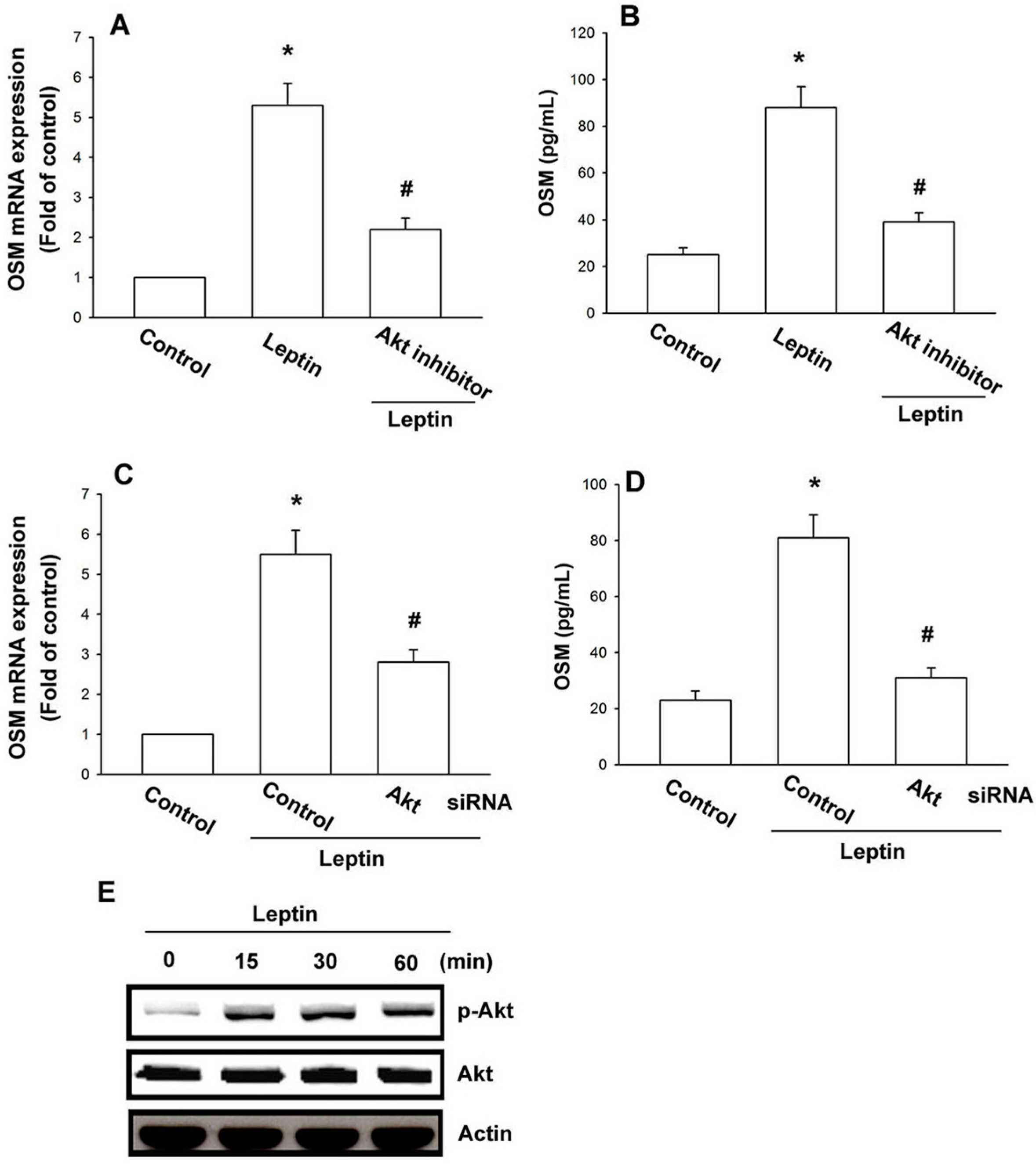
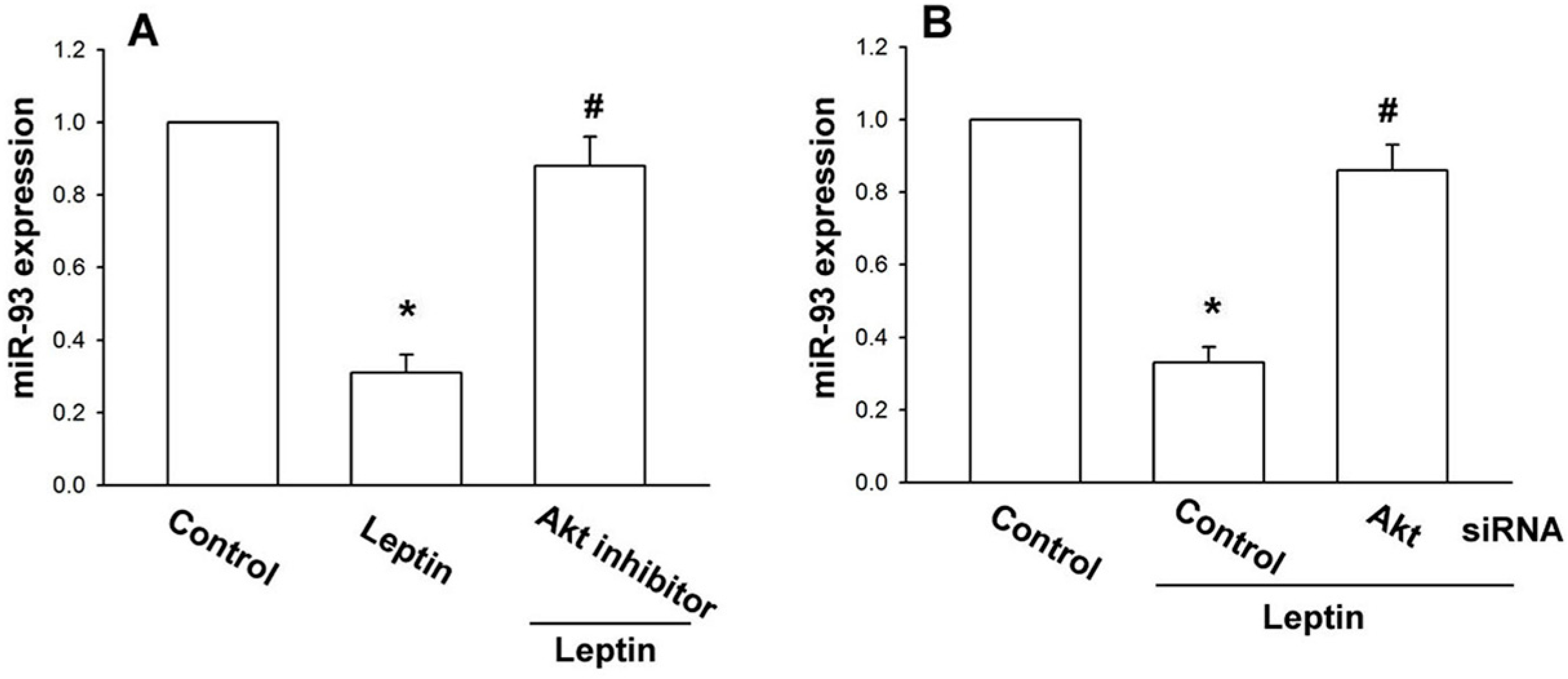
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Cell Culture
4.3. Measurement of OSM Production
4.4. Real-Time Quantitative PCR of mRNA and miRNA
4.5. Western Blot Analysis
4.6. Synthesis of OBRl and OBRs Decoy Oligonucleotide
4.7. Statistical Analysis
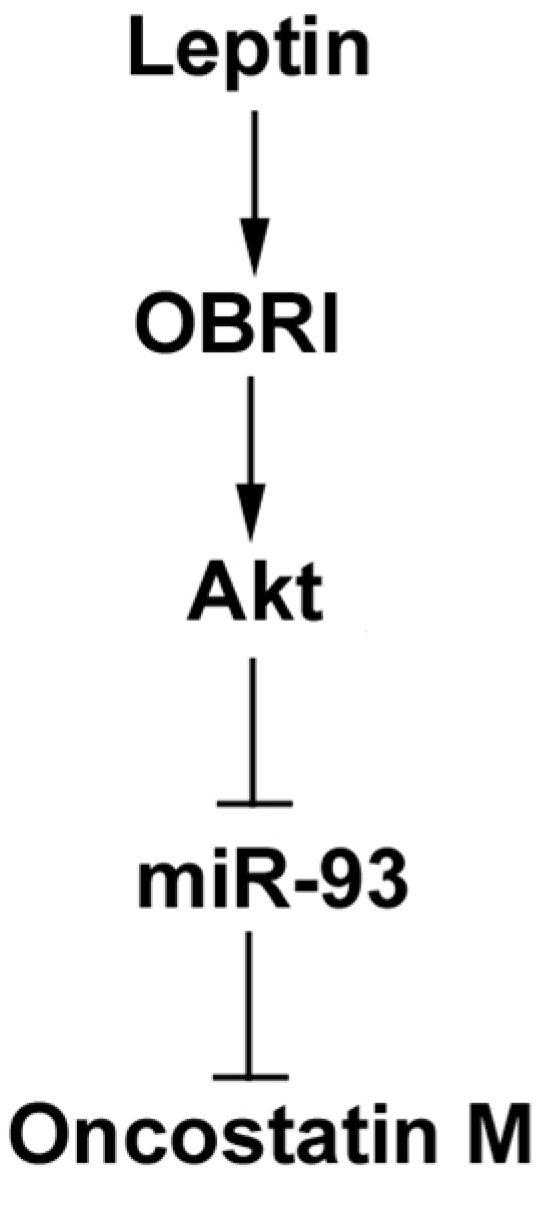
5. Conclusions
Acknowledgments
Authors Contribution
Conflicts of Interest
References
- Bresnihan, B. Pathogenesis of joint damage in rheumatoid arthritis. J. Rheumatol. 1999, 26, 717–719. [Google Scholar]
- Grossman, J.M.; Brahn, E. Rheumatoid arthritis: Current clinical and research directions. J. Women’s Health 1997, 6, 627–638. [Google Scholar] [CrossRef]
- Altman, R.; Asch, E.; Bloch, D.; Bole, G.; Borenstein, D.; Brandt, K.; Christy, W.; Cooke, T.D.; Greenwald, R.; Hochberg, M.; et al. Development of criteria for the classification and reporting of osteoarthritis.Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheumatol. 1986, 29, 1039–1049. [Google Scholar] [CrossRef]
- Bai, T.; Chen, C.C.; Lau, L.F. Matricellular protein CCN1 activates a proinflammatory genetic program in murine macrophages. J. Immunol. 2010, 184, 3223–3232. [Google Scholar] [CrossRef]
- Barksby, H.E.; Hui, W.; Wappler, I.; Peters, H.H.; Milner, J.M.; Richards, C.D.; Cawston, T.E.; Rowan, A.D. Interleukin-1 in combination with oncostatin M up-regulates multiple genes in chondrocytes: Implications for cartilage destruction and repair. Arthritis Rheumatol. 2006, 54, 540–550. [Google Scholar] [CrossRef]
- Lisignoli, G.; Toneguzzi, S.; Pozzi, C.; Piacentini, A.; Riccio, M.; Ferruzzi, A.; Gualtieri, G.; Facchini, A. Proinflammatory cytokines and chemokine production and expression by human osteoblasts isolated from patients with rheumatoid arthritis and osteoarthritis. J. Rheumatol. 1999, 26, 791–799. [Google Scholar]
- Karmakar, S.; Kay, J.; Gravallese, E.M. Bone damage in rheumatoid arthritis: Mechanistic insights and approaches to prevention. Rheum. Dis. Clin. N. Am. 2010, 36, 385–404. [Google Scholar] [CrossRef]
- Mladenovic, Z.; Saurel, A.S.; Berenbaum, F.; Jacques, C. Potential role of hyaluronic acid on bone in osteoarthritis: Matrix metalloproteinases, aggrecanases, and RANKL expression are partially prevented by hyaluronic acid in interleukin 1beta-stimulated osteoblasts. J. Rheumatol. 2014, 41, 945–954. [Google Scholar] [CrossRef]
- Langdon, C.; Kerr, C.; Hassen, M.; Hara, T.; Arsenault, A.L.; Richards, C.D. Murine oncostatin M stimulates mouse synovial fibroblasts in vitro and induces inflammation and destruction in mouse joints in vivo. Am. J. Pathol. 2000, 157, 1187–1196. [Google Scholar] [CrossRef]
- Richards, C.D. The enigmatic cytokine oncostatin M and roles in disease. ISRN Inflamm 2013. [Google Scholar] [CrossRef]
- Houssiau, F.A.; Devogelaer, J.P.; van Damme, J.; de Deuxchaisnes, C.N.; van Snick, J. Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheumatol. 1988, 31, 784–788. [Google Scholar] [CrossRef]
- Jay, P.R.; Centrella, M.; Lorenzo, J.; Bruce, A.G.; Horowitz, M.C. Oncostatin-M: A new bone active cytokine that activates osteoblasts and inhibits bone resorption. Endocrinology 1996, 137, 1151–1158. [Google Scholar]
- Hui, W.; Bell, M.; Carroll, G. Detection of oncostatin M in synovial fluid from patients with rheumatoid arthritis. Ann. Rheum. Dis. 1997, 56, 184–187. [Google Scholar] [CrossRef]
- Kotake, S.; Sato, K.; Kim, K.J.; Takahashi, N.; Udagawa, N.; Nakamura, I.; Yamaguchi, A.; Kishimoto, T.; Suda, T.; Kashiwazaki, S. Interleukin-6 and soluble interleukin-6 receptors in the synovial fluids from rheumatoid arthritis patients are responsible for osteoclast-like cell formation. J. Bone Miner. Res. 1996, 11, 88–95. [Google Scholar]
- Manicourt, D.H.; Poilvache, P.; van Egeren, A.; Devogelaer, J.P.; Lenz, M.E.; Thonar, E.J. Synovial fluid levels of tumor necrosis factor alpha and oncostatin M correlate with levels of markers of the degradation of crosslinked collagen and cartilage aggrecan in rheumatoid arthritis but not in osteoarthritis. Arthritis Rheumatol. 2000, 43, 281–288. [Google Scholar] [CrossRef]
- Fearon, U.; Mullan, R.; Markham, T.; Connolly, M.; Sullivan, S.; Poole, A.R.; FitzGerald, O.; Bresnihan, B.; Veale, D.J. Oncostatin M induces angiogenesis and cartilage degradation in rheumatoid arthritis synovial tissue and human cartilage cocultures. Arthritis Rheumatol. 2006, 54, 3152–3162. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Elmquist, J.K.; Maratos-Flier, E.; Saper, C.B.; Flier, J.S. Unraveling the central nervous system pathways underlying responses to leptin. Nat. Neurosci. 1998, 1, 445–450. [Google Scholar] [CrossRef]
- Ahima, R.S.; Prabakaran, D.; Mantzoros, C.; Qu, D.; Lowell, B.; Maratos-Flier, E.; Flier, J.S. Role of leptin in the neuroendocrine response to fasting. Nature 1996, 382, 250–252. [Google Scholar] [CrossRef]
- Tartaglia, L.A.; Dembski, M.; Weng, X.; Deng, N.; Culpepper, J.; Devos, R.; Richards, G.J.; Campfield, L.A.; Clark, F.T.; Deeds, J.; et al. Identification and expression cloning of a leptin receptor, OB-R. Cell 1995, 83, 1263–1271. [Google Scholar] [CrossRef]
- Zarkesh-Esfahani, H.; Pockley, G.; Metcalfe, R.A.; Bidlingmaier, M.; Wu, Z.; Ajami, A.; Weetman, A.P.; Strasburger, C.J.; Ross, R.J. High-dose leptin activates human leukocytes via receptor expression on monocytes. J. Immunol. 2001, 167, 4593–4599. [Google Scholar] [CrossRef]
- Sweeney, G. Leptin signalling. Cell Signal 2002, 14, 655–663. [Google Scholar] [CrossRef]
- Zhang, Q.; Jeang, K.-T. Long noncoding RNAs and viral infections. BioMedicine 2013, 3, 34–42. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, H.; Yang, X.; Wang, L.; Su, L.; Liu, K.; Gu, Q.; Xu, X. MicroRNA-93 inhibits inflammatory cytokine production in LPS-stimulated murine macrophages by targeting IRAK4. FEBS Lett. 2014, 588, 1692–1698. [Google Scholar] [CrossRef]
- Cartmell, S.H.; Rupani, A.; Balint, R. Osteoblasts and their applications in bone tissue engineering. Cell Health Cytoskelet. 2012, 4, 49–61. [Google Scholar]
- Lisignoli, G.; Piacentini, A.; Toneguzzi, S.; Grassi, F.; Cocchini, B.; Ferruzzi, A.; Gualtieri, G.; Facchini, A. Osteoblasts and stromal cells isolated from femora in rheumatoid arthritis (RA) and osteoarthritis (OA) patients express IL-11, leukaemia inhibitory factor and oncostatin M. Clin. Exp. Immunol. 2000, 119, 346–353. [Google Scholar] [CrossRef]
- Langdon, C.; Leith, J.; Smith, F.; Richards, C.D. Oncostatin M stimulates monocyte chemoattractant protein-1- and interleukin-1-induced matrix metalloproteinase-1 production by human synovial fibroblasts in vitro. Arthritis Rheumatol. 1997, 40, 2139–2146. [Google Scholar] [CrossRef]
- Presle, N.; Pottie, P.; Dumond, H.; Guillaume, C.; Lapicque, F.; Pallu, S.; Mainard, D.; Netter, P.; Terlain, B. Differential distribution of adipokines between serum and synovial fluid in patients with osteoarthritis.Contribution of joint tissues to their articular production. Osteoarthr. Cartil. 2006, 14, 690–695. [Google Scholar] [CrossRef]
- Del Rey, M.J.; Izquierdo, E.; Usategui, A.; Gonzalo, E.; Blanco, F.J.; Acquadro, F.; Pablos, J.L. The transcriptional response of normal and rheumatoid arthritis synovial fibroblasts to hypoxia. Arthritis Rheumatol. 2010, 62, 3584–3594. [Google Scholar] [CrossRef]
- Simopoulou, T.; Malizos, K.N.; Iliopoulos, D.; Stefanou, N.; Papatheodorou, L.; Ioannou, M.; Tsezou, A. Differential expression of leptin and leptin’s receptor isoform (Ob-Rb) mRNA between advanced and minimally affected osteoarthritic cartilage; effect on cartilage metabolism. Osteoarthr. Cartil. 2007, 15, 872–883. [Google Scholar] [CrossRef]
- He, X.; Jing, Z.; Cheng, G. MicroRNAs: New regulators of Toll-like receptor signalling pathways. BioMed. Res. Int. 2014. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, Y.; Wang, N. New paradigms in inflammatory signaling in vascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H317–H325. [Google Scholar] [CrossRef]
- Metlakunta, A.S.; Sahu, M.; Yasukawa, H.; Dhillon, S.S.; Belsham, D.D.; Yoshimura, A.; Sahu, A. Neuronal suppressor of cytokine signaling-3 deficiency enhances hypothalamic leptin-dependent phosphatidylinositol 3-kinase signaling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R1185–R1193. [Google Scholar] [CrossRef]
- Russo, V.C.; Metaxas, S.; Kobayashi, K.; Harris, M.; Werther, G.A. Antiapoptotic effects of leptin in human neuroblastoma cells. Endocrinology 2004, 145, 4103–4112. [Google Scholar] [CrossRef]
- Niswender, K.D.; Morton, G.J.; Stearns, W.H.; Rhodes, C.J.; Myers, M.G., Jr.; Schwartz, M.W. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature 2001, 413, 794–795. [Google Scholar]
- Bamber, B.; Reife, R.A.; Haugen, H.S.; Clegg, C.H. Oncostatin M stimulates excessive extracellular matrix accumulation in a transgenic mouse model of connective tissue disease. J. Mol. Med. 1998, 76, 61–69. [Google Scholar] [CrossRef]
- Fantuzzi, G.; Faggioni, R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J. Leukoc. Biol. 2000, 68, 437–446. [Google Scholar]
- Gkretsi, V.; Simopoulou, T.; Tsezou, A. Lipid metabolism and osteoarthritis: lessons from atherosclerosis. Prog. Lipid. Res. 2011, 50, 133–140. [Google Scholar] [CrossRef]
- Chen, P.C.; Cheng, H.C.; Wang, J.; Wang, S.W.; Tai, H.C.; Lin, C.W.; Tang, C.H. Prostate cancer-derived CCN3 induces M2 macrophage infiltration and contributes to angiogenesis in prostate cancer microenvironment. Oncotarget 2014, 5, 1595–1608. [Google Scholar]
- Yu, H.S.; Wang, S.W.; Chang, A.C.; Tai, H.C.; Yeh, H.I.; Lin, Y.M.; Tang, C.H. Bradykinin promotes vascular endothelial growth factor expression and increases angiogenesis in human prostate cancer cells. Biochem. Pharmacol. 2014, 87, 243–253. [Google Scholar] [CrossRef]
- Tu, H.J.; Lin, T.H.; Chiu, Y.C.; Tang, C.H.; Yang, R.S.; Fu, W.M. Enhancement of placenta growth factor expression by oncostatin M in human rheumatoid arthritis synovial fibroblasts. J. Cell Physiol. 2013, 228, 983–990. [Google Scholar] [CrossRef]
- Nazarians-Armavil, A.; Menchella, J.A.; Belsham, D.D. Cellular insulin resistance disrupts leptin-mediated control of neuronal signaling and transcription. Mol. Endocrinol. 2013, 27, 990–1003. [Google Scholar] [CrossRef]
- Olofsson, L.E.; Unger, E.K.; Cheung, C.C.; Xu, A.W. Modulation of AgRP-neuronal function by SOCS3 as an initiating event in diet-induced hypothalamic leptin resistance. Proc. Natl. Acad. Sci. USA 2013, 110, E697–E706. [Google Scholar] [CrossRef]
- Yu, H.S.; Lin, T.H.; Tang, C.H. Bradykinin enhances cell migration in human prostate cancer cells through B2 receptor/PKCdelta/c-Src dependent signaling pathway. Prostate 2013, 73, 89–100. [Google Scholar]
- Ozanne, B.W.; Spence, H.J.; McGarry, L.C.; Hennigan, R.F. Transcription factors control invasion: AP-1 the first among equals. Oncogene 2007, 26, 1–10. [Google Scholar] [CrossRef]
- Tzeng, H.E.; Tsai, C.H.; Chang, Z.L.; Su, C.M.; Wang, S.W.; Hwang, W.L.; Tang, C.H. Interleukin-6 induces vascular endothelial growth factor expression and promotes angiogenesis through apoptosis signal-regulating kinase 1 in human osteosarcoma. Biochem. Pharmacol. 2013, 85, 531–540. [Google Scholar] [CrossRef]
- Lerner, I.; Baraz, L.; Pikarsky, E.; Meirovitz, A.; Edovitsky, E.; Peretz, T.; Vlodavsky, I.; Elkin, M. Function of heparanase in prostate tumorigenesis: potential for therapy. Clin. Cancer Res. 2008, 14, 668–676. [Google Scholar] [CrossRef]
- Tang, C.H.; Hsu, C.J.; Fong, Y.C. The CCL5/CCR5 axis promotes interleukin-6 production in human synovial fibroblasts. Arthritis Rheumatol. 2010, 62, 3615–3624. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chen, S.Y.; Tsai, H.C.; Hsu, H.C.; Tang, C.H. Thrombin induces epidermal growth factor receptor transactivation and CCL2 expression in human osteoblasts. Arthritis Rheumatol. 2012, 64, 3344–3354. [Google Scholar] [CrossRef]
- Samuels, J.; Krasnokutsky, S.; Abramson, S.B. Osteoarthritis: A tale of three tissues. Bull. NYU Hosp. Jt. Dis. 2008, 66, 244–250. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yang, W.-H.; Tsai, C.-H.; Fong, Y.-C.; Huang, Y.-L.; Wang, S.-J.; Chang, Y.-S.; Tang, C.-H. Leptin Induces Oncostatin M Production in Osteoblasts by Downregulating miR-93 through the Akt Signaling Pathway. Int. J. Mol. Sci. 2014, 15, 15778-15790. https://doi.org/10.3390/ijms150915778
Yang W-H, Tsai C-H, Fong Y-C, Huang Y-L, Wang S-J, Chang Y-S, Tang C-H. Leptin Induces Oncostatin M Production in Osteoblasts by Downregulating miR-93 through the Akt Signaling Pathway. International Journal of Molecular Sciences. 2014; 15(9):15778-15790. https://doi.org/10.3390/ijms150915778
Chicago/Turabian StyleYang, Wei-Hung, Chun-Hao Tsai, Yi-Chin Fong, Yuan-Li Huang, Shoou-Jyi Wang, Yung-Sen Chang, and Chih-Hsin Tang. 2014. "Leptin Induces Oncostatin M Production in Osteoblasts by Downregulating miR-93 through the Akt Signaling Pathway" International Journal of Molecular Sciences 15, no. 9: 15778-15790. https://doi.org/10.3390/ijms150915778
APA StyleYang, W.-H., Tsai, C.-H., Fong, Y.-C., Huang, Y.-L., Wang, S.-J., Chang, Y.-S., & Tang, C.-H. (2014). Leptin Induces Oncostatin M Production in Osteoblasts by Downregulating miR-93 through the Akt Signaling Pathway. International Journal of Molecular Sciences, 15(9), 15778-15790. https://doi.org/10.3390/ijms150915778




