Rumphellols A and B, New Caryophyllene Sesquiterpenoids from a Formosan Gorgonian Coral, Rumphella antipathies
Abstract
:1. Introduction
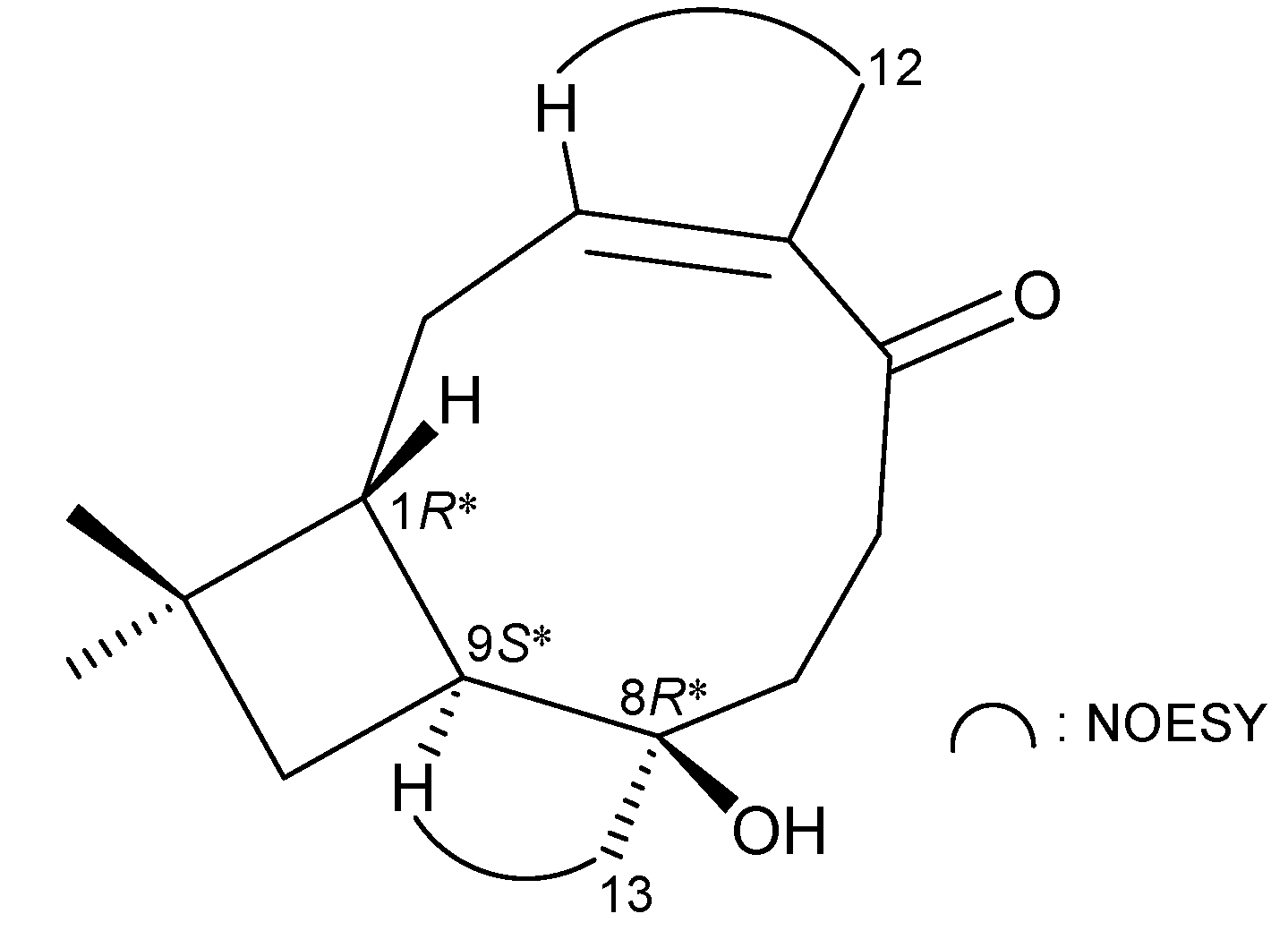
2. Results and Discussion
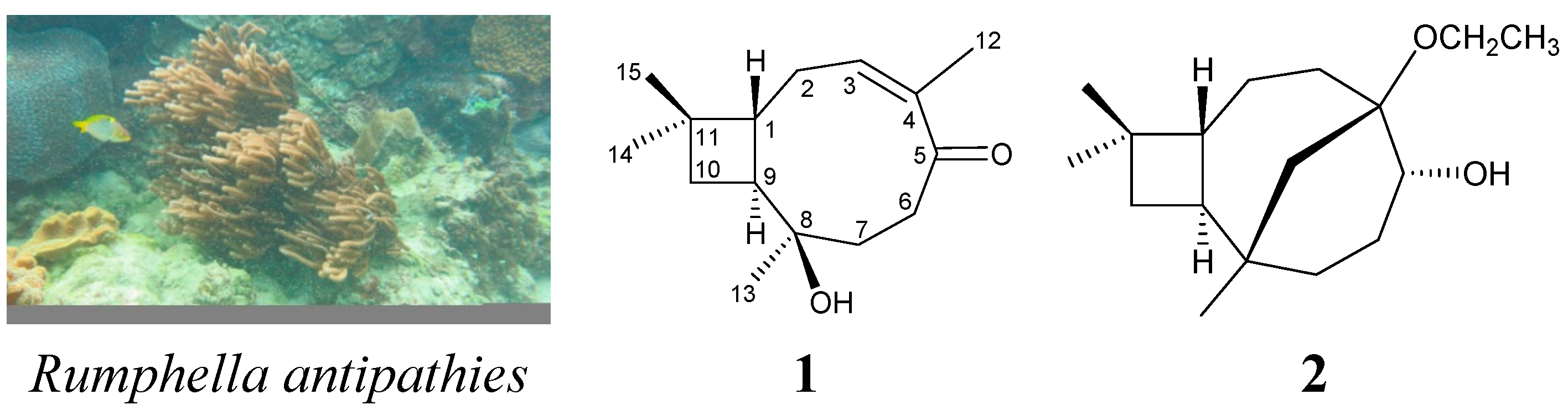
| C/H | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC (H→C) |
|---|---|---|---|---|
| 1 | 1.83 m | 44.4, CH | H2-2, H-9 | C-8, -9, -11 |
| 2 | 1.84 m | 27.6, CH2 | H-1, H-3 | C-1, -3, -4, -9 |
| 2.20 m | ||||
| 3 | 5.64 dd (8.4, 8.4) | 128.8, CH | H2-2 | C-2, -5, -12 |
| 4 | 138.4, C | |||
| 5 | 212.7, C | |||
| 6 | 2.19 ddd (16.0, 12.0, 1.6) | 38.0, CH2 | H2-7 | C-4, -5, -7, -8 |
| 2.53 ddd (16.0, 8.8, 2.0) | ||||
| 7 | 1.88 ddd (12.0, 8.8, 1.6) | 37.7, CH2 | H2-6 | C-5, -6, -8, -9, -13 |
| 2.03 ddd (12.0, 12.0, 2.0) | ||||
| 8 | 72.6, C | |||
| 9 | 1.95 ddd (9.2, 9.2, 9.2) | 44.9, CH | H-1, H2-10 | C-1, -2, -10 |
| 10 | 1.63 dd (10.8, 9.2) | 33.6, CH2 | H-9 | C-1, -8, -9, -11, -14, -15 |
| 1.56 dd (10.8, 9.2) | ||||
| 11 | 32.8, C | |||
| 12 | 1.82 s | 19.8, CH3 | C-3, -4, -5 | |
| 13 | 1.02 s | 25.3, CH3 | C-7, -8, -9 | |
| 14 | 0.96 s | 29.6, CH3 | C-1, -10, -11, -15 | |
| 15 | 0.96 s | 23.2, CH3 | C-1, -10, -11, -14 |
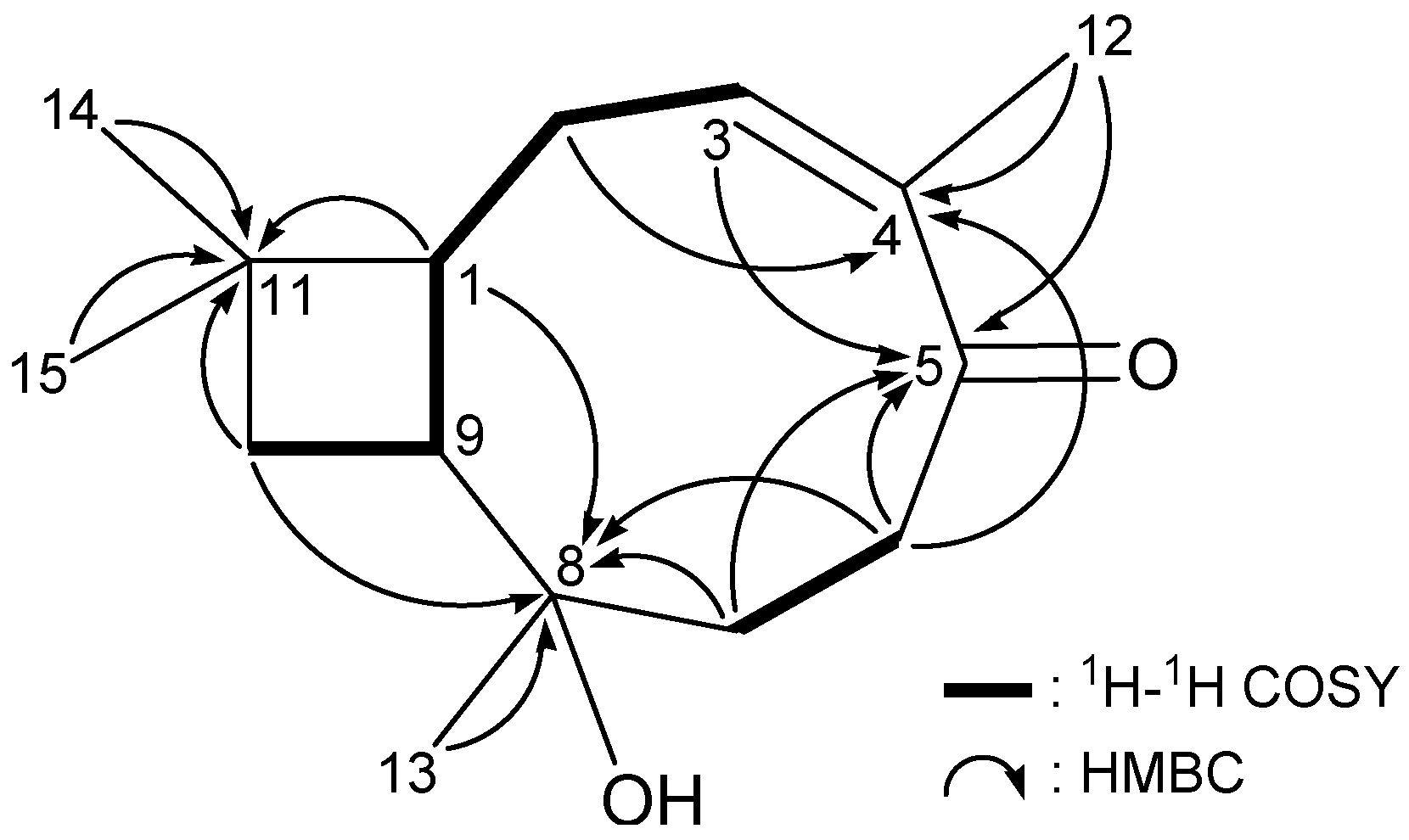
| C/H | δH (J in Hz) | δCb | 1H–1H COSY | HMBC (H→C) |
|---|---|---|---|---|
| 1 | 1.73 m | 44.0, CH | H2-2, H-9 | C-2, -8, -9, -10, -11, -14, -15 |
| 2 | 1.69 m | 21.7, CH2 | H-1, H2-3 | C-1, -3, -4, -11 |
| 1.53 m | ||||
| 3 | 1.91 m | 29.3, CH2 | H2-2 | C-1, -2, -4, -5 |
| 1.56 m | ||||
| 4 | 80.2, C | |||
| 5 | 3.57 dd (11.2, 6.0) | 76.8, CH | H2-6 | C-3, -4, -6 |
| 6 | 1.61–1.80 m | 27.1, CH2 | H-5, H2-7 | C-4, -5, -7, -8 |
| 7 | 1.22 ddd (13.2, 13.2, 5.2) | 36.6, CH2 | H2-6 | C-5, -6, -8, -9, -12 |
| 1.38 dddd (13.2, 4.4, 2.8, 2.8) | ||||
| 8 | 32.8, C | |||
| 9 | 2.08 ddd (11.6, 10.0, 8.0) | 36.5, CH | H-1, H2-10 | C-1, -2, -7, -8, -11, -13 |
| 10 | 1.28 dd (10.0, 9.6) | 35.5, CH2 | H-9 | C-1, -8, -9, -11, -14, -15 |
| 1.46 dd (9.6, 8.0) | ||||
| 11 | 34.9, C | |||
| 12 | 0.92 d (12.8) | 42.7, CH2 | C-3, -4, -5, -7, -8, -9 | |
| 1.88 d (12.8) | ||||
| 13 | 0.80 s | 26.2, CH3 | C-7, -8, -9, -12 | |
| 14 | 0.98 s | 30.7, CH3 | C-1, -10, -11, -15 | |
| 15 | 0.97 s | 20.8, CH3 | C-1, -10, -11, -14 | |
| 4-OEt | 3.42 dq (8.8, 7.2) | 56.3, CH2 | H3-2' | C-4, -2' |
| 3.49 dq (8.8, 7.2) | ||||
| 1.13 t (7.2) | 16.4 CH3 | H2-1' | C-1' |
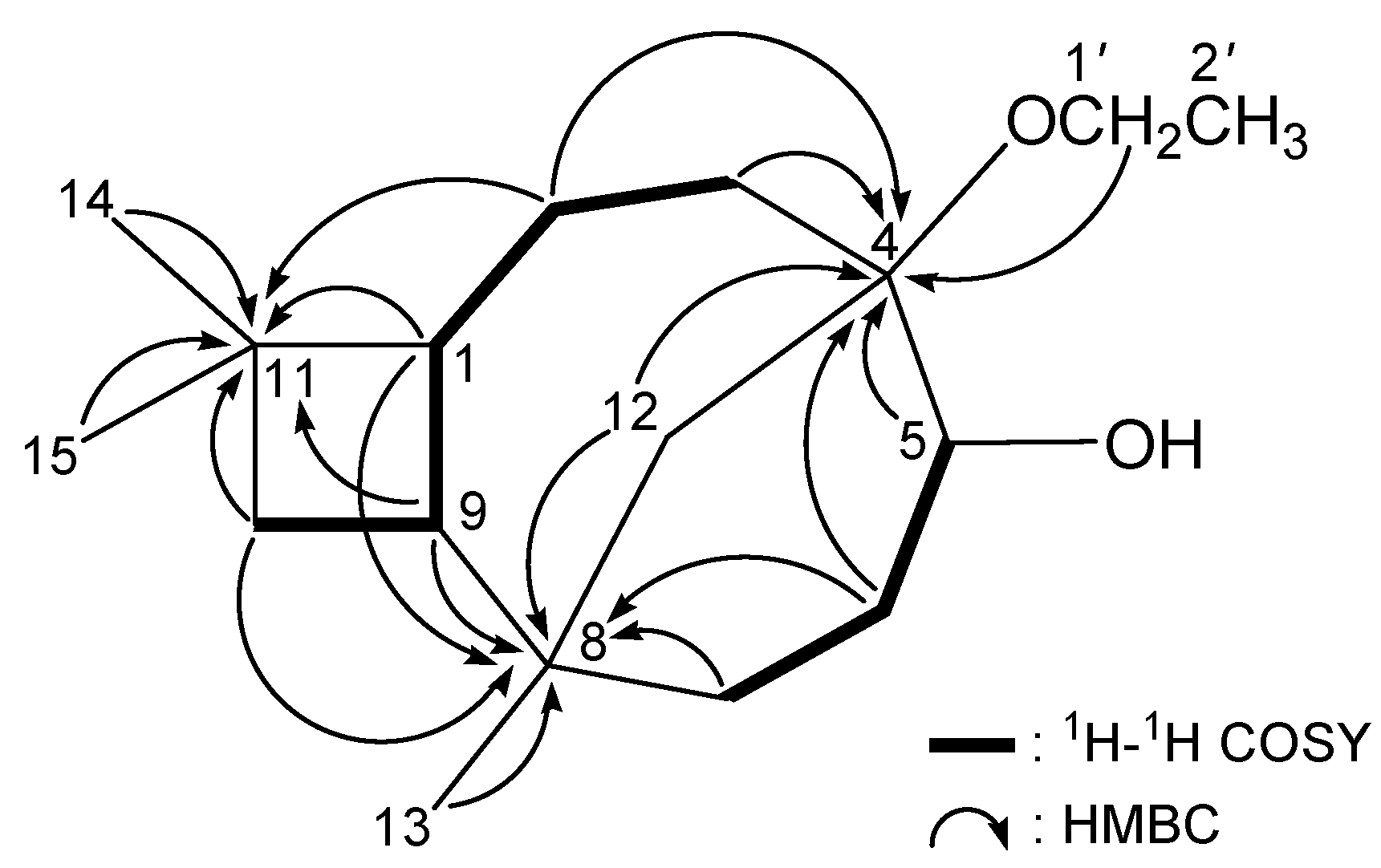
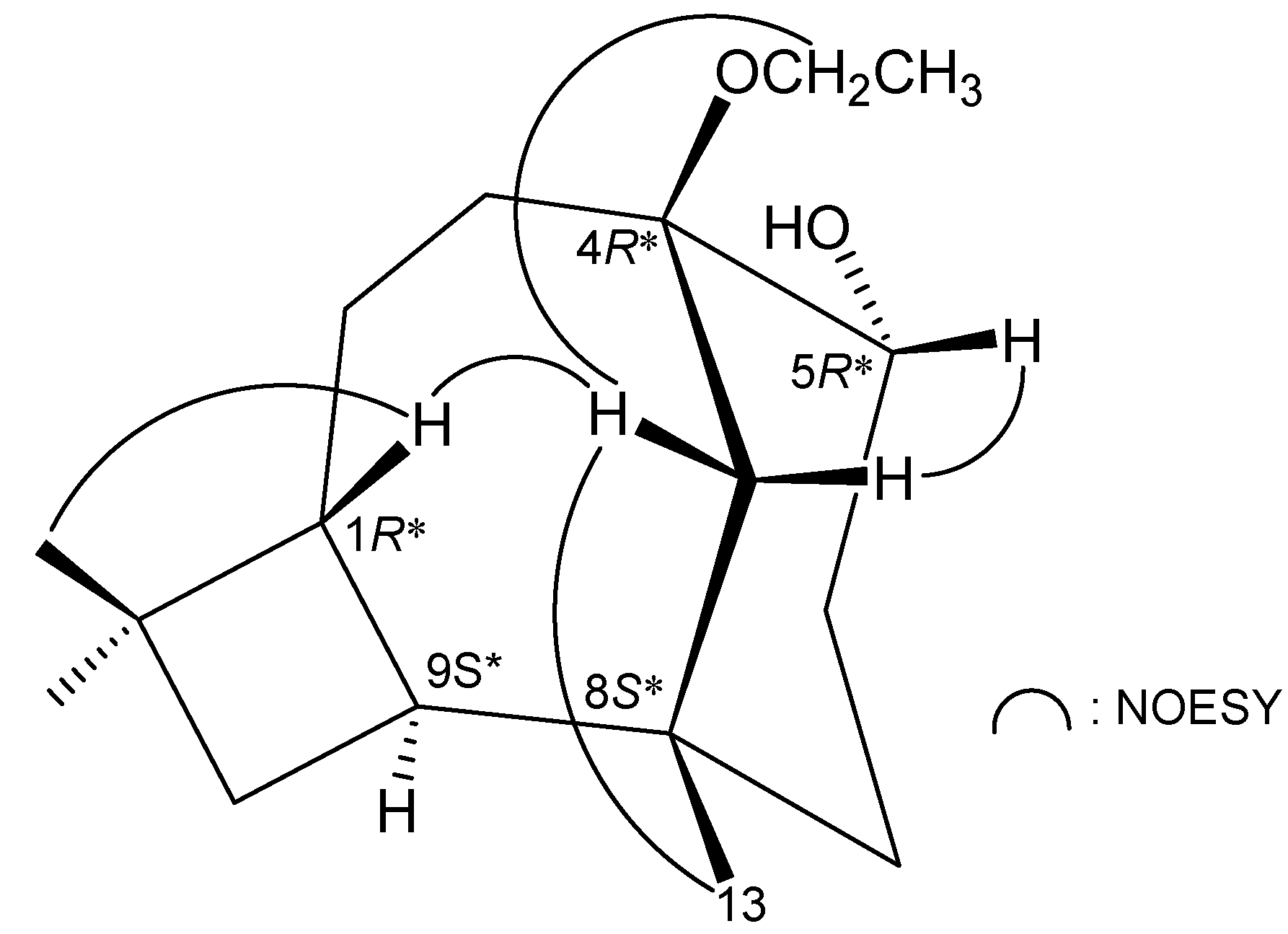
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
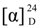 −55 (c 0.04, CHCl3); IR (neat) νmax 3429, 1724 cm−1; 1H NMR (CDCl3, 400 MHz) and 13C NMR (CDCl3, 100 MHz) data, see Table 1; ESIMS m/z 237 [M + H]+; HRESIMS m/z 237.1836 (calcd. for C15H24O2 + H, 237.1849).
−55 (c 0.04, CHCl3); IR (neat) νmax 3429, 1724 cm−1; 1H NMR (CDCl3, 400 MHz) and 13C NMR (CDCl3, 100 MHz) data, see Table 1; ESIMS m/z 237 [M + H]+; HRESIMS m/z 237.1836 (calcd. for C15H24O2 + H, 237.1849).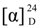 +12 (c 0.27, CHCl3); IR (neat) νmax 3441 cm−1; 1H NMR(CDCl3, 400 MHz) and 13C NMR (CDCl3, 100 MHz) data, see Table 2; ESIMS m/z 289 [M + Na]+; HRESIMS m/z 289.2128 (calcd for C17H30O2 + Na, 289.2138).
+12 (c 0.27, CHCl3); IR (neat) νmax 3441 cm−1; 1H NMR(CDCl3, 400 MHz) and 13C NMR (CDCl3, 100 MHz) data, see Table 2; ESIMS m/z 289 [M + Na]+; HRESIMS m/z 289.2128 (calcd for C17H30O2 + Na, 289.2138).3.4. Human Neutrophil Superoxide Anion Generation and Elastase Release
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rocha, J.; Peixe, L.; Gomes, N.C.M.; Calado, R. Cnidarians as new marine bioactive compounds—An overview of the last decade and future steps for bioprospeting. Mar. Drugs 2011, 9, 1860–1886. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2014, 31, 160–258. [Google Scholar] [CrossRef]
- Rahman, M.A.; Isa, Y.; Uehara, T. Proteins of calcified endoskeleton: II partial amino acid sequences of endoskeletal proteins and the characterization of proteinaceous orgnic matrix of spicules from the alcyonarian, Synularia polydactyla. Proteomics 2005, 5, 885–893. [Google Scholar] [CrossRef]
- Green, D.W.; Padula, M.P.; Santos, J.; Chou, J.; Milthorpe, B.; Ben-Nissan, B. A therapeutic potential for marine skeletal proteins in bone regeneration. Mar. Drugs 2013, 11, 1203–1220. [Google Scholar] [CrossRef]
- Bayer, F.M. Key to the genera of Octocorallia exclusive of Pennatulacea (Coelenterata: Anthozoa), with diagnoses of new taxa. Proc. Biol. Soc. Wash. 1995, 94, 902–947. [Google Scholar]
- Sung, P.-J.; Chuang, L.-F.; Kuo, J.; Chen, J.-J.; Fan, T.-Y.; Li, J.-J.; Fang, L.-S.; Wang, W.-H. Rumphellolides A–F, six new caryophyllane-related derivatives from the Formosan gorgonian coral Rumphella. antipathies. Chem. Pharm. Bull. 2007, 55, 1296–1301. [Google Scholar] [CrossRef]
- Sung, P.-J.; Chuang, L.-F.; Fan, T.-Y.; Chou, H.-N.; Kuo, J.; Fang, L.-S.; Wang, W.-H. Rumphellolide G, a new caryophyllane-type tetrahydropyran norsesquiterpenoid from the gorgonian coral Rumphella. antipathies (Gorgoniidae). Chem. Lett. 2007, 36, 1322–1323. [Google Scholar]
- Hwang, T.-L.; Su, Y.-D.; Hu, W.-P.; Chuang, L.-F.; Sung, P.-J. Rumphellolide H, a new natural caryophyllane from the gorgonian Rumphella. antipathies. Heterocycles 2009, 78, 1563–1567. [Google Scholar]
- Sung, P.-J.; Su, Y.-D.; Hwang, T.-L.; Chuang, L.-F.; Chung, H.-M.; Chen, J.-J.; Li, J.-J.; Fang, L.-S.; Wang, W.-H. Rumphellolide I, a novel caryophyllane-related tetrahydropyran norsesquiterpenoid from gorgonian coral Rumphella. antipathies. Chem. Lett. 2009, 38, 282–283. [Google Scholar] [CrossRef]
- Sung, P.-J.; Chuang, L.-F.; Kuo, J.; Fan, T.-Y.; Hu, W.-P. Rumphellatin A, the first chloride-containing caryophyllane-type norsesquiterpenoid from Rumphella. antipathies. Tetrahedron Lett. 2007, 48, 3987–3989. [Google Scholar]
- Sung, P.-J.; Chuang, L.-F.; Hu, W.-P. Rumphellatins B and C, two new caryophyllane-type hemiketal norsesquiterpenoids from the Formosan gorgonian coral Rumphella. antipathies. Bull. Chem. Soc. Jpn. 2007, 80, 2395–2399. [Google Scholar]
- Sung, P.-J.; Su, Y.-D.; Hwang, T.-L.; Chuang, L.-F.; Chen, J.-J.; Li, J.-J.; Fang, L.-S.; Wang, W.-H. Rumphellatin D, a novel chlorinated caryophyllane from gorgonian coral Rumphella. antipathies. Chem. Lett. 2008, 37, 1244–1245. [Google Scholar] [CrossRef]
- Chung, H.-M.; Chen, Y.-H.; Lin, M.-R.; Su, J.-H.; Wang, W.-H.; Sung, P.-J. Rumphellaone A, a novel caryophyllane-related derivative from the gorgonian coral Rumphella antipathies. Tetrahedron Lett. 2010, 51, 6025–6027. [Google Scholar]
- Chung, H.-M.; Wang, W.-H.; Hwang, T.-L.; Li, J.-J.; Fang, L.-S.; Wu, Y.-C.; Sung, P.-J. Rumphellaones B and C, new 4,5-seco-carophyllane sesquiterpenoids from Rumphella. antipathies. Molecules 2014, 19, 12320–12327. [Google Scholar] [CrossRef]
- Chuang, L.-F.; Fan, T.-Y.; Li, J.-J.; Sung, P.-J. Kobusone: Occurrence of a norsesquiterpenoid in the gorgonian coral Rumphella. antipathies (Gorgoniidae). Biochem. Syst. Ecol. 2007, 35, 470–471. [Google Scholar] [CrossRef]
- Chuang, L.-F.; Fan, T.-Y.; Li, J.-J.; Kuo, J.; Fang, L.-S.; Wang, W.-H.; Sung, P.-J. Isokobusone, a caryophyllane-type norsesquiterpenoid from the gorgonian coral Rumphella. antipathies (Gorgoniidae). Platax 2007, 4, 61–67. [Google Scholar]
- Chung, H.-M.; Chen, Y.-H.; Hwang, T.-L.; Chuang, L.-F.; Wang, W.-H.; Sung, P.-J. Rumphellclovane A, a novel clovane-related sesquiterpenoid from the gorgonian coral Rumphella. antipathies. Tetrahedron Lett. 2010, 51, 2734–2736. [Google Scholar]
- Chung, H.-M.; Hwang, T.-L.; Chen, Y.-H.; Su, J.-H.; Lu, M.-C.; Chen, J.-J.; Li, J.-J.; Fang, L.-S.; Wang, W.-H.; Sung, P.-J. Rumphellclovane B, a novel clovane analogue from the gorgonian coral Rumphella. antipathies. Bull. Chem. Soc. Jpn. 2011, 84, 119–121. [Google Scholar] [CrossRef]
- Chung, H.-M.; Su, J.-H.; Hwang, T.-L.; Li, J.-J.; Chen, J.-J.; Chen, Y.-H.; Chang, Y.-C.; Su, Y.-D.; Chen, Y.-H.; Fang, L.-S.; et al. Rumphellclovanes C–E, new clovane-type sesquiterpenoids from the gorgonian coral Rumphella. antipathies. Tetrahedron 2013, 69, 2740–2744. [Google Scholar] [CrossRef]
- Chung, H.-M.; Wang, W.-H.; Hwang, T.-L.; Wu, Y.-C.; Sung, P.-J. Natural clovanes from the gorgonian coral Rumphella. antipathies. Nat. Prod. Commun. 2013, 8, 1037–1040. [Google Scholar]
- Fraga, B.M. Natural sesquiterpenoids. Nat. Prod. Rep. 2013, 30, 1226–1264. [Google Scholar]
- Kernan, M.R.; Cambie, R.C.; Bergquist, P.R. Chemistry of sponges, X. New sesquiterpenes from a marine sponge of the genus Eurypon. J. Nat. Prod. 1990, 53, 1353–1356. [Google Scholar]
- Wang, G.-H.; Ahmed, A.F.; Sheu, J.-H.; Duh, C.-Y.; Shen, Y.-C.; Wang, L.-T. Suberosols A–D, four new sesquiterpenes with β-caryophyllene skeletons from a Taiwanese gorgonian coral Subergorgia. suberosa. J. Nat. Prod. 2002, 65, 887–891. [Google Scholar]
- Ahmed, A.F.; Su, J.-H.; Shiue, R.-T.; Pan, X.-J.; Dai, C.-F.; Kuo, Y.-H.; Sheu, J.-H. New β-caryophyllene-derived terpenoids from the soft coral Sinularia. nanolobata. J. Nat. Prod. 2004, 67, 592–597. [Google Scholar] [CrossRef]
- Yang, S.-C.; Chung, P.-J.; Ho, C.-M.; Kuo, C.-Y.; Hung, M.-F.; Huang, Y.-T.; Chang, W.-Y.; Chang, Y.-W.; Chan, K.-H.; Hwang, T.-L. Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide-activated human neutrophils by blocking formyl peptide receptor 1. J. Immunol. 2013, 190, 6511–6519. [Google Scholar]
- Yu, H.-P.; Hsieh, P.-W.; Chang, Y.-J.; Chung, P.-J.; Kuo, L.-M.; Hwang, T.-L. 2-(2-Fluorobenzamido)benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radic. Biol. Med. 2011, 50, 1737–1748. [Google Scholar]
- Anjaneyulu, V.; Rao, K.N.; Kobayashi, M. (24S)-24-Methylcholest-4-ene-3β,6β-diol from a gorgonian (Rumphella. aggregata) of the Andaman and Nicobar islands. Indian J. Chem. 1995, 34B, 78–80. [Google Scholar]
- Puglisi, M.P.; Paul, V.J.; Biggs, J.; Slattery, M. Co-occurrence of chemical and structural defenses in the gorgonian corals of Guam. Mar. Ecol. Prog. Ser. 2002, 239, 105–114. [Google Scholar]
- Nourry, M.; Urvois, P.-A.; Tomasoni, C.; Biard, J.F.; Verbist, J.F.; Roussakis, C. Antiproliferative effects of a product isolated from the gorgonian Rumphella. aggregata. Anticancer Res. 1999, 19, 1881–1885. [Google Scholar]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chung, H.-M.; Wang, W.-H.; Hwang, T.-L.; Chen, J.-J.; Fang, L.-S.; Wen, Z.-H.; Wang, Y.-B.; Wu, Y.-C.; Sung, P.-J. Rumphellols A and B, New Caryophyllene Sesquiterpenoids from a Formosan Gorgonian Coral, Rumphella antipathies. Int. J. Mol. Sci. 2014, 15, 15679-15688. https://doi.org/10.3390/ijms150915679
Chung H-M, Wang W-H, Hwang T-L, Chen J-J, Fang L-S, Wen Z-H, Wang Y-B, Wu Y-C, Sung P-J. Rumphellols A and B, New Caryophyllene Sesquiterpenoids from a Formosan Gorgonian Coral, Rumphella antipathies. International Journal of Molecular Sciences. 2014; 15(9):15679-15688. https://doi.org/10.3390/ijms150915679
Chicago/Turabian StyleChung, Hsu-Ming, Wei-Hsien Wang, Tsong-Long Hwang, Jih-Jung Chen, Lee-Shing Fang, Zhi-Hong Wen, Yu-Bao Wang, Yang-Chang Wu, and Ping-Jyun Sung. 2014. "Rumphellols A and B, New Caryophyllene Sesquiterpenoids from a Formosan Gorgonian Coral, Rumphella antipathies" International Journal of Molecular Sciences 15, no. 9: 15679-15688. https://doi.org/10.3390/ijms150915679
APA StyleChung, H.-M., Wang, W.-H., Hwang, T.-L., Chen, J.-J., Fang, L.-S., Wen, Z.-H., Wang, Y.-B., Wu, Y.-C., & Sung, P.-J. (2014). Rumphellols A and B, New Caryophyllene Sesquiterpenoids from a Formosan Gorgonian Coral, Rumphella antipathies. International Journal of Molecular Sciences, 15(9), 15679-15688. https://doi.org/10.3390/ijms150915679








