Oral Administration of Bovine Milk from Cows Hyperimmunized with Intestinal Bacterin Stimulates Lamina Propria T Lymphocytes to Produce Th1-Biased Cytokines in Mice
Abstract
:1. Introduction
2. Results
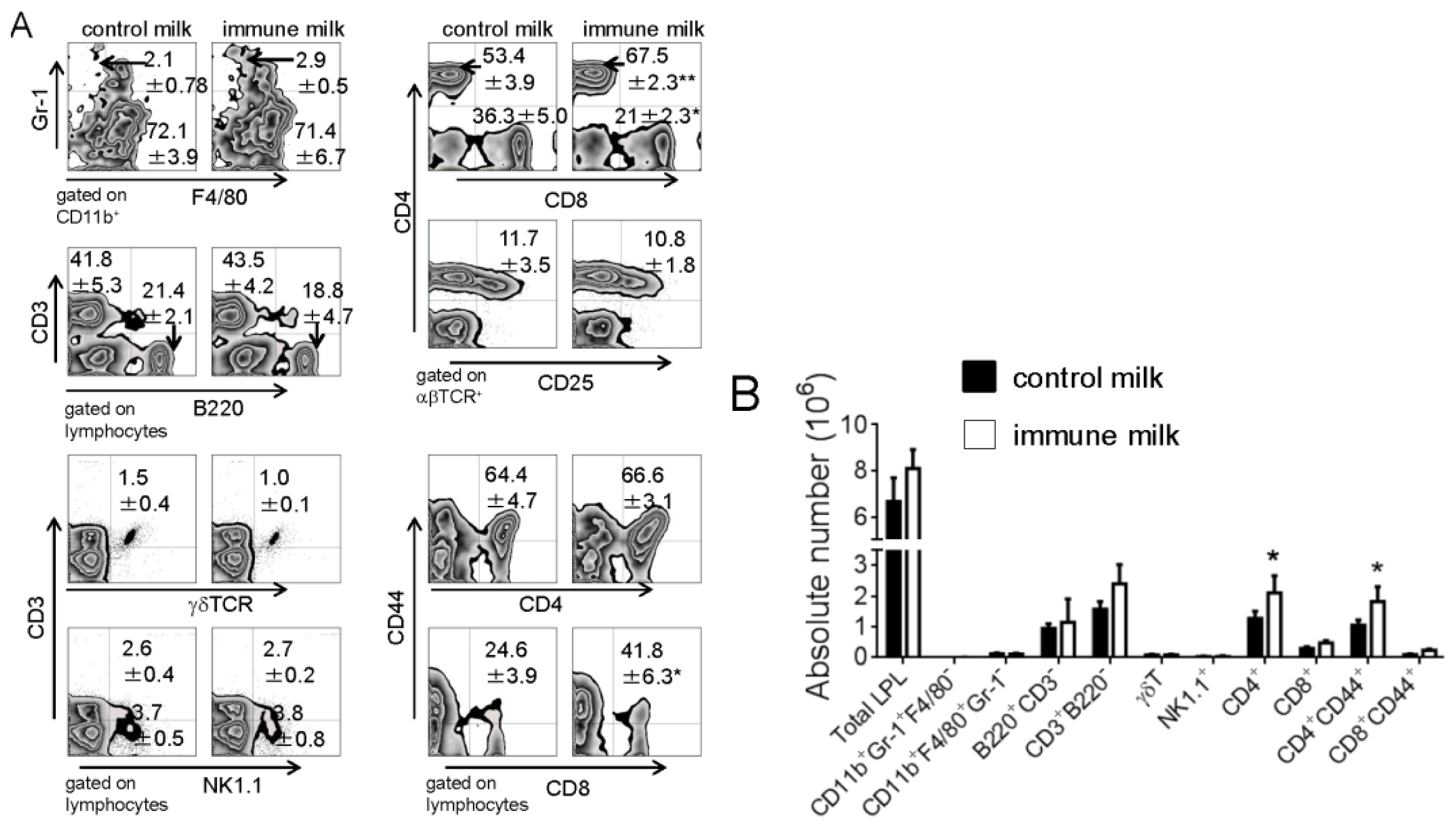
2.1. Populations of LPL in Large Intestine of Mice Fed Immune Milk
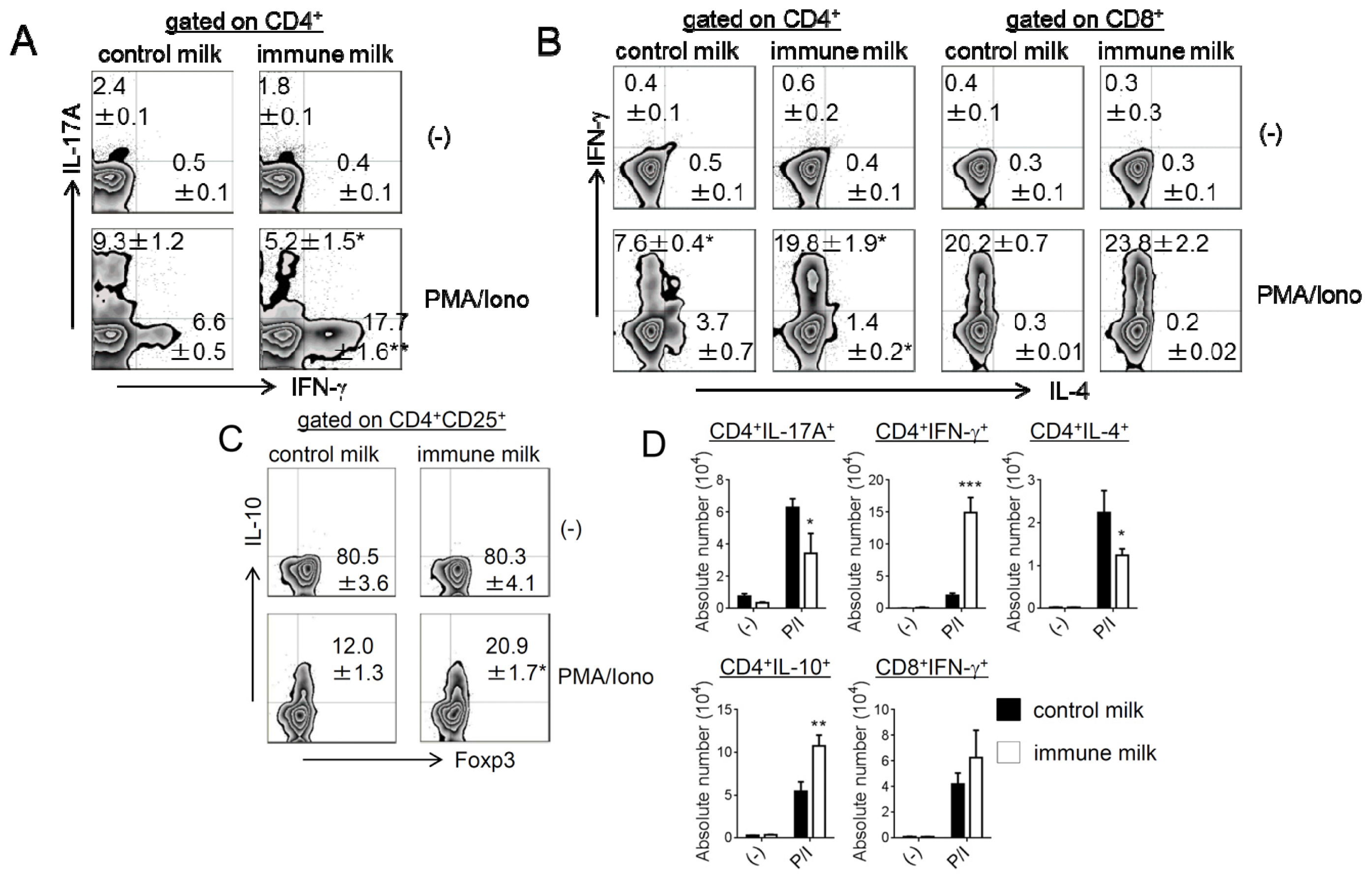
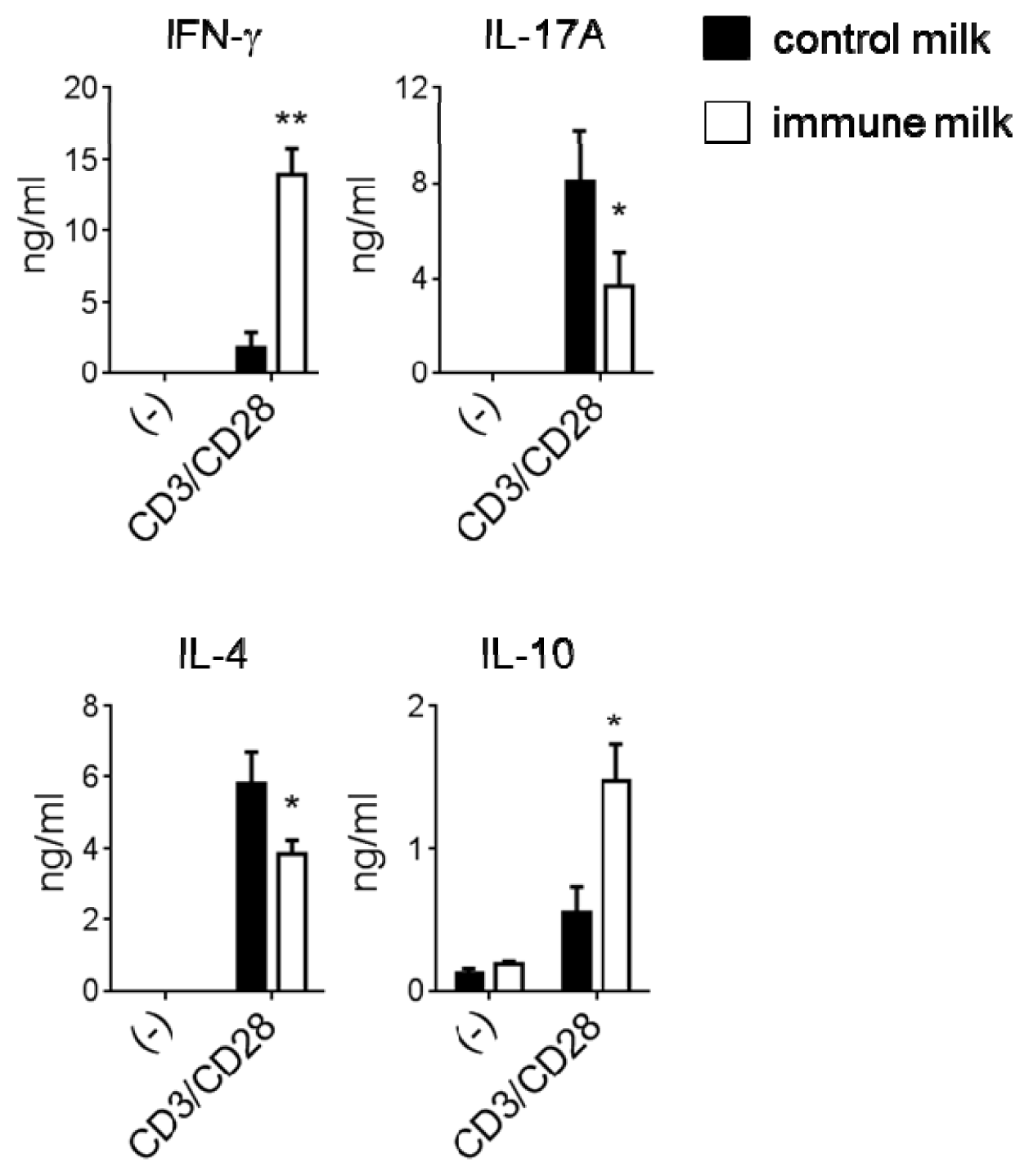
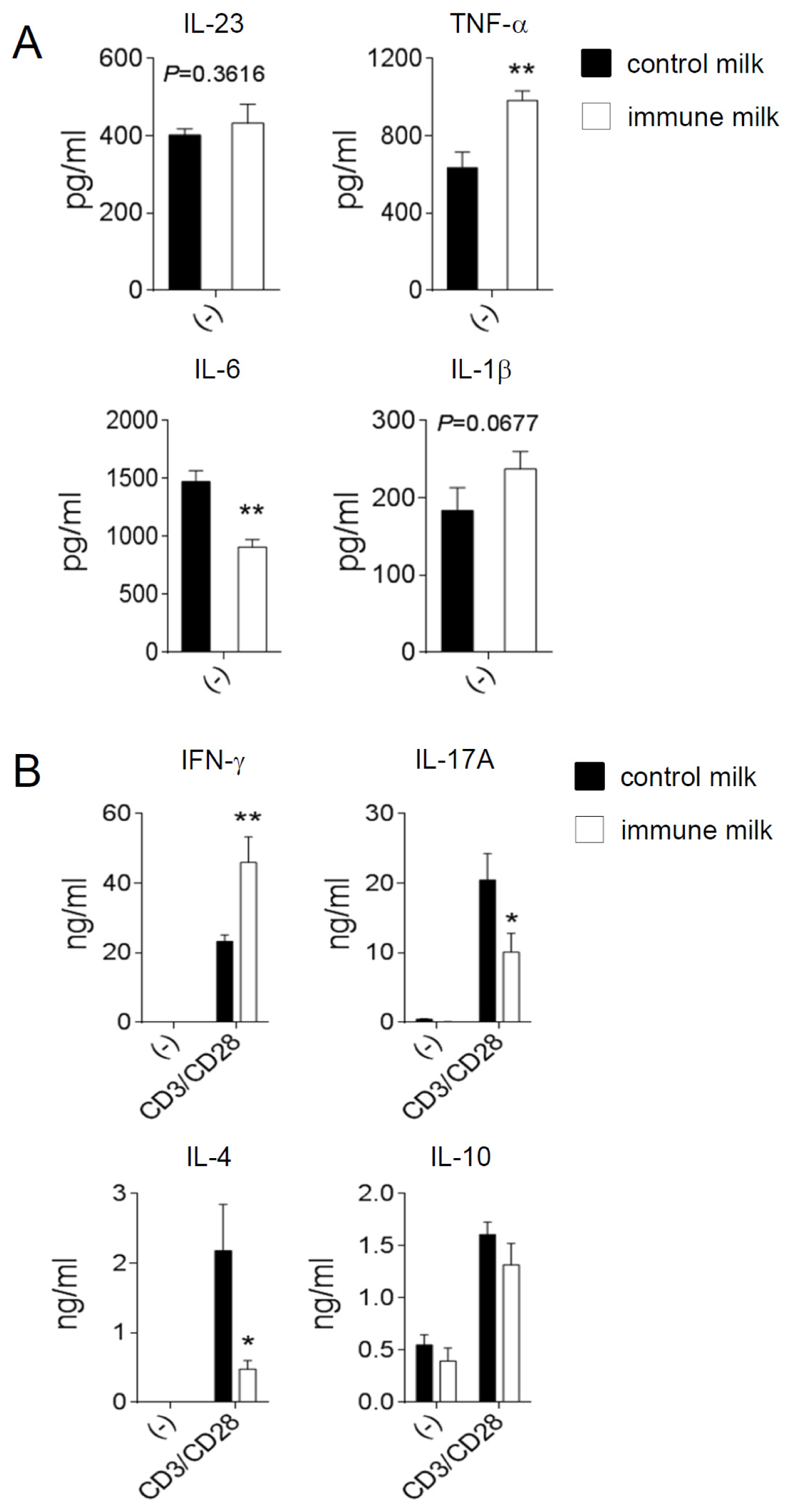
2.2. Cytokine Production by T-LPL Cells in Mice Fed Immune Milk
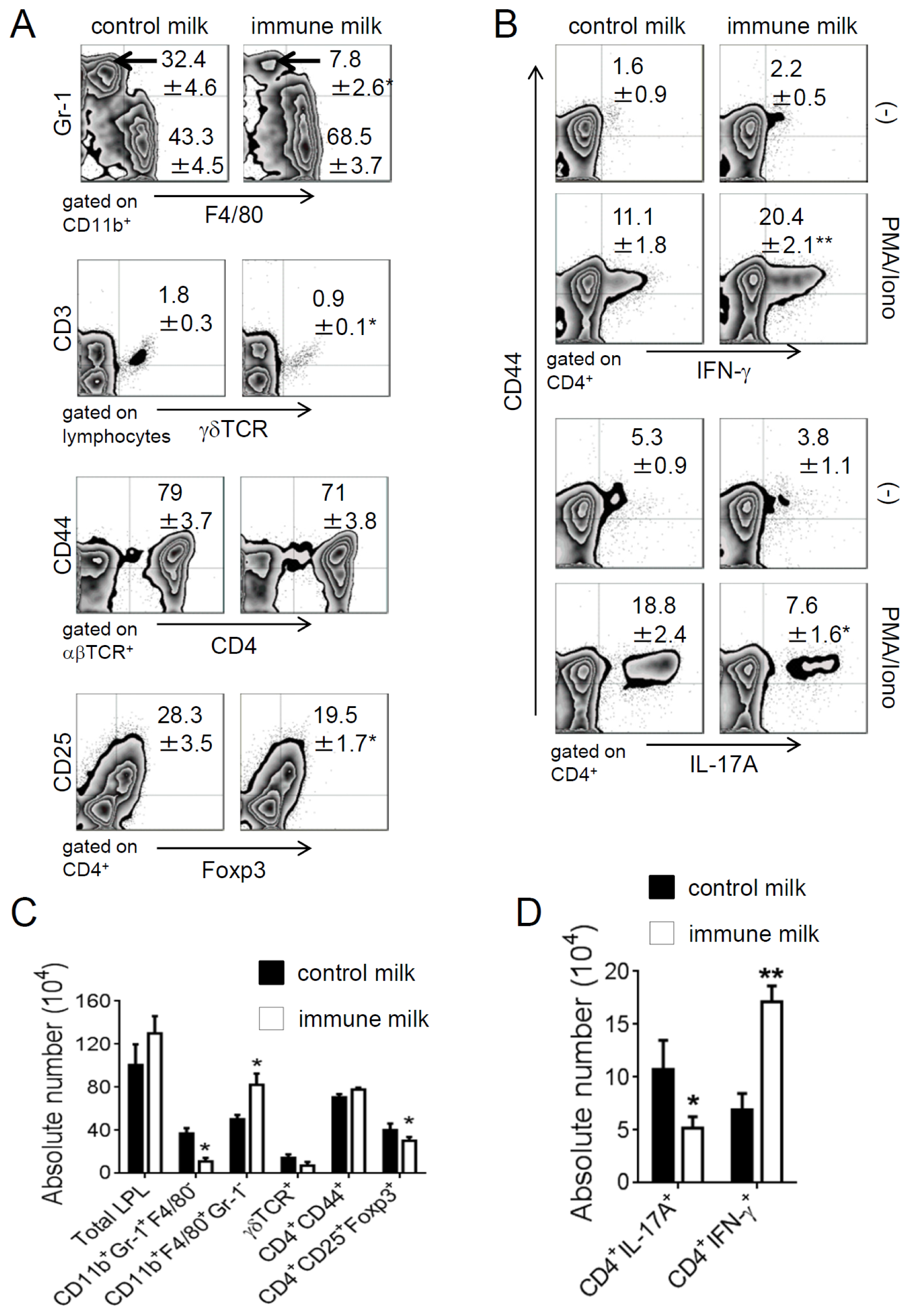
2.3. Immune Milk-Fed Mice Are not Susceptible to Induce Acute Colitis by DSS
3. Discussion
4. Materials and Methods
4.1. Milk Samples
4.2. Mice
4.3. Abs and Reagents
4.4. Cell Preparation
4.5. Flow Cytometry
4.6. Cytokine Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Induction of Acute Colitis by DSS
4.8. Histology Assessment of Colitis
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Conflicts of Interest
- Author ContributionsY.W., L.L. did experimental analyses; C.Y. did establishment and evaluation on of DSS-induced colitis model; C.L. involved in research and discussion; S.O., K.A. provided the immune milk and control milk; X.S., Y.Y. wrote and edited the manuscript.
References
- Hooper, L.Y.; Littman, D.R.; Macpherson, A.J. Interaction between the microbiota and the immune system. Science 2012, 336, 1268–1273. [Google Scholar]
- McLoughlin, R.M.; Mills, K.H. Influence of gastrointestinal commensal bacteria on the immune responses that mediate allergy and asthma. J. Allergy Clin. Immunol 2011, 127, 1097–1107. [Google Scholar]
- Kaser, A.; Zeissig, S.; Blumberg, R.S. Inflammatory bowel disease. Annu. Rev. Immunol 2010, 28, 573–621. [Google Scholar]
- Dieleman, L.; Ridwan, A.B.U.; Ennyson, G.S.; Beagley, K.W.; Bucy, R.P.; Elson, C.O. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology 1994, 107, 1643–1652. [Google Scholar]
- Dieleman, L.; Palmen, A.M.J.; Akol, H.; Bloemena, E.; Peña, A.S.; Meuwissen, S.G.; van Rees, E.P. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol 1998, 114, 385–391. [Google Scholar]
- Fina, D.; Sarra, M.; Fantini, M.C.; Rizzo, A.; Caruso, R.; Caprioli, F.; Stolfi, C.I.; Cardolini, I.; Dottori, M.; Boirivant, M.; et al. Regulation of gut inflammation and Th17 cell response by interleukin-21. Gastroenterology 2008, 134, 1038–1048. [Google Scholar]
- O’Connor, W.J.; Kamanaka, M.; Booth, C.J.; Town, T.; Nakae, S.; Iwakura, Y.; Kolls, J.K.; Flavell, R.A. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat. Immunol 2009, 10, 603–609. [Google Scholar]
- Boden, E.K.; Snapper, S.B. Regulatory T cells in inflammatory bowel disease. Curr. Opin. Gastroenterol 2008, 24, 733–741. [Google Scholar]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011, 331, 337–341. [Google Scholar]
- Boirivant, M.; Strober, W. The mechanism of action of probiotics. Curr. Opin. Gastroenterol 2007, 23, 679–692. [Google Scholar]
- Ivanov, I.I.; Atarashi, K.; Manel, N.; Brodie, E.L.; Shima, T.; Karaoz, U.; Wei, D.; Goldfarb, K.C.; Santee, C.A.; Lynch, S.V.; et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009, 139, 485–498. [Google Scholar]
- Mazmanian, S.K.; Liu, C.H.; Tzianabos, A.O.; Kasper, D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005, 122, 107–118. [Google Scholar]
- Lee, Y.K.; Mazmanian, S.K. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science 2010, 330, 1768–1773. [Google Scholar]
- Ishida, A.; Yoshikai, Y.; Murosaki, S.; Hidaka, Y.; Nomoto, K. Administration of milk from cows immunized with intestinal bacteria protects mice from radiation-induced lethality. Biotherapy 1992, 5, 215–225. [Google Scholar]
- Nomoto, K.; Matsuoka, Y.; Hayakawa, K.; Ohwaki, M.; Kan, T.; Yoshikai, Y.; Nomoto, K. Antibacterial effect of bovine milk antibody against Escherichia coli in a mouse indigenous infection model. Med. Microbiol. Immunol 1992, 181, 87–98. [Google Scholar]
- Murosaki, S.; Yoshikai, Y.; Kubo, C.; Oshida, A.; Matsuzaki, G.; Sato, T.; Endo, K.; Nomoto, K. Influence of intake of skim milk from cows immunized with intestinal bacterial antigens on onset of renal disease in (NZB x NZW) F1 mice fed ad libitum or restricted in energy intake. J. Nutr 1991, 121, 1860–1868. [Google Scholar]
- Sun, X.; Yamada, H.; Shibata, K.; Muta, H.; Tani, K.; Podack, E.R.; Iwakura, Y.; Yoshikai, Y. CD30 ligand is a target for a novel biological therapy against colitis associated with Th17 responses. J. Immunol 2010, 185, 7671–7680. [Google Scholar]
- Strober, W.; Fuss, I.J.; Blumberg, R.S. The immunology of mucosal models of inflammation. Annu. Rev. Immunol 2002, 20, 495–549. [Google Scholar]
- Ishida, A.; Yoshikai, Y.; Murosaki, S.; Kubo, C.; Hidaka, Y.; Nomoto, K. Consumption of milk from cows immunized with intestinal bacteria influences age-related changes in immune competence in mice. J. Nutr 1992, 122, 1875–1883. [Google Scholar]
- Palmer, D.C.; Restifo, N.P. Suppressors of cytokine signaling (SOCS) in T cell differentiation, maturation, and function. Trends Immunol 2009, 30, 592–602. [Google Scholar]
- Sun, X.; Somada, S.; Shibata, K.; Muta, H.; Yamada, H.; Yoshihara, H.; Honda, K.; Nakamura, K.; Takayanagi, R.; Tani, K.; et al. A critical role of CD30 ligand/CD30 in controlling inflammatory bowel diseases in mice. Gastroenterology 2008, 134, 447–458. [Google Scholar]

| Bacterial antigen | ATCC No. 2 | IgG 3 (μg/g milk) | |
|---|---|---|---|
| Control milk | Immune milk | ||
| Staphylococcus simulans | 11631 | 10.8 | NT |
| Staphylococcus epidermidis | 155 | NT | 15.2 |
| Staphylococcus pyogenes, Type 1 | 8671 | NT | 13.6 |
| Staphylococcus pyogenes, Type 3 | 10389 | 1.9 | 90.6 |
| Staphylococcus pyogenes, Type 5 | 12347 | 2.2 | 24.4 |
| Staphylococcus pyogenes, Type 8 | 12349 | 11.4 | 34 |
| Staphylococcus pyogenes, Type 12 | 11434 | 2.5 | 77 |
| Staphylococcus pyogenes, Type 14 | 12972 | 5.3 | 80.0 |
| Staphylococcus pyogenes, Type 18 | 12357 | 10.7 | 88.4 |
| Staphylococcus pyogenes, Type 22 | 10403 | 12.4 | 106.2 |
| Aerobacter aerogenes | 884 | 14.2 | 52.5 |
| Escherichia coli | 26 | 16.0 | 109.0 |
| Salmonella enteritidis | 13076 | 12.5 | 158.3 |
| Pseudomonas aeruginosa | 7700 | 18.7 | 68.4 |
| Klebsiella pneumoniae | 9590 | 42.9 | 305.0 |
| Salmonella typhimurium | 13311 | 21.5 | 46.2 |
| Haemophilus influenzae | 9333 | 36.3 | 50.9 |
| Streptococcus mitis | 6249 | 9.8 | 36.4 |
| Proteus vulgaris | 13315 | 16.2 | 28.1 |
| Sigella dysenteriae | 11835 | 12.5 | 43.4 |
| Propionibacterium acnes | 11827 | 12.6 | 20.9 |
| Streptococcus sanguis | 10556 | 12.5 | 44.0 |
| Streptococcus salivarius | 13419 | 6.8 | 30.6 |
| Streptococcus mutans | 25175 | 9.1 | 31.0 |
| Streptococcus agalactiae | 13813 | 6.2 | 30.6 |
| Streptococcus pneumoniae | 6303 | 6.2 | 31.0 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, Y.; Lin, L.; Yin, C.; Othtani, S.; Aoyama, K.; Lu, C.; Sun, X.; Yoshikai, Y. Oral Administration of Bovine Milk from Cows Hyperimmunized with Intestinal Bacterin Stimulates Lamina Propria T Lymphocytes to Produce Th1-Biased Cytokines in Mice. Int. J. Mol. Sci. 2014, 15, 5458-5471. https://doi.org/10.3390/ijms15045458
Wang Y, Lin L, Yin C, Othtani S, Aoyama K, Lu C, Sun X, Yoshikai Y. Oral Administration of Bovine Milk from Cows Hyperimmunized with Intestinal Bacterin Stimulates Lamina Propria T Lymphocytes to Produce Th1-Biased Cytokines in Mice. International Journal of Molecular Sciences. 2014; 15(4):5458-5471. https://doi.org/10.3390/ijms15045458
Chicago/Turabian StyleWang, Yuanyuan, Lianjie Lin, Chunming Yin, Satoru Othtani, Katsuhiko Aoyama, Changlong Lu, Xun Sun, and Yasunobu Yoshikai. 2014. "Oral Administration of Bovine Milk from Cows Hyperimmunized with Intestinal Bacterin Stimulates Lamina Propria T Lymphocytes to Produce Th1-Biased Cytokines in Mice" International Journal of Molecular Sciences 15, no. 4: 5458-5471. https://doi.org/10.3390/ijms15045458
APA StyleWang, Y., Lin, L., Yin, C., Othtani, S., Aoyama, K., Lu, C., Sun, X., & Yoshikai, Y. (2014). Oral Administration of Bovine Milk from Cows Hyperimmunized with Intestinal Bacterin Stimulates Lamina Propria T Lymphocytes to Produce Th1-Biased Cytokines in Mice. International Journal of Molecular Sciences, 15(4), 5458-5471. https://doi.org/10.3390/ijms15045458




