Synthesis and Thermotropic Studies of Two Novel Series of Kinked Liquid Crystals: 2-(4'-Alkoxybiphen-4-yl)-6-methylquinolines and 2-(6-Alkoxynaphthalen-2-yl)-6-methylquinolines
Abstract
:1. Introduction
2. Results and Discussion
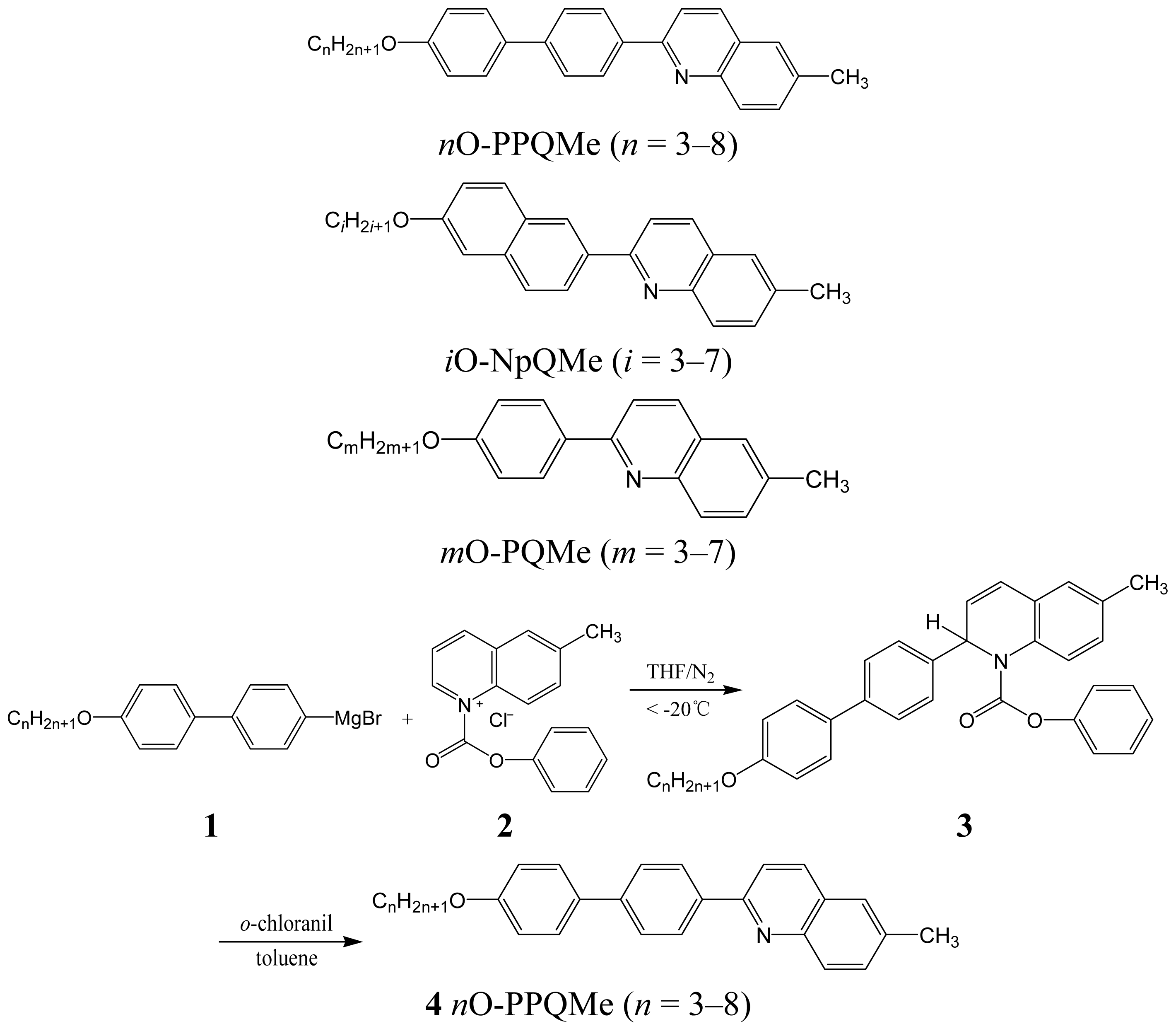
2.1. Synthesis
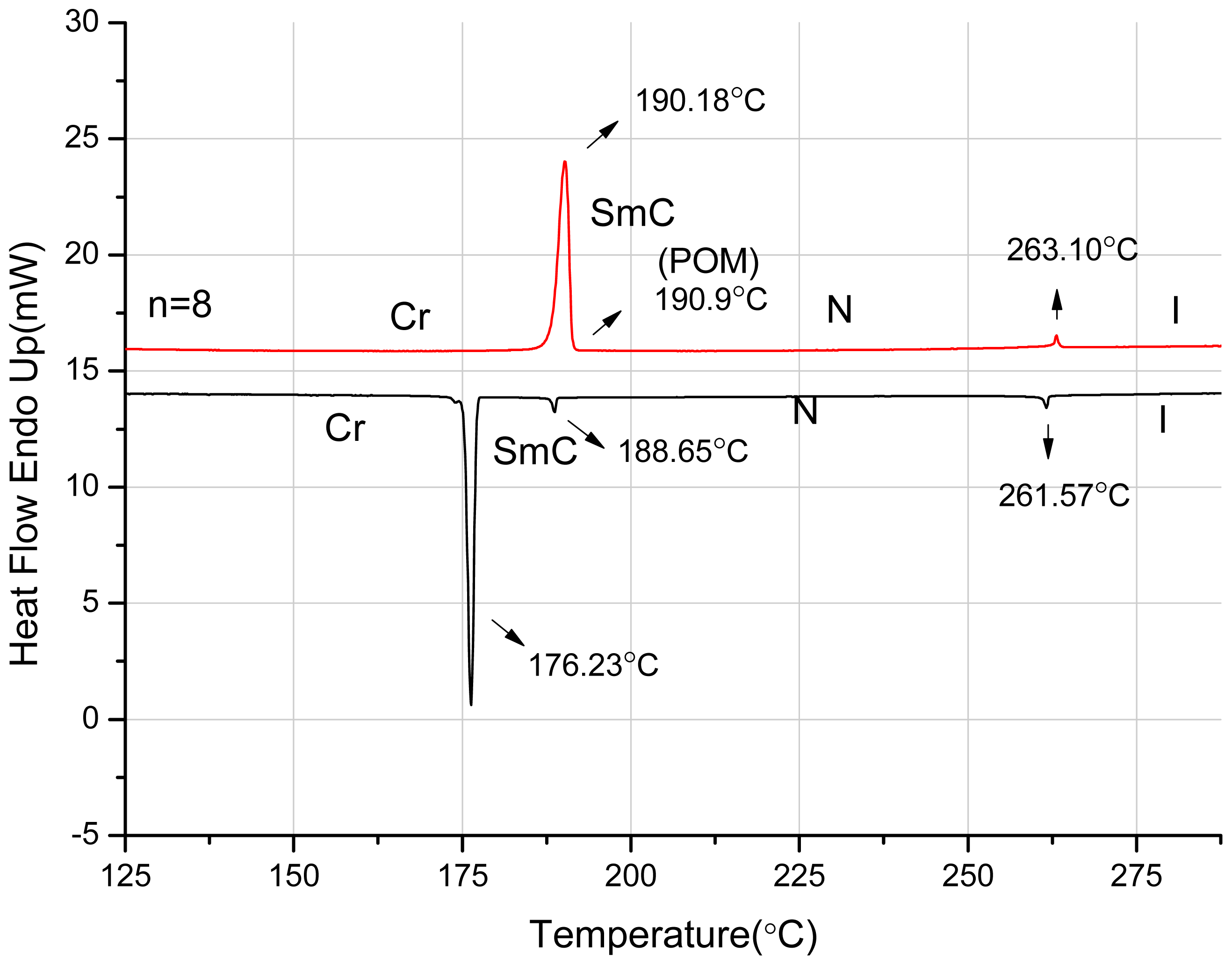
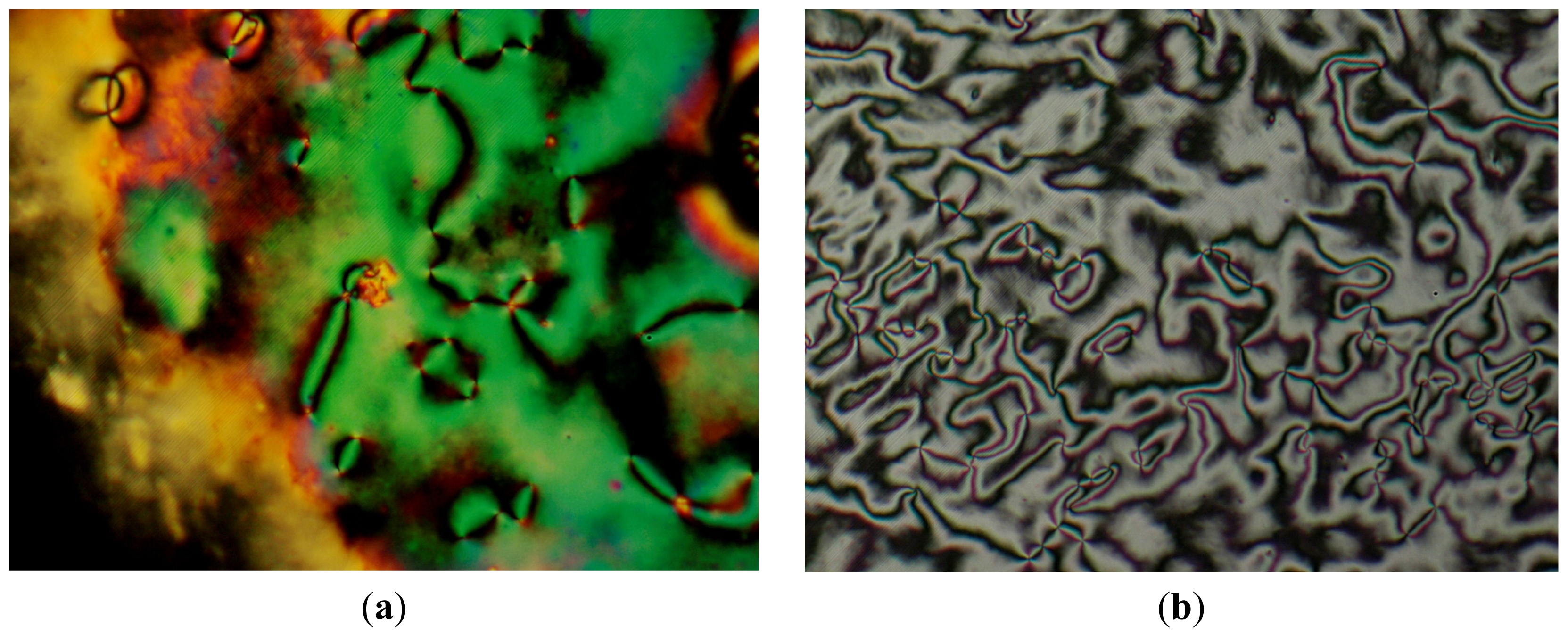
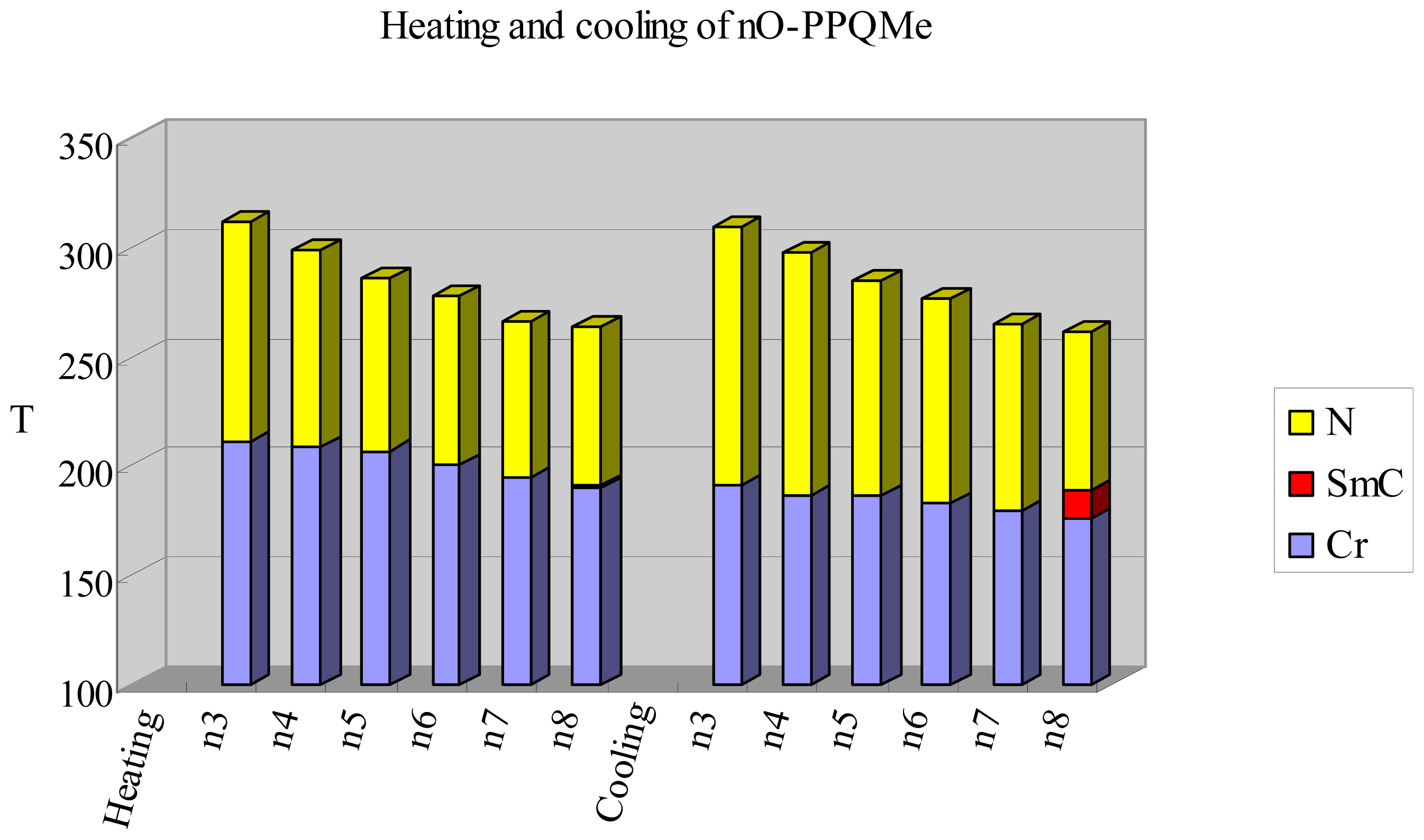
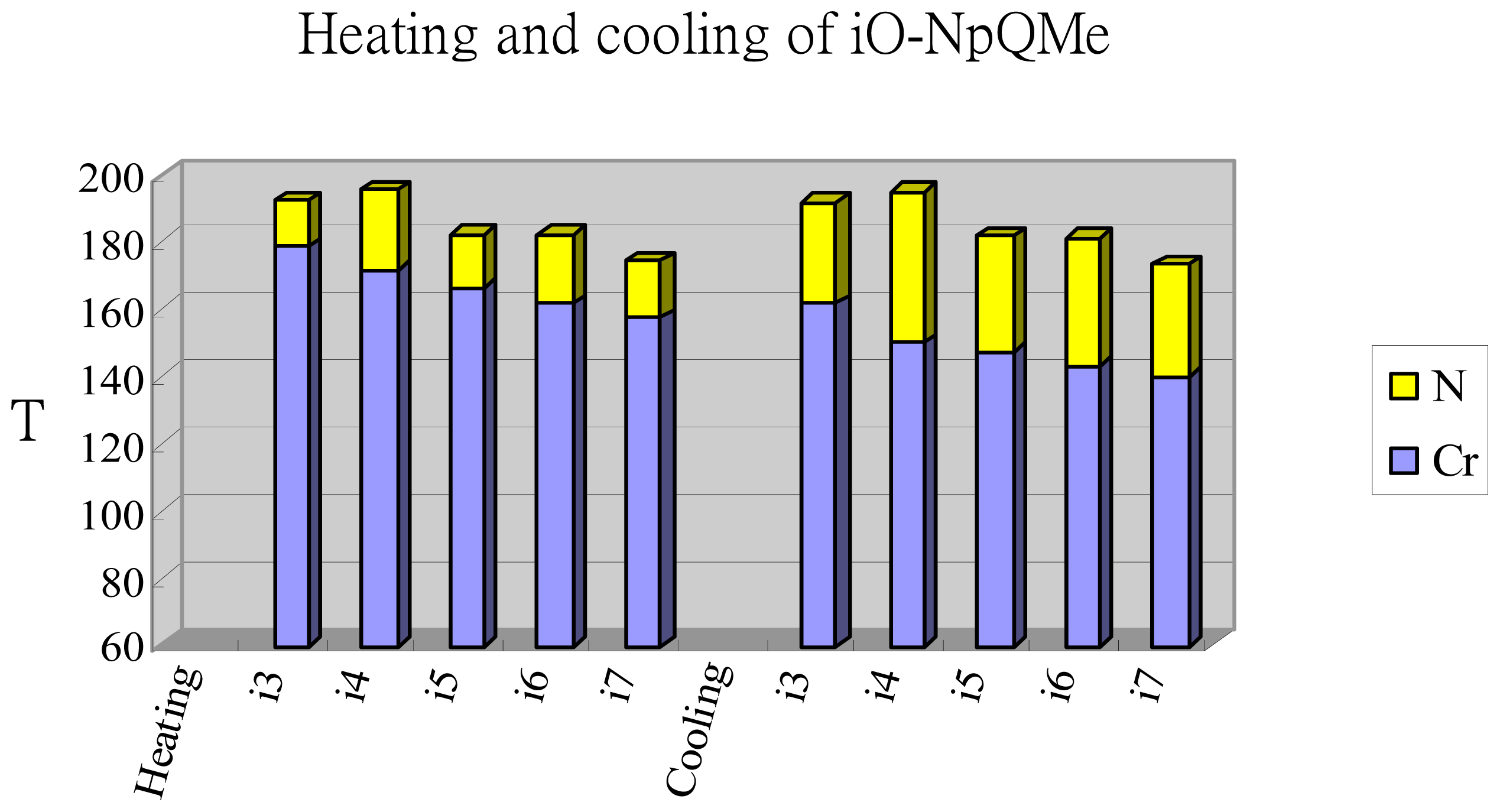
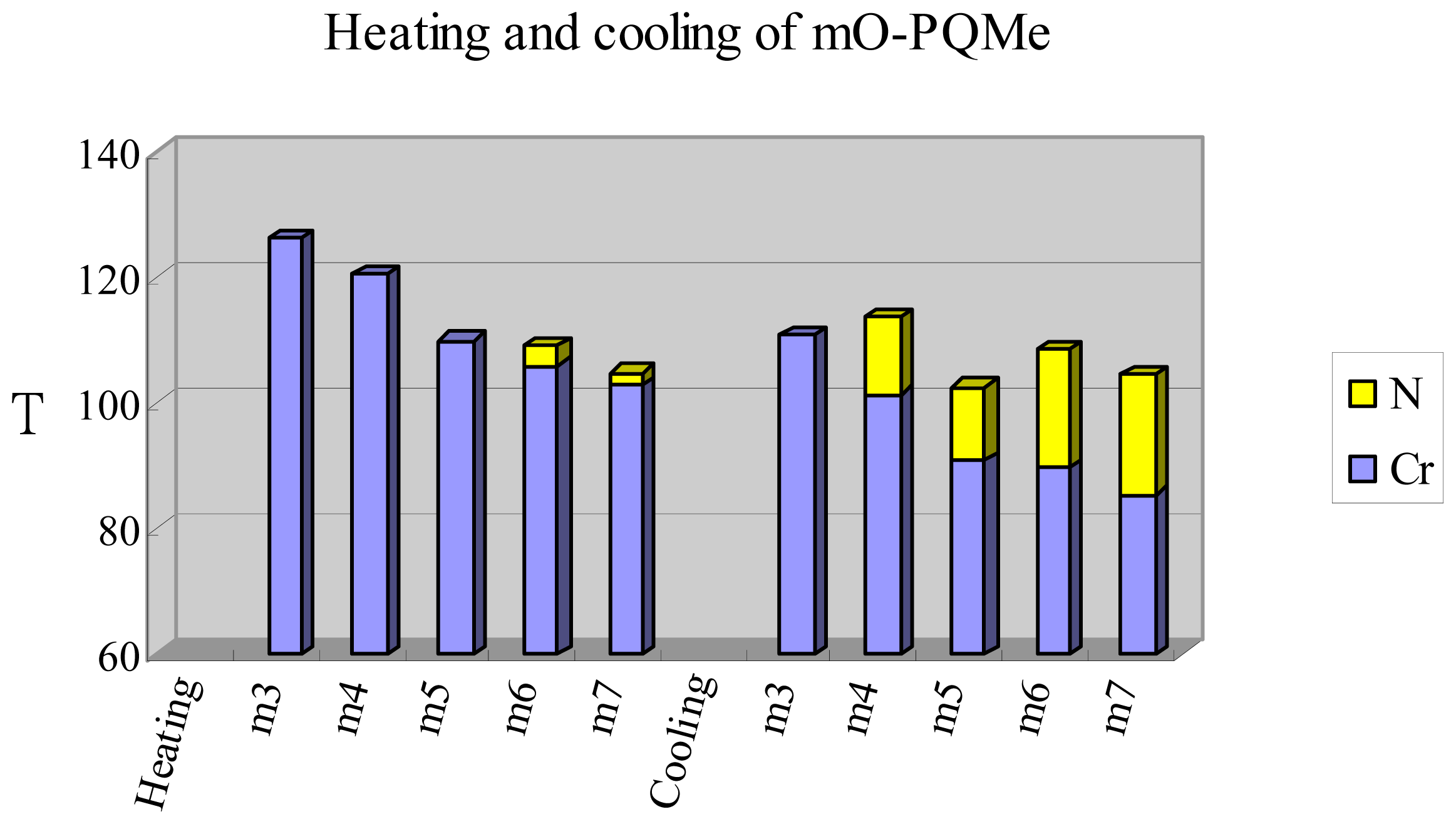
2.2. Thermotropic Studies
3. Experimental Section
3.1. General
3.2. Synthesis
3.2.1. Syntheses of 2-(4′-Alkoxybiphen-4-yl)-6-methylquinolines (nO-PPQMe, n = 3–8)
3.2.1.1. 2-(4′-Propoxybiphen-4-yl)-6-methylquinoline (3O-PPQMe)
3.2.1.2. 2-(4′-Butoxybiphen-4-yl)-6-methylquinoline (4O-PPQMe)
3.2.1.3. 2-(4′-Pentoxybiphen-4-yl)-6-methylquinoline (5O-PPQMe)
3.2.1.4. 2-(4′-Hexoxybiphen-4-yl)-6-methylquinoline (6O-PPQMe)
3.2.1.5. 2-(4′-Heptoxybiphen-4-yl)-6-methylquinoline (7O-PPQMe)
3.2.1.6. 2-(4′-Octoxybiphen-4-yl)-6-methylquinoline (8O-PPQMe)
3.2.2. 2-(6-Alkoxynaphthalen-2-yl)-6-methylquinolines (iO-NpQMe, i = 3–7)
3.2.2.1. 2-(6-Propyloxynaphthalen-2-yl)-6-methylquinolines (3O-NpQMe)
3.2.2.2. 2-(6-Butyloxynaphthalen-2-yl)-6-methylquinolines (4O-NpQMe)
3.2.2.3. 2-(6-Pentyloxynaphthalen-2-yl)-6-methylquinolines (5O-NpQMe)
3.2.2.4. 2-(6-Hexyloxynaphthalen-2-yl)-6-methylquinolines (6O-NpQMe)
3.2.2.5. 2-(6-Heptyloxynaphthalen-2-yl)-6-methylquinolines (7O-NpQMe)
4. Conclusions
Acknowledgments
Conflicts of Interest
- Author ContributionsW.-L.C. conceived the idea of synthesis, supervised students to carry out the experiments, drew and summarized the figures, and finalized the preparation of the manuscript. K.-N.K. and S.-H.L. synthesized the series of nO-PPQMe, n = 3–8 and iO-NpQMe, i = 3–7, respectively, and did measurements on their thermotropic properties.
References
- Li, Q. Liquid Crystals beyond Displays Chemistry, Physics, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Donaldson, T.; Staesche, H.; Lu, Z.B.; Henderson, P.A.; Achard, M.F.; Imrie, C.T. Symmetric and non-symmetric chiral liquid crystal dimers. Liq. Cryst 2010, 37, 1097–1110. [Google Scholar]
- Paraskos, P.A.; Swager, T.M. Effects of desymmetrization on thiophene-based bent-rod mesogens. Chem. Mater 2002, 14, 4543–4549. [Google Scholar]
- Sluckin, T.J.; Dunmur, D.A.; Stegemeyer, H. Crystals that Flow: Classic Papers from the History of Liquid Crystals; Gray, G.W., Goodby, J.W., Fukuda, A., Eds.; Taylor & Francis: London, UK, 2004; pp. 3–21. [Google Scholar]
- Leardini, R.; Nanni, D.; Pedulli, G.F.; Tundo, A.; Zanardi, G. Liquid-crystalline quinoline derivatives. Liq. Cryst 1987, 2, 625–631. [Google Scholar]
- Yokoyama, A.; Nishiyama, I.; Yoshizawa, A. 6-Alkyl-2-(4-alkyloxyphenyl)quinoline: A new smectic C base material. Ferroelectrics 1993, 148, 139–145. [Google Scholar]
- Zuniga, C.; Bartulin, J.; Muller, H.J.; Schumacher, E.; Taylor, T.R. Synthesis and mesomorphic properties of 2-N-hexyl-6-(4-alkoxybenzoyloxy)quinoline. Mol. Cryst. Liq. Cryst 1991, 206, 131–137. [Google Scholar]
- Zuniga, C.; Belmar, J.; Parra, M.; Ramirez, A.; Decap, J.; Ros, B.; Serrano, J.L. Synthesis and mesomorphic properties of 6-N-decyloxy-2-[4′-N-alkoxyphenylimino)methyl]quinolines (IV). Liq. Cryst 1996, 20, 253–260. [Google Scholar]
- Belmar, J.; Parra, M.; Zuniga, C.; Fuentes, G.; Marcos, M.; Serrano, J.L. Synthesis and mesomorphic properties of 2,6-disubstituted derivatives of quinoline: Amides and esters. Liq. Cryst 1999, 26, 9–15. [Google Scholar]
- Eisch, J.J.; Dluzniewski, T. Mechanism of the Skraup and Doebner-von Miller quinoline synthesis: Cyclization of a,b-unsaturated N-aryliminium salts via 1,3-diazetidinium ion intermediates. J. Org. Chem 1989, 54, 1269–1274. [Google Scholar]
- Gilchrist, T.L. Heterocyclic Chemistry; Longman Scientific & Technical: Harlow, UK, 1985; pp. 270–272. [Google Scholar]
- Lin, H.-C.; Lai, L.-L.; Hsieh, W.-P.; Huang, W.-Y. A novel class of heterocyclic liquid crystals with broad smectic C phase. Liq. Cryst 1997, 22, 661–667. [Google Scholar]
- Meth-Cohn, O.; Narine, B.; Tarnowski, B. A versatile new synthesis of quinolines and related fused pyridines, Part II. Tetrahedron Lett 1979, 33, 3111–3114. [Google Scholar]
- Lai, L.-L.; Wang, C.-H.; Hsieh, W.P.; Lin, H.-C. Synthesis and characterization of liquid crystalline molecules containing the quinoline unit. Mol. Cryst. Liq. Cryst 1996, 287, 177–181. [Google Scholar]
- Sato, K.; Kitayama, H.; Shinjo, K.; Nakamura, S.; Nakamura, K. Liquid crystal composition, liquid crystal device using the composition, liquid crystal apparatus and display method. U.S. Patent 5,948,317, 7 September 1999. [Google Scholar]
- Kosaka, Y.; Takiguchi, T.; Iwaki, T.; Togano, T.; Nakamura, S. Mesomorphic compound, liquid crystal composition containing the compound, liquid crystal device using the composition, liquid crystal apparatus and display method. U.S. Patent 5,695,684, 9 December 1997. [Google Scholar]
- Yokoyama, A.; Yoshizawa, A.; Hirai, T. New phenylquinoline compound and liquid crystal composition containing the same. Jpn Kokai Tokkyo Koho JP 04316555, 6 November 1992. [Google Scholar]
- Chia, W.L.; Liao, K.H.; Ho, C.I. Synthesis and mesomorphic properties on the series of 2-(4-alkylphenyl)-6-methylquinolines and 2-(4-alkoxyphenyl)-6-methylquinolines. Liq. Cryst 2009, 36, 557–563. [Google Scholar]
- Chia, W.-L.; Chang, C.H. Synthesis and mesomorphic studies of a series of liquid crystalline 2-(4-alkylphenyl)-6-methoxyquinolines. Mol. Cryst. Liq. Cryst 2009, 506, 47–55. [Google Scholar]
- Chia, W.L.; Ye, F.J.; Chen, E.C. Synthesis and mesomorphic behaviour of the series of 2-(4-alkoxyphenyl)-6-methoxyquinolines and 2-(4-alkoxybiphenyl-4′-yl)-6-methoxyquinoline. Liq. Cryst 2013, 40, 989–997. [Google Scholar]
- Gray, G.W.; Mosley, A. Trends in the nematic-isotropic liquid transition temperatures for the homologous series of 4-N-alkoxy and 4-N-alkyl-4′ cyanobiphenyls. J. Chem. Soc. Perkin Trans. II 1976, 97–102. [Google Scholar]
- Chia, W.L.; Lin, C.W. Synthesis and thermotropic studies of a novel series of nematogenic liquid crystals 2-(6-alkoxynaphthalen-2-yl)-5-cyanopyridines. Liq. Cryst 2013, 40, 922–931. [Google Scholar]
| Entry (n) | Alkyl group | Yield a (%) |
|---|---|---|
| n = 3 | Propyl | 37 |
| n = 4 | Butyl | 35 |
| n = 5 | Pentyl | 30 |
| n = 6 | Hexyl | 39 |
| n = 7 | Heptyl | 40 |
| n = 8 | Octyl | 37 |
| i = 3 | Propyl | 51 |
| i = 4 | Butyl | 52 |
| i = 5 | Pentyl | 52 |
| i = 6 | Hexyl | 57 |
| i = 7 | Heptyl | 55 |
| Compound nO-PPQMe or iO-NpQMe | Phase transition temperatures (°C) and their corresponding transition enthalpies (kJ·mol−1) | |
|---|---|---|
| Heating a | Cooling a | |
| n = 3 | Cr 210.6(26.3) N 311 b I | I 309 b N 191.5(22.3) Cr |
| n = 4 | Cr 209.0(30.7) N 299.1(0.9) I | I 297.7(0.8) N 186.7(27.9) Cr |
| n = 5 | Cr 206.9(35.8) N 286.0(0.8) I | I 284.4(0.8) N 186.6(33.7) Cr |
| n = 6 | Cr 200.0(28.9) N 278.1(0.8) I | I 276.7(0.8) N 182.7(27.0) Cr |
| n = 7 | Cr 194.5(27.7) N 266.4(0.4) I | I 264.4(0.5) N 179.4(26.6) Cr |
| n = 8 | Cr 190.2(32.8) SmC 190.9 c N 263.1(0.8) I | I 261.6(1.0) N 188.7(0.9) SmC 176.2(30.4) Cr |
| i = 3 | Cr 178.5(36.6) N 192.1(0.4) I | I 191.5(0.4) N 161.7(32.5) Cr |
| i = 4 | Cr 171.5(32.5) N 195.6(0.4) I | I 194.9(0.5) N 150.3(31.2) Cr |
| i = 5 | Cr 166.3(32.9) N 182.3(0.5) I | I 181.6(0.4) N 147.2(28.2) Cr |
| i = 6 | Cr 161.7(31.9) N 182.0(0.5) I | I 181.2(0.5) N 143.1(29.8) Cr |
| i = 7 | Cr 157.6(32.2) N 174.1(0.5) I | I 173.2(0.4) N 140.2(31.3) Cr |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chia, W.-L.; Kuo, K.-N.; Lin, S.-H. Synthesis and Thermotropic Studies of Two Novel Series of Kinked Liquid Crystals: 2-(4'-Alkoxybiphen-4-yl)-6-methylquinolines and 2-(6-Alkoxynaphthalen-2-yl)-6-methylquinolines. Int. J. Mol. Sci. 2014, 15, 7579-7593. https://doi.org/10.3390/ijms15057579
Chia W-L, Kuo K-N, Lin S-H. Synthesis and Thermotropic Studies of Two Novel Series of Kinked Liquid Crystals: 2-(4'-Alkoxybiphen-4-yl)-6-methylquinolines and 2-(6-Alkoxynaphthalen-2-yl)-6-methylquinolines. International Journal of Molecular Sciences. 2014; 15(5):7579-7593. https://doi.org/10.3390/ijms15057579
Chicago/Turabian StyleChia, Win-Long, Ker-Non Kuo, and Shao-Hsun Lin. 2014. "Synthesis and Thermotropic Studies of Two Novel Series of Kinked Liquid Crystals: 2-(4'-Alkoxybiphen-4-yl)-6-methylquinolines and 2-(6-Alkoxynaphthalen-2-yl)-6-methylquinolines" International Journal of Molecular Sciences 15, no. 5: 7579-7593. https://doi.org/10.3390/ijms15057579
APA StyleChia, W.-L., Kuo, K.-N., & Lin, S.-H. (2014). Synthesis and Thermotropic Studies of Two Novel Series of Kinked Liquid Crystals: 2-(4'-Alkoxybiphen-4-yl)-6-methylquinolines and 2-(6-Alkoxynaphthalen-2-yl)-6-methylquinolines. International Journal of Molecular Sciences, 15(5), 7579-7593. https://doi.org/10.3390/ijms15057579




