Exercise Pretreatment Promotes Mitochondrial Dynamic Protein OPA1 Expression after Cerebral Ischemia in Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
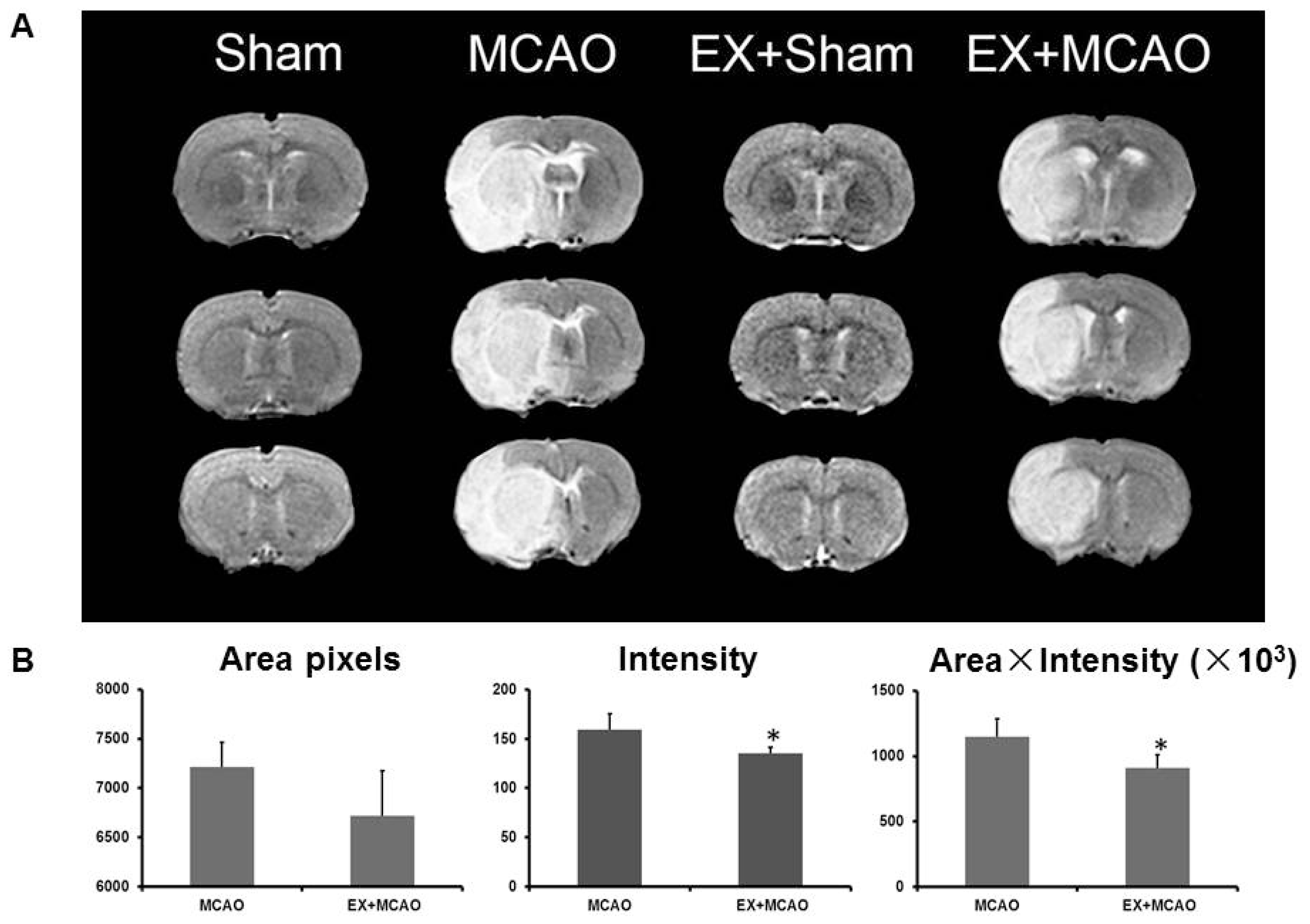
2.1.1. Exercise Alleviates Brain Edema after Ischemic Injury
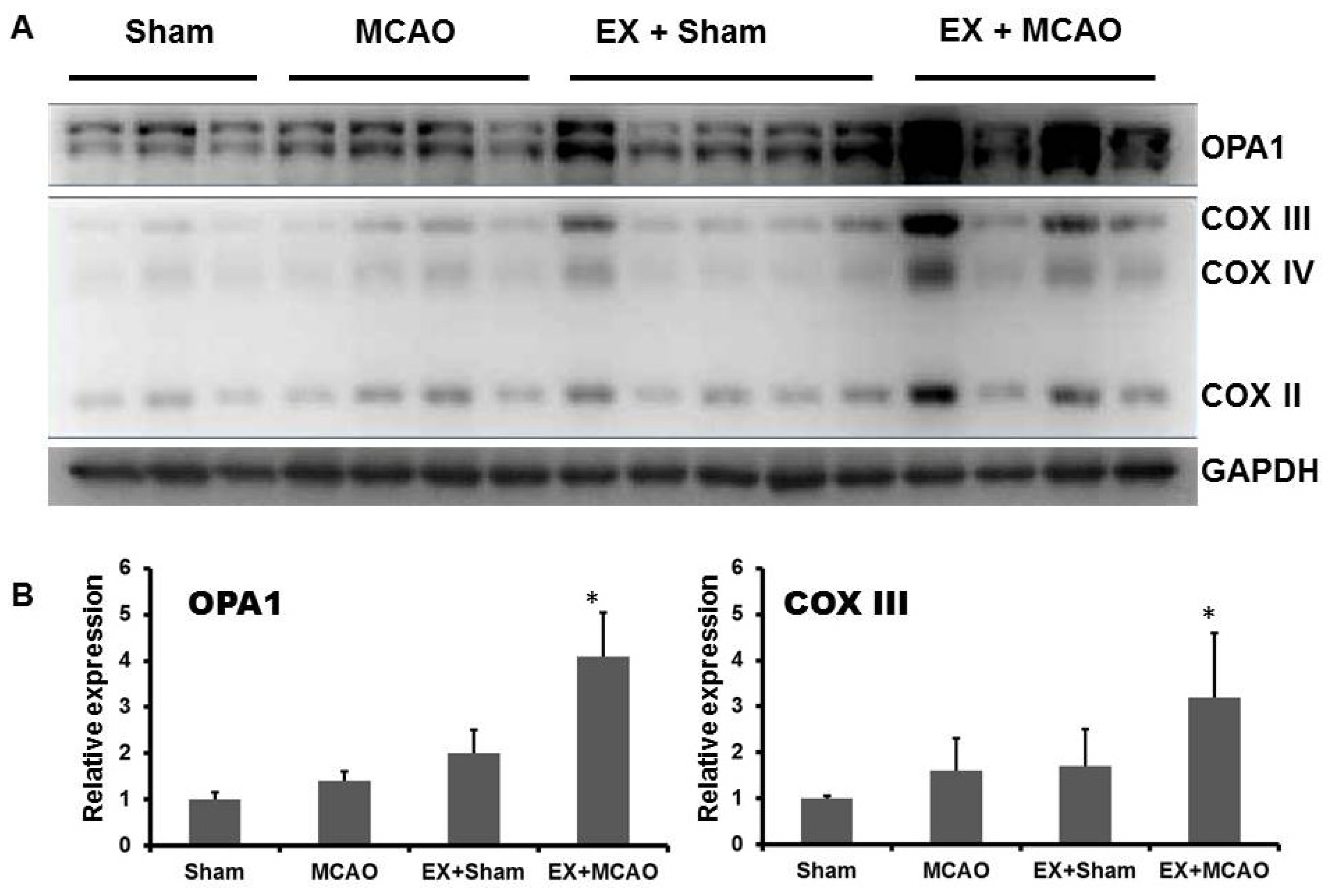
2.1.2. Exercise Up-Regulates Mitochondrial COX Subunits and Mitochondria-Fusion GTPase OPA1
2.1.3. The Effects of Exercise on Mitochondrial Dynamic Proteins in the MCAO Model
2.2. Discussion
3. Experimental Section
3.1. Animals
3.2. Exercise Pretreatment
3.3. Transient Cerebral Focal Ischemia (Transient Middle Cerebral Artery Occlusion, tMCAO)
3.4. Magnetic Resonance Imaging (MRI)
3.5. Western Blotting
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
- Author ContributionsDesign the study: Li Zhang, Zhijie He, Jie Jia; Perform the experiments: Li Zhang, Zhijie He, Qi Zhang, Xiaojiao Yang, Wenxiu Niu; Analysis the data: Li Zhang, Zhijie He, Yi Wu, Jie Jia; Write the manuscript: Li Zhang, Zhijie He, Yongshan Hu, Jie Jia.
References
- Goldstein, L.B.; Bushnell, C.D.; Adams, R.J.; Appel, L.J.; Braun, L.T.; Chaturvedi, S.; Creager, M.A.; Culebras, A.; Eckel, R.H.; Hart, R.G.; et al. Guidelines for the primary prevention of stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011, 42, 517–584. [Google Scholar]
- Furie, K.L.; Kasner, S.E.; Adams, R.J.; Albers, G.W.; Bush, R.L.; Fagan, S.C.; Halperin, J.L.; Johnston, S.C.; Katzan, I.; Kernan, W.N.; et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke 2011, 42, 227–276. [Google Scholar]
- Lee, C.D.; Folsom, A.R.; Blair, S.N. Physical activity and stroke risk: A meta-analysis. Stroke 2003, 34, 2475–2481. [Google Scholar]
- Hu, F.B.; Stampfer, M.J.; Colditz, G.A.; Ascherio, A.; Rexrode, K.M.; Willett, W.C.; Manson, J.E. Physical activity and risk of stroke in women. JAMA 2000, 283, 2961–2967. [Google Scholar]
- Lee, I.M.; Hennekens, C.H.; Berger, K.; Buring, J.E.; Manson, J.E. Exercise and risk of stroke in male physicians. Stroke 1999, 30, 1–6. [Google Scholar]
- Zhang, F.; Wu, Y.; Jia, J. Exercise preconditioning and brain ischemic tolerance. Neuroscience 2011, 177, 170–176. [Google Scholar]
- Ang, E.T.; Wong, P.T.; Moochhala, S.; Ng, Y.K. Neuroprotection associated with running: Is it a result of increased endogenous neurotrophic factors? Neuroscience 2003, 118, 335–345. [Google Scholar]
- DeBow, S.B.; Davies, M.L.; Clarke, H.L.; Colbourne, F. Constraint-induced movement therapy and rehabilitation exercises lessen motor deficits and volume of brain injury after striatal hemorrhagic stroke in rats. Stroke 2003, 34, 1021–1026. [Google Scholar]
- Marin, R.; Williams, A.; Hale, S.; Burge, B.; Mense, M.; Bauman, R.; Tortella, F. The effect of voluntary exercise exposure on histological and neurobehavioral outcomes after ischemic brain injury in the rat. Physiol. Behav. 2003, 80, 167–175. [Google Scholar]
- Zhang, Q.; Wu, Y.; Sha, H.; Zhang, P.; Jia, J.; Hu, Y.; Zhu, J. Early exercise affects mitochondrial transcription factors expression after cerebral ischemia in rats. Int. J. Mol. Sci. 2012, 13, 1670–1679. [Google Scholar]
- Zhang, Q.; Wu, Y.; Zhang, P.; Sha, H.; Jia, J.; Hu, Y.; Zhu, J. Exercise induces mitochondrial biogenesis after brain ischemia in rats. Neuroscience 2012, 205, 10–17. [Google Scholar]
- Goldenthal, M.J.; Marin-Garcia, J. Mitochondrial signaling pathways: A receiver/integrator organelle. Mol. Cell. Biochem. 2004, 262, 1–16. [Google Scholar]
- Abe, K.; Aoki, M.; Kawagoe, J.; Yoshida, T.; Hattori, A.; Kogure, K.; Itoyama, Y. Ischemic delayed neuronal death A mitochondrial hypothesis. Stroke 1995, 26, 1478–1489. [Google Scholar]
- Chan, D.C. Fusion and fission: Interlinked processes critical for mitochondrial health. Annu. Rev. Genet. 2012, 46, 265–287. [Google Scholar]
- Koshiba, T.; Detmer, S.A.; Kaiser, J.T.; Chen, H.; McCaffery, J.M.; Chan, D.C. Structural basis of mitochondrial tethering by mitofusin complexes. Science 2004, 305, 858–862. [Google Scholar]
- Song, Z.; Ghochani, M.; McCaffery, J.M.; Frey, T.G.; Chan, D.C. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol. Biol. Cell 2009, 20, 3525–3532. [Google Scholar]
- Otera, H.; Wang, C.; Cleland, M.M.; Setoguchi, K.; Yokota, S.; Youle, R.J.; Mihara, K. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J. Cell Biol. 2010, 191, 1141–1158. [Google Scholar]
- Yoon, Y.; Krueger, E.W.; Oswald, B.J.; McNiven, M.A. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol. Cell. Biol. 2003, 23, 5409–5420. [Google Scholar]
- Detmer, S.A.; Chan, D.C. Functions and dysfunctions of mitochondrial dynamics. Nat. Rev. Mol. Cell Biol. 2007, 8, 870–879. [Google Scholar]
- Itoh, K.; Nakamura, K.; Iijima, M.; Sesaki, H. Mitochondrial dynamics in neurodegeneration. Trends Cell Biol. 2013, 23, 64–71. [Google Scholar]
- Hayashi, T.; Abe, K. Ischemic neuronal cell death and organellae damage. Neurol. Res. 2004, 26, 827–834. [Google Scholar]
- He, Z.; Wang, X.; Wu, Y.; Jia, J.; Hu, Y.; Yang, X.; Li, J.; Fan, M.; Zhang, L.; Guo, J.; et al. Treadmill pre-training ameliorates brain edema in ischemic stroke via down-regulation of aquaporin-4: An MRI study in rats. PLoS One 2014, 9, e84602. [Google Scholar]
- Guo, M.; Lin, V.; Davis, W.; Huang, T.; Carranza, A.; Sprague, S.; Reyes, R.; Jimenez, D.; Ding, Y. Preischemic induction of TNF-alpha by physical exercise reduces blood-brain barrier dysfunction in stroke. J. Cereb. Blood Flow Metab. 2008, 28, 1422–1430. [Google Scholar]
- Guo, M.; Cox, B.; Mahale, S.; Davis, W.; Carranza, A.; Hayes, K.; Sprague, S.; Jimenez, D.; Ding, Y. Pre-ischemic exercise reduces matrix metalloproteinase-9 expression and ameliorates blood-brain barrier dysfunction in stroke. Neuroscience 2008, 151, 340–351. [Google Scholar]
- Jia, J.; Hu, Y.S.; Wu, Y.; Liu, G.; Yu, H.X.; Zheng, Q.P.; Zhu, D.N.; Xia, C.M.; Cao, Z.J. Pre-ischemic treadmill training affects glutamate and gamma aminobutyric acid levels in the striatal dialysate of a rat model of cerebral ischemia. Life Sci. 2009, 84, 505–511. [Google Scholar]
- Liu, W.; Tian, F.; Kurata, T.; Morimoto, N.; Abe, K. Dynamic changes of mitochondrial fusion and fission proteins after transient cerebral ischemia in mice. J. Neurosci. Res. 2012, 90, 1183–1189. [Google Scholar]
- Olichon, A.; Baricault, L.; Gas, N.; Guillou, E.; Valette, A.; Belenguer, P.; Lenaers, G. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity leading to cytochrome c release and apoptosis. J. Biol. Chem. 2003, 278, 7743–7746. [Google Scholar]
- Sarzi, E.; Angebault, C.; Seveno, M.; Gueguen, N.; Chaix, B.; Bielicki, G.; Boddaert, N.; Mausset-Bonnefont, A.L.; Cazevieille, C.; Rigau, V.; et al. The human OPA1delTTAG mutation induces premature age-related systemic neurodegeneration in mouse. Brain 2012, 135, 3599–3613. [Google Scholar]
- Grohm, J.; Kim, S.W.; Mamrak, U.; Tobaben, S.; Cassidy-Stone, A.; Nunnari, J.; Plesnila, N.; Culmsee, C. Inhibition of Drp1 provides neuroprotection in vitro and in vivo. Cell Death Differ. 2012, 19, 1446–1458. [Google Scholar]
- Neuspiel, M.; Zunino, R.; Gangaraju, S.; Rippstein, P.; McBride, H. Activated mitofusin 2 signals mitochondrial fusion interferes with Bax activation and reduces susceptibility to radical induced depolarization. J. Biol. Chem. 2005, 280, 25060–25070. [Google Scholar]
- Sugioka, R.; Shimizu, S.; Tsujimoto, Y. Fzo1 a protein involved in mitochondrial fusion inhibits apoptosis. J. Biol. Chem. 2004, 279, 52726–52734. [Google Scholar]
- Chan, D.C. Dissecting mitochondrial fusion. Dev. Cell 2006, 11, 592–594. [Google Scholar]
- Caffin, F.; Prola, A.; Piquereau, J.; Novotova, M.; David, D.J.; Garnier, A.; Fortin, D.; Alavi, M.V.; Veksler, V.; Ventura-Clapier, R.; et al. Altered skeletal muscle mitochondrial biogenesis but improved endurance capacity in trained OPA1-deficient mice. J. Physiol. 2013, 591, 6017–6037. [Google Scholar]
- Ding, H.; Jiang, N.; Liu, H.; Liu, X.; Liu, D.; Zhao, F.; Wen, L.; Liu, S.; Ji, L.L.; Zhang, Y. Response of mitochondrial fusion and fission protein gene expression to exercise in rat skeletal muscle. Biochim. Biophys. Acta 2010, 1800, 250–256. [Google Scholar]
- Meeusen, S.; DeVay, R.; Block, J.; Cassidy-Stone, A.; Wayson, S.; McCaffery, J.M.; Nunnari, J. Mitochondrial inner-membrane fusion and crista maintenance requires the dynamin-related GTPase Mgm1. Cell 2006, 127, 383–395. [Google Scholar]
- Yamaguchi, R.; Lartigue, L.; Perkins, G.; Scott, R.T.; Dixit, A.; Kushnareva, Y.; Kuwana, T.; Ellisman, M.H.; Newmeyer, D.D. Opa1-mediated cristae opening is Bax/Bak and BH3 dependent required for apoptosis and independent of Bak oligomerization. Mol. Cell 2008, 31, 557–569. [Google Scholar]
- Di Meo, S.; Venditti, P. Mitochondria in exercise-induced oxidative stress. Biol. Signals Recept. 2001, 10, 125–140. [Google Scholar]
- Yang, X.; He, Z.; Zhang, Q.; Wu, Y.; Hu, Y.; Wang, X.; Li, M.; Wu, Z.; Guo, Z.; Guo, J.; et al. Pre-Ischemic treadmill training for prevention of ischemic brain injury via regulation of glutamate and its transporter GLT-1. Int. J. Mol. Sci. 2012, 13, 9447–9459. [Google Scholar]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar]

© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, L.; He, Z.; Zhang, Q.; Wu, Y.; Yang, X.; Niu, W.; Hu, Y.; Jia, J. Exercise Pretreatment Promotes Mitochondrial Dynamic Protein OPA1 Expression after Cerebral Ischemia in Rats. Int. J. Mol. Sci. 2014, 15, 4453-4463. https://doi.org/10.3390/ijms15034453
Zhang L, He Z, Zhang Q, Wu Y, Yang X, Niu W, Hu Y, Jia J. Exercise Pretreatment Promotes Mitochondrial Dynamic Protein OPA1 Expression after Cerebral Ischemia in Rats. International Journal of Molecular Sciences. 2014; 15(3):4453-4463. https://doi.org/10.3390/ijms15034453
Chicago/Turabian StyleZhang, Li, Zhijie He, Qi Zhang, Yi Wu, Xiaojiao Yang, Wenxiu Niu, Yongshan Hu, and Jie Jia. 2014. "Exercise Pretreatment Promotes Mitochondrial Dynamic Protein OPA1 Expression after Cerebral Ischemia in Rats" International Journal of Molecular Sciences 15, no. 3: 4453-4463. https://doi.org/10.3390/ijms15034453
APA StyleZhang, L., He, Z., Zhang, Q., Wu, Y., Yang, X., Niu, W., Hu, Y., & Jia, J. (2014). Exercise Pretreatment Promotes Mitochondrial Dynamic Protein OPA1 Expression after Cerebral Ischemia in Rats. International Journal of Molecular Sciences, 15(3), 4453-4463. https://doi.org/10.3390/ijms15034453




