Influence of Interferon-Alpha Combined with Chemo (Radio) Therapy on Immunological Parameters in Pancreatic Adenocarcinoma
Abstract
:1. Introduction
2. Results
2.1. Human Study: Clinical Data
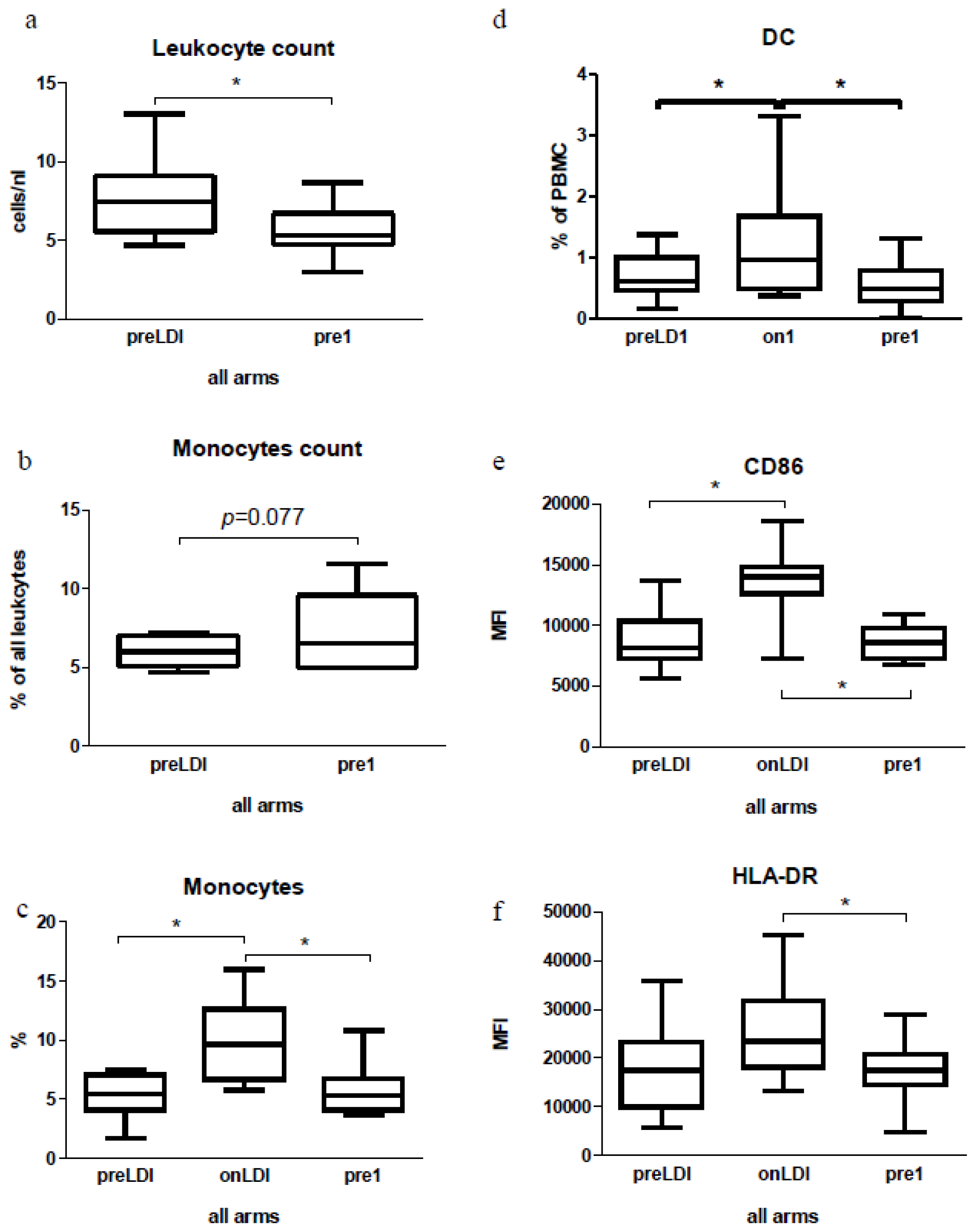
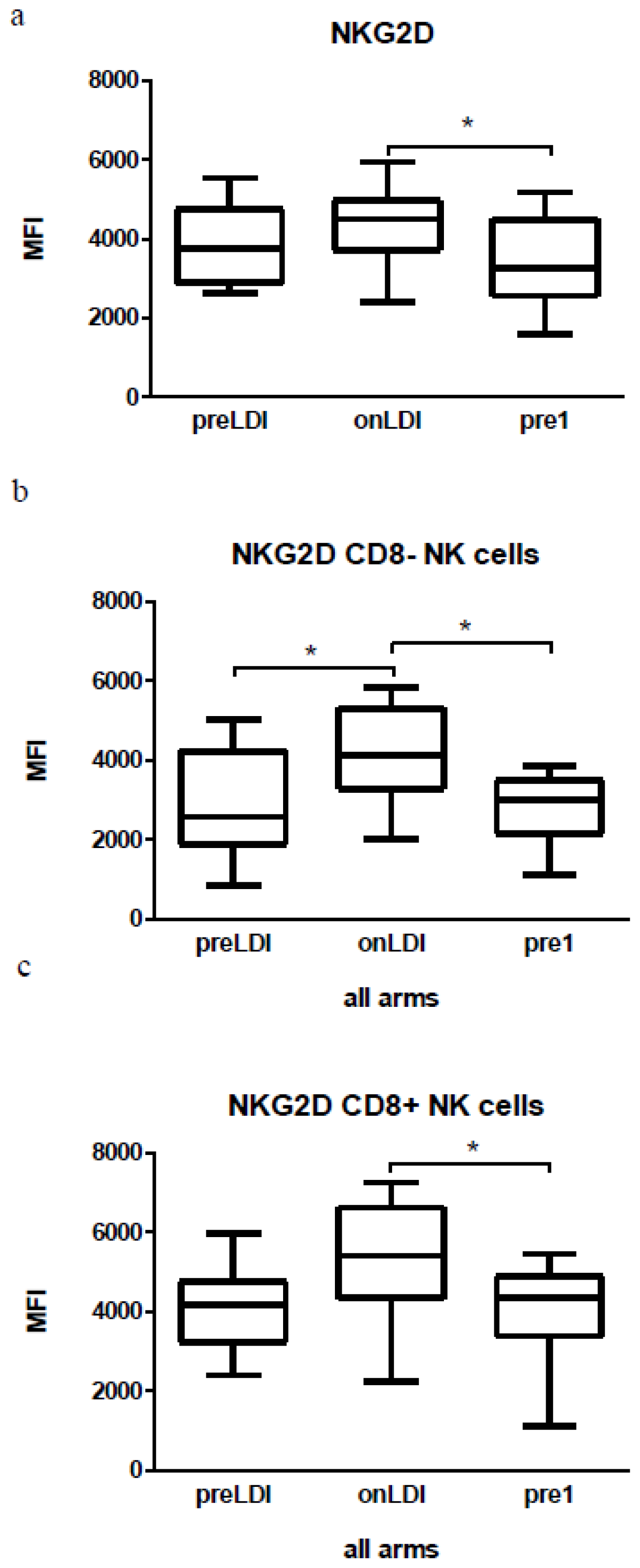
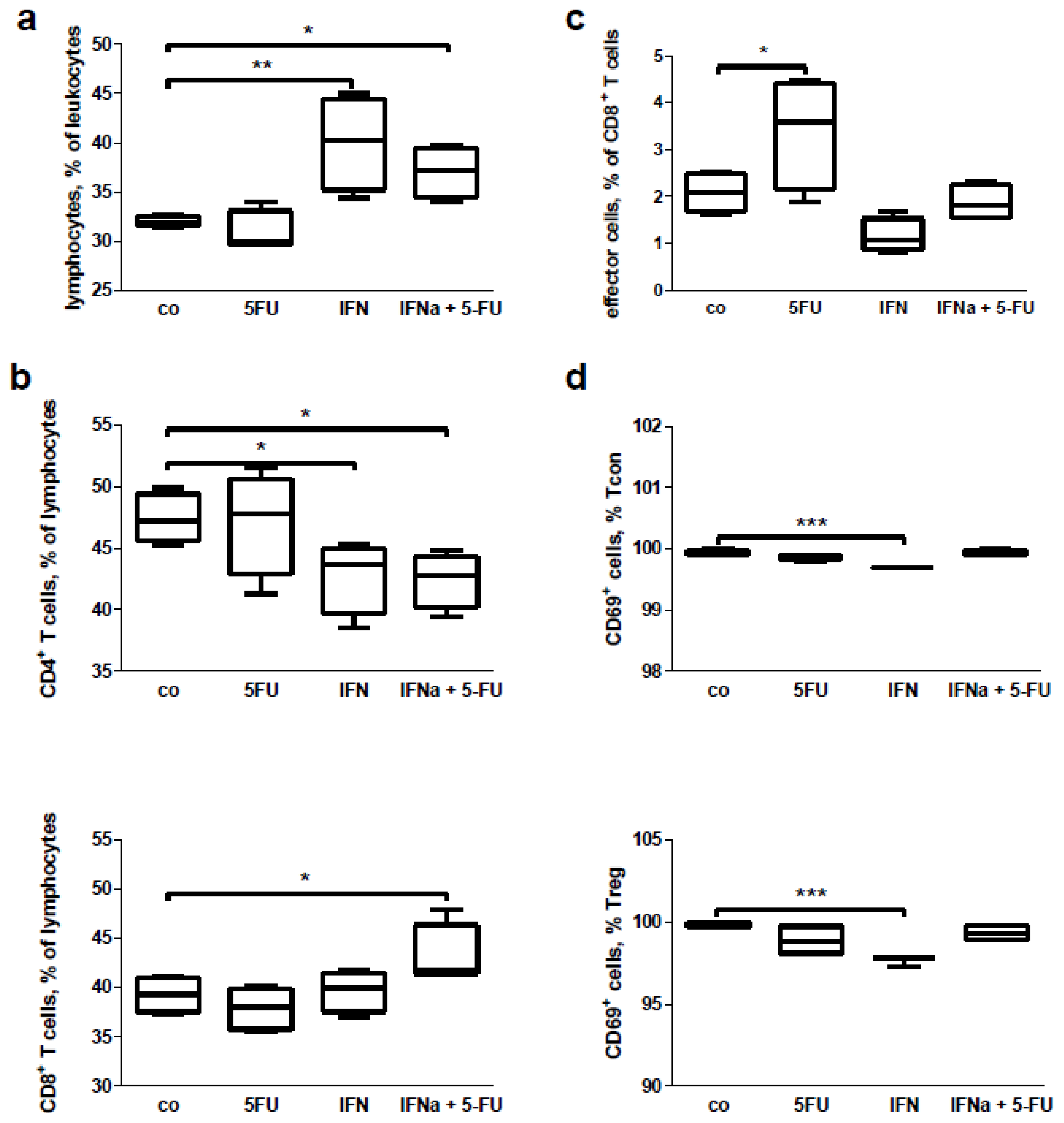
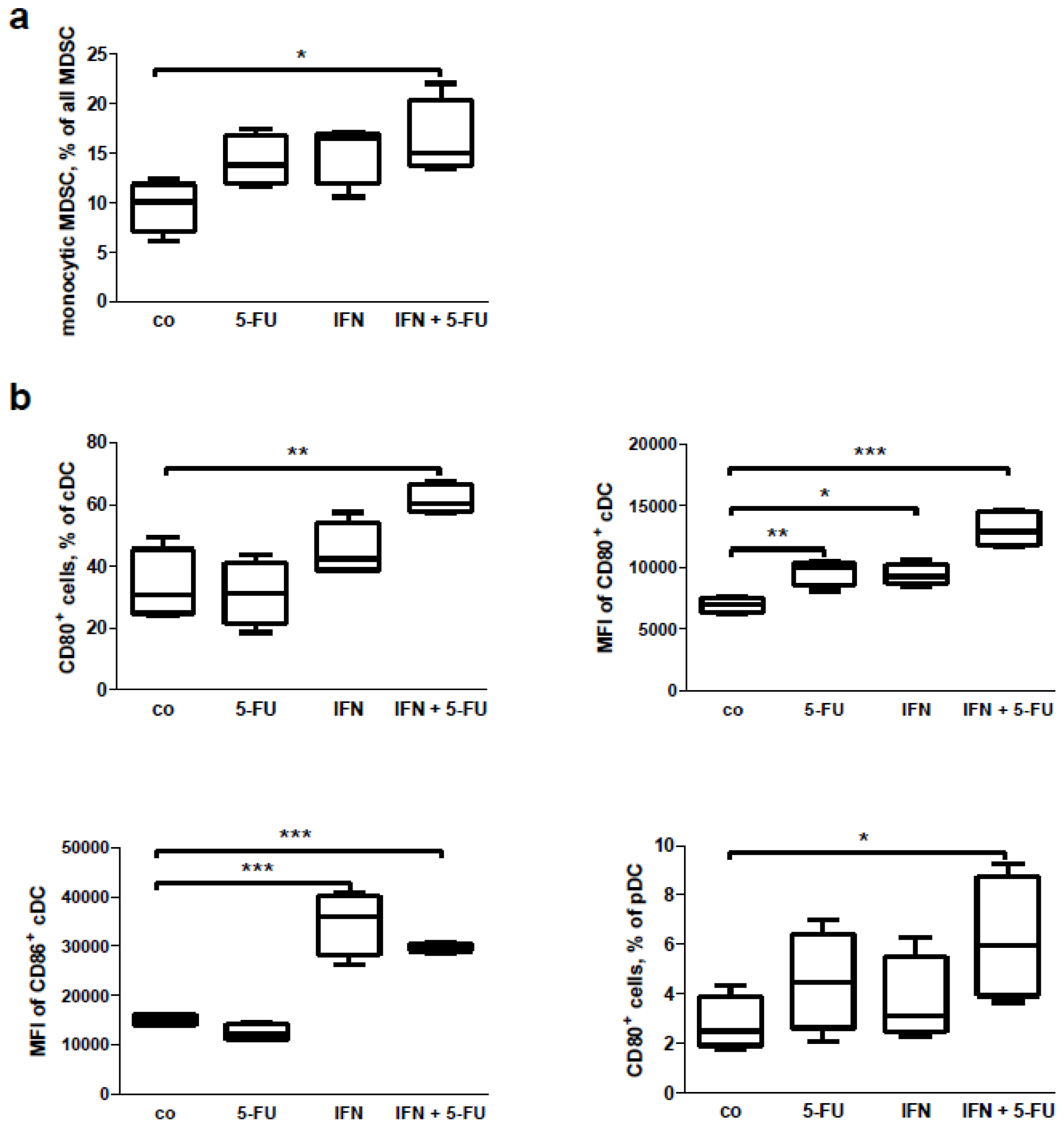
2.2. Human Study: Immunological Effects of IFN Only
2.3. Human Study: Effects of Chemo-Radio-Immunotherapy on Immunological Parameters
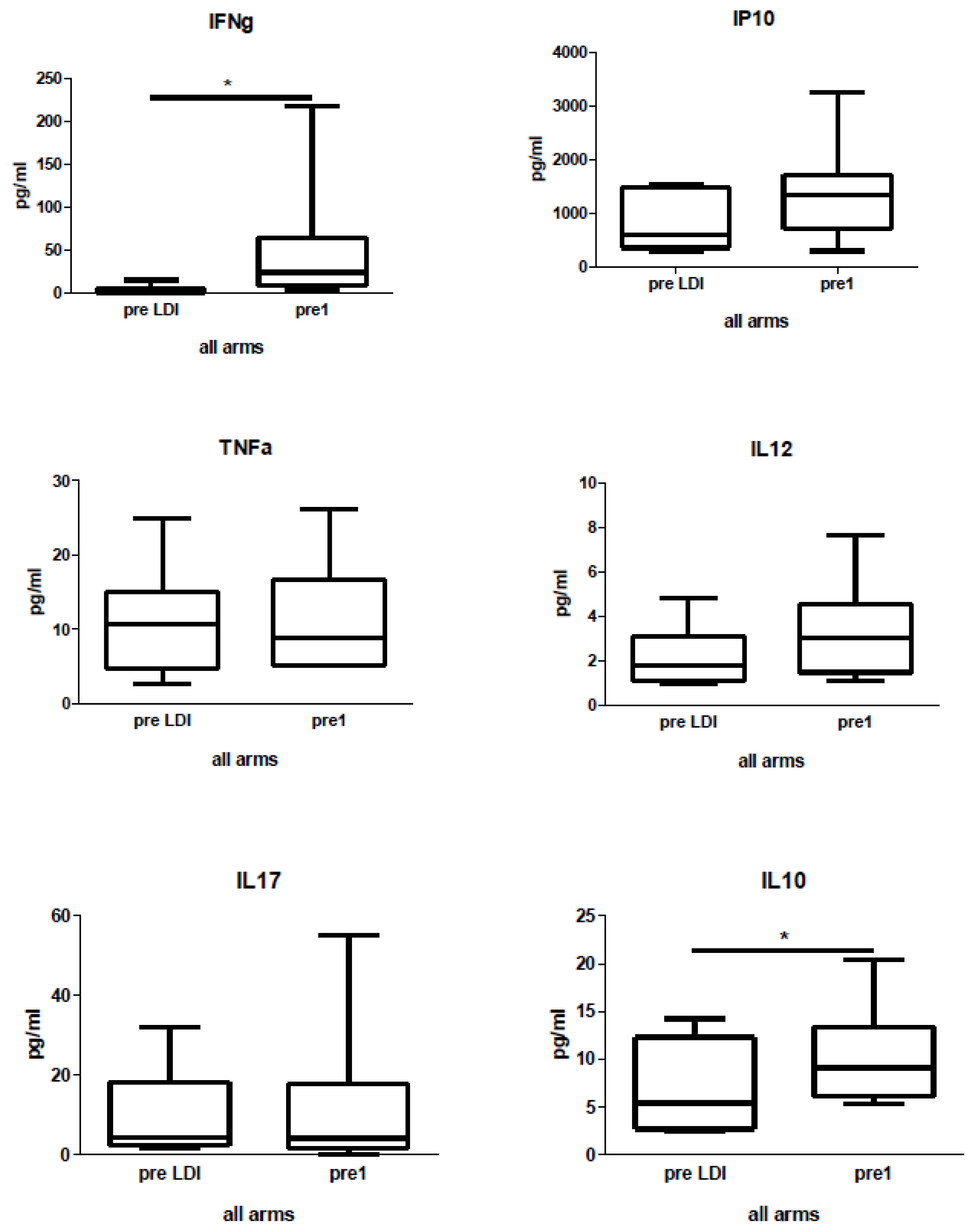
2.4. Human Study: Effects of Chemo-Radio-Immunotherapy on Serum Cytokines
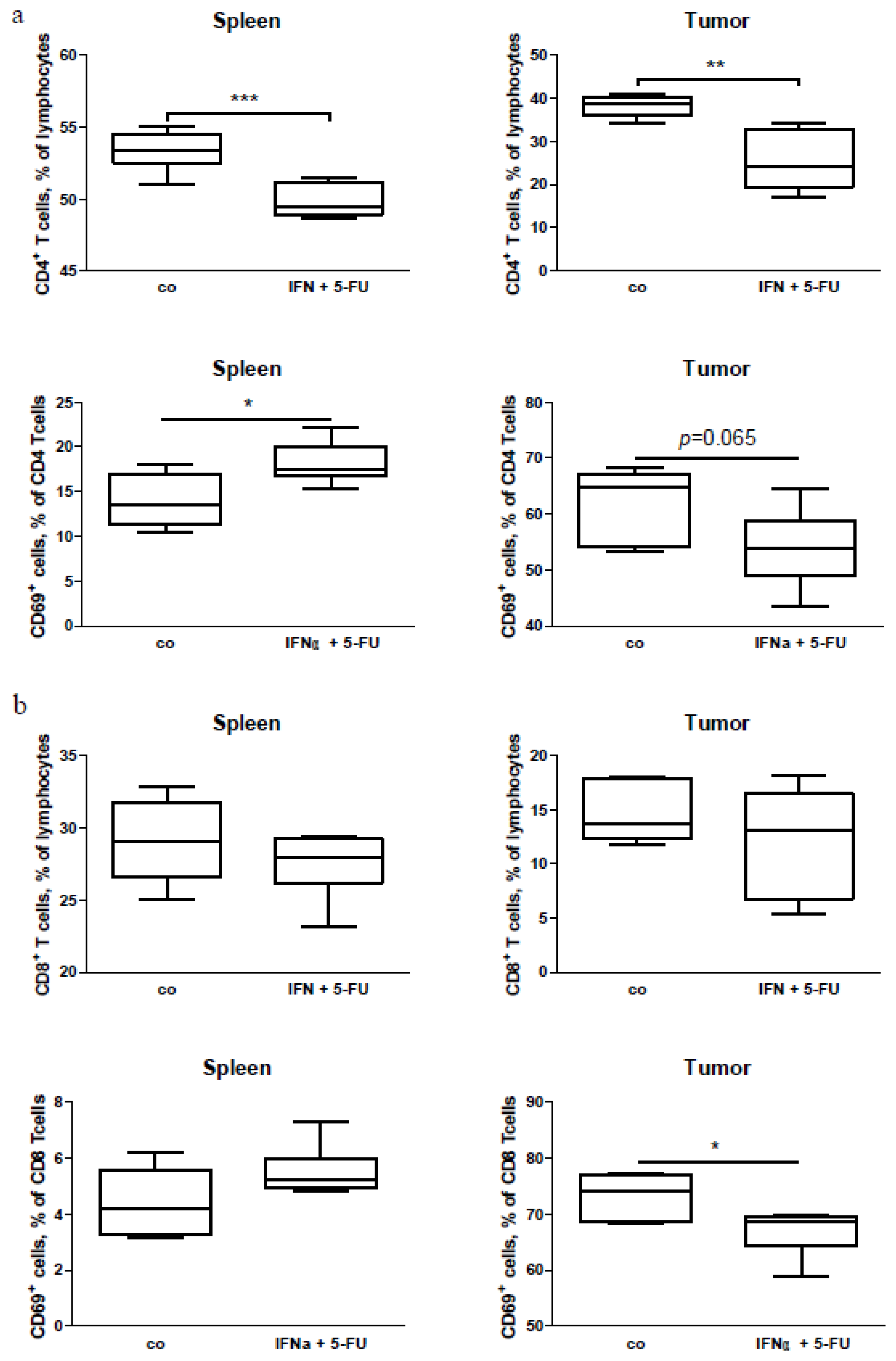
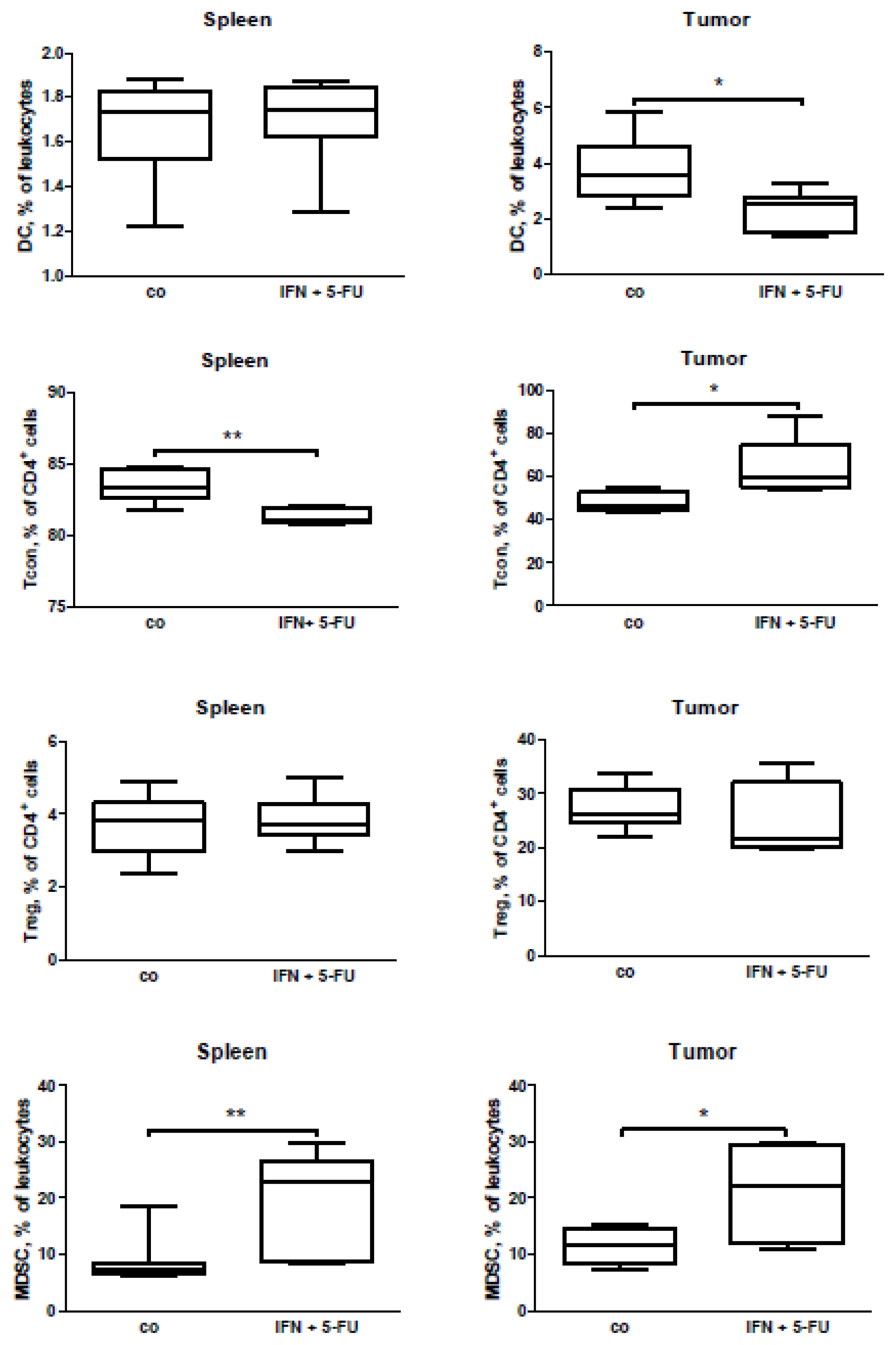
2.5. Mouse Study: In Vivo Effects of Chemo-Immunotherapy on Immunological Parameters
2.6. Mouse Study: In Vitro Effects of Chemo-Immunotherapy on Immunological Parameters
3. Discussion
4. Experimental Section
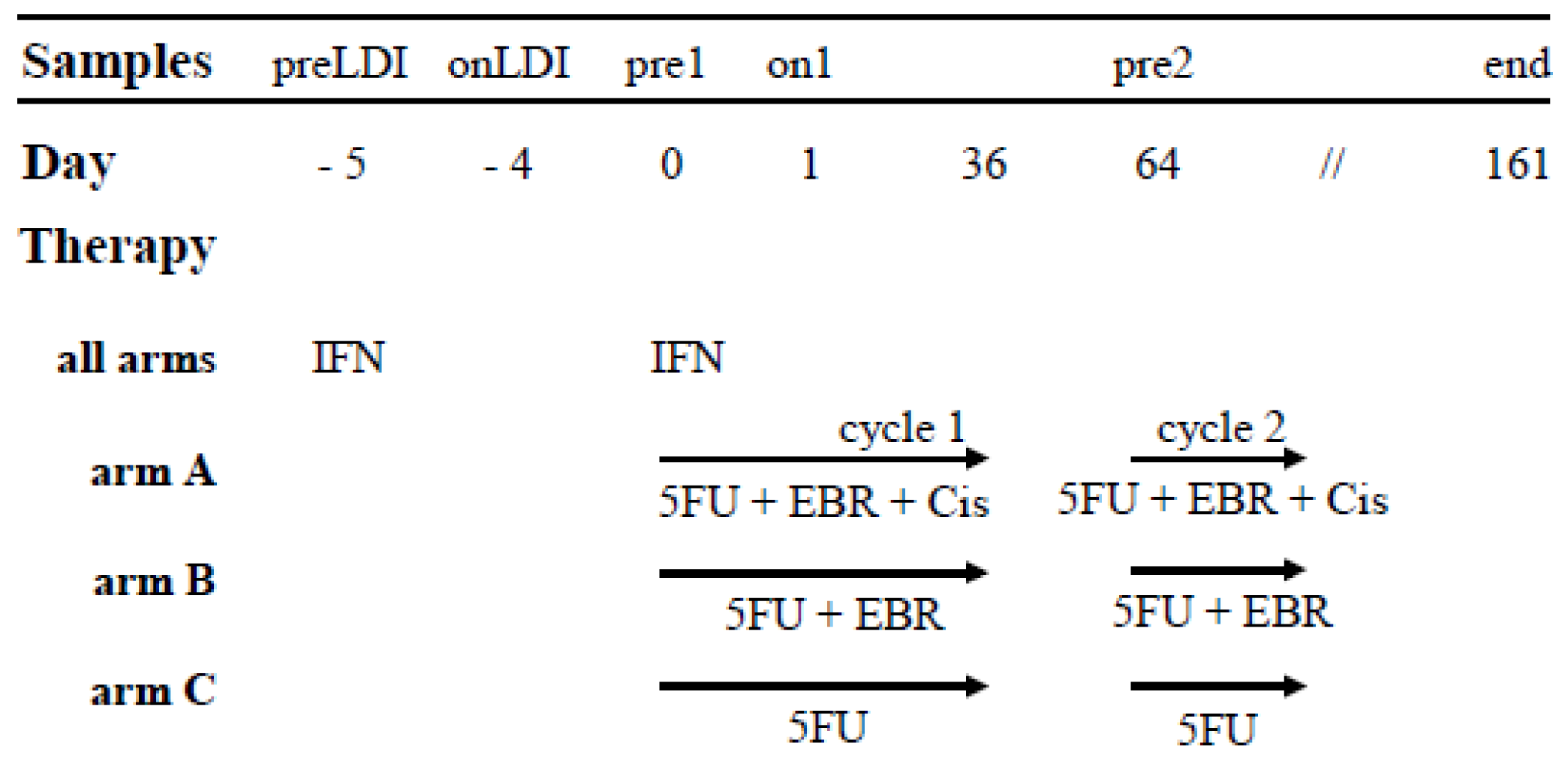
4.1. Patients Characteristics and Study Set up
4.2. Time Point of Analysis and Sample Preparation
4.3. Cell Culture
4.4. Mice
4.5. Orthotopic Mouse Model of Pancreatic Carcinoma
4.6. Flow Cell Biology Analysis of Human Blood Samples
4.7. Flow Cell Biology Analysis of Murine Splenocyte and Tumor Samples
4.8. In Vitro Treatment of Murine Splenocytes
4.9. Analysis of Human Cytokines with LUMINEX
4.10. Cytotoxicity Assay
4.11. ELIspot
4.12. Statistical Analysis
5. Conclusions
Supplementary Information
ijms-15-04104-s001.pdfAcknowledgments
Conflicts of Interest
References
- Jemal, A.; Tiwari, R.C.; Murray, T.; Ghafoor, A.; Samuels, A.; Ward, E.; Feuer, E.J.; Thun, M.J. Cancer statistics 2004. CA Cancer J. Clin. 2004, 54, 8–29. [Google Scholar]
- Neoptolemos, J.P.; Stocken, D.D.; Friess, H.; Bassi, C.; Dunn, J.A.; Hickey, H.; Beger, H.; Fernandez-Cruz, L.; Dervenis, C.; Lacaine, F.; et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N. Engl. J. Med. 2004, 350, 1200–1210. [Google Scholar]
- Oettle, H.; Post, S.; Neuhaus, P.; Gellert, K.; Langrehr, J.; Ridwelski, K.; Schramm, H.; Fahlke, J.; Zuelke, C.; Burkart, C.; et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: A randomized controlled trial. JAMA 2007, 297, 267–277. [Google Scholar]
- Neoptolemos, J.P.; Stocken, D.D.; Bassi, C.; Ghaneh, P.; Cunningham, D.; Goldstein, D.; Padbury, R.; Moore, M.J.; Gallinger, S.; Mariette, C.; et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: A randomized controlled trial. JAMA 2010, 304, 1073–1081. [Google Scholar]
- Dillman, R.O. Cancer immunotherapy. Cancer Biother. Radiopharm. 2011, 26, 1–64. [Google Scholar]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar]
- Lake, R.A.; Robinson, B.W. Immunotherapy and chemotherapy—A practical partnership. Nat. Rev. Cancer 2005, 5, 397–405. [Google Scholar]
- Knaebel, H.P.; Marten, A.; Schmidt, J.; Hoffmann, K.; Seiler, C.; Lindel, K.; Schmitz-Winnenthal, H.; Fritz, S.; Herrmann, T.; Goldschmidt, H.; et al. Phase III trial of postoperative cisplatin interferon alpha-2b and 5-FU combined with external radiation treatment vs 5-FU alone for patients with resected pancreatic adenocarcinoma—Capri: Study protocol [isrctn62866759]. BMC Cancer 2005, 5, 37. [Google Scholar]
- Atkins, M.B.; Hsu, J.; Lee, S.; Cohen, G.I.; Flaherty, L.E.; Sosman, J.A.; Sondak, V.K.; Kirkwood, J.M. Phase III trial comparing concurrent biochemotherapy with cisplatin vinblastine dacarbazine interleukin-2 and interferon alfa-2b with cisplatin vinblastine and dacarbazine alone in patients with metastatic malignant melanoma (e3695): A trial coordinated by the eastern cooperative oncology group. J. Clin. Oncol. 2008, 26, 5748–5754. [Google Scholar]
- Garbe, C.; Radny, P.; Linse, R.; Dummer, R.; Gutzmer, R.; Ulrich, J.; Stadler, R.; Weichenthal, M.; Eigentler, T.; Ellwanger, U.; et al. Adjuvant low-dose interferon {alpha}2a with or without dacarbazine compared with surgery alone: A prospective-randomized phase III decog trial in melanoma patients with regional lymph node metastasis. Ann. Oncol. 2008, 19, 1195–1201. [Google Scholar]
- Hauschild, A.; Weichenthal, M.; Rass, K.; Linse, R.; Ulrich, J.; Stadler, R.; Volkenandt, M.; Grabbe, S.; Proske, U.; Schadendorf, D.; et al. Prospective randomized multicenter adjuvant dermatologic cooperative oncology group trial of low-dose interferon alfa-2b with or without a modified high-dose interferon alfa-2b induction phase in patients with lymph node-negative melanoma. J. Clin. Oncol. 2009, 27, 3496–3502. [Google Scholar]
- Hudes, G.; Carducci, M.; Tomczak, P.; Dutcher, J.; Figlin, R.; Kapoor, A.; Staroslawska, E.; Sosman, J.; McDermott, D.; Bodrogi, I.; et al. Temsirolimus interferon alfa or both for advanced renal-cell carcinoma. N. Engl. J. Med. 2007, 356, 2271–2281. [Google Scholar]
- Iacopino, F.; Ferrandina, G.; Scambia, G.; Benedetti-Panici, P.; Mancuso, S.; Sica, G. Interferons inhibit egf-stimulated cell growth and reduce egf binding in human breast cancer cells. Anticancer Res. 1996, 16, 1919–1924. [Google Scholar]
- Vitale, G.; van Eijck, C.H.; van Koetsveld Ing, P.M.; Erdmann, J.I.; Speel, E.J.; van der Wansem Ing, K.; Mooij, D.M.; Colao, A.; Lombardi, G.; Croze, E.; et al. Type I interferons in the treatment of pancreatic cancer: Mechanisms of action and role of related receptors. Ann. Surg. 2007, 246, 259–268. [Google Scholar]
- Caraglia, M.; Marra, M.; Tagliaferri, P.; Lamberts, S.W.; Zappavigna, S.; Misso, G.; Cavagnini, F.; Facchini, G.; Abbruzzese, A.; Hofland, L.J.; et al. Emerging strategies to strengthen the anti-tumour activity of type I interferons: Overcoming survival pathways. Curr. Cancer Drug Targets 2009, 9, 690–704. [Google Scholar]
- Vitale, G.; Zappavigna, S.; Marra, M.; Dicitore, A.; Meschini, S.; Condello, M.; Arancia, G.; Castiglioni, S.; Maroni, P.; Bendinelli, P.; et al. The ppar-gamma agonist troglitazone antagonizes survival pathways induced by stat-3 in recombinant interferon-beta treated pancreatic cancer cells. Biotechnol. Adv. 2012, 30, 169–184. [Google Scholar]
- Dicitore, A.; Caraglia, M.; Gaudenzi, G.; Manfredi, G.; Amato, B.; Mari, D.; Persani, L.; Arra, C.; Vitale, G. Type I interferon-mediated pathway interacts with peroxisome proliferator activated receptor-gamma (ppar-gamma): At the cross-road of pancreatic cancer cell proliferation. Biochim. Biophys. Acta 2013, 1845, 42–52. [Google Scholar]
- Hoffmann, K.; Mehrle, S.; Schmidt, J.; Buchler, M.W.; Marten, A. Interferon-alpha restitutes the chemosensitivity in pancreatic cancer. Anticancer Res. 2008, 28, 1499–1507. [Google Scholar]
- Solorzano, C.C.; Hwang, R.; Baker, C.H.; Bucana, C.D.; Pisters, P.W.; Evans, D.B.; Killion, J.J.; Fidler, I.J. Administration of optimal biological dose and schedule of interferon alpha combined with gemcitabine induces apoptosis in tumor-associated endothelial cells and reduces growth of human pancreatic carcinoma implanted orthotopically in nude mice. Clin. Cancer Res. 2003, 9, 1858–1867. [Google Scholar]
- Pfeffer, L.M.; Dinarello, C.A.; Herberman, R.B.; Williams, B.R.; Borden, E.C.; Bordens, R.; Walter, M.R.; Nagabhushan, T.L.; Trotta, P.P.; Pestka, S. Biological properties of recombinant alpha-interferons: 40th anniversary of the discovery of interferons. Cancer Res. 1998, 58, 2489–2499. [Google Scholar]
- Schmidt, J.; Abel, U.; Debus, J.; Harig, S.; Hoffmann, K.; Herrmann, T.; Bartsch, D.; Klein, J.; Mansmann, U.; Jager, D.; et al. Open-label multicenter randomized phase iii trial of adjuvant chemoradiation plus interferon alfa-2b vs fluorouracil and folinic acid for patients with resected pancreatic adenocarcinoma. J. Clin. Oncol. 2012, 30, 4077–4083. [Google Scholar]
- Marten, A.; Schmidt, J.; Ose, J.; Harig, S.; Abel, U.; Munter, M.W.; Jager, D.; Friess, H.; Mayerle, J.; Adler, G.; et al. A randomized multicentre phase ii trial comparing adjuvant therapy in patients with interferon alpha-2b and 5-FU alone or in combination with either external radiation treatment and cisplatin (capri) or radiation alone regarding event-free survival—Capri-2. BMC Cancer 2009, 9, 160. [Google Scholar]
- Esposito, I.; Kleeff, J.; Bergmann, F.; Reiser, C.; Herpel, E.; Friess, H.; Schirmacher, P.; Buchler, M.W. Most pancreatic cancer resections are r1 resections. Ann. Surg. Oncol. 2008, 15, 1651–1660. [Google Scholar]
- Corssmit, E.P.; Heijligenberg, R.; Hack, C.E.; Endert, E.; Sauerwein, H.P.; Romijn, J.A. Effects of interferon-alpha (ifn-alpha) administration on leucocytes in healthy humans. Clin. Exp. Immunol. 1997, 107, 359–363. [Google Scholar]
- Colombatto, P.; Oliveri, F.; Leandro, G.; Baldi, M.; Capalbo, M.; Rocca, G.; Brunetto, M.R.; Bonino, F. Platelet and white blood cell counts during therapy with different types of alpha interferon in patients with chronic viral hepatitis Investigators of the alpha interferon study group of piemonte Italy. Ital. J. Gastroenterol. Hepatol. 1997, 29, 441–447. [Google Scholar]
- Toccaceli, F.; Rosati, S.; Scuderi, M.; Iacomi, F.; Picconi, R.; Laghi, V. Leukocyte and platelet lowering by some interferon types during viral hepatitis treatment. Hepatogastroenterology 1998, 45, 1748–1752. [Google Scholar]
- Bellone, G.; Novarino, A.; Vizio, B.; Brondino, G.; Addeo, A.; Prati, A.; Giacobino, A.; Campra, D.; Fronda, G.R.; Ciuffreda, L. Impact of surgery and chemotherapy on cellular immunity in pancreatic carcinoma patients in view of an integration of standard cancer treatment with immunotherapy. Int. J. Oncol. 2009, 34, 1701–1715. [Google Scholar]
- Luft, T.; Pang, K.C.; Thomas, E.; Hertzog, P.; Hart, D.N.; Trapani, J.; Cebon, J. Type I ifns enhance the terminal differentiation of dendritic cells. J. Immunol. 1998, 161, 1947–1953. [Google Scholar]
- Gallucci, S.; Lolkema, M.; Matzinger, P. Natural adjuvants: Endogenous activators of dendritic cells. Nat. Med. 1999, 5, 1249–1255. [Google Scholar]
- Hervas-Stubbs, S.; Perez-Gracia, J.L.; Rouzaut, A.; Sanmamed, M.F.; Le Bon, A.; Melero, I. Direct effects of type I interferons on cells of the immune system. Clin. Cancer Res. 2011, 17, 2619–2627. [Google Scholar]
- Zamai, L.; Ponti, C.; Mirandola, P.; Gobbi, G.; Papa, S.; Galeotti, L.; Cocco, L.; Vitale, M. NK cells and cancer. J. Immunol. 2007, 178, 4011–4016. [Google Scholar]
- De Gast, G.C.; Klumpen, H.J.; Vyth-Dreese, F.A.; Kersten, M.J.; Verra, N.C.; Sein, J.; Batchelor, D.; Nooijen, W.J.; Schornagel, J.H. Phase I trial of combined immunotherapy with subcutaneous granulocyte macrophage colony-stimulating factor low-dose interleukin 2 and interferon alpha in progressive metastatic melanoma and renal cell carcinoma. Clin. Cancer Res. 2000, 6, 1267–1272. [Google Scholar]
- Naing, A.; Reuben, J.M.; Camacho, L.H.; Gao, H.; Lee, B.N.; Cohen, E.N.; Verschraegen, C.; Stephen, S.; Aaron, J.; Hong, D.; et al. Phase I dose escalation study of sodium stibogluconate (ssg) a protein tyrosine phosphatase inhibitor combined with interferon alpha for patients with solid tumors. J. Cancer 2011, 2, 81–89. [Google Scholar]
- Ernstoff, M.S.; Fusi, S.; Kirkwood, J.M. Parameters of interferon action: II Immunological effects of recombinant leukocyte interferon (ifn-alpha 2) in phase I-II trials. J. Biol. Response Mod. 1983, 2, 540–547. [Google Scholar]
- Bekisz, J.; Sato, Y.; Johnson, C.; Husain, S.R.; Puri, R.K.; Zoon, K.C. Immunomodulatory effects of interferons in malignancies. J. Interferon Cytokine Res. 2013, 33, 154–161. [Google Scholar]
- Ng, T.H.; Britton, G.J.; Hill, E.V.; Verhagen, J.; Burton, B.R.; Wraith, D.C. Regulation of adaptive immunity; The role of interleukin-10. Front. Immunol. 2013, 4, 129. [Google Scholar]
- Rousset, F.; Garcia, E.; Defrance, T.; Peronne, C.; Vezzio, N.; Hsu, D.H.; Kastelein, R.; Moore, K.W.; Banchereau, J. Interleukin 10 is a potent growth and differentiation factor for activated human b lymphocytes. Proc. Natl. Acad. Sci. USA 1992, 89, 1890–1893. [Google Scholar]
- Tatsugami, K.; Eto, M.; Naito, S. Influence of immunotherapy with interferon-alpha on regulatory t cells in renal cell carcinoma patients. J. Interferon Cytokine Res. 2010, 30, 43–48. [Google Scholar]
- Tarhini, A.A.; Butterfield, L.H.; Shuai, Y.; Gooding, W.E.; Kalinski, P.; Kirkwood, J.M. Differing patterns of circulating regulatory T cells and myeloid-derived suppressor cells in metastatic melanoma patients receiving anti-ctla4 antibody and interferon-alpha or tlr-9 agonist and gm-csf with peptide vaccination. J. Immunother. 2012, 35, 702–710. [Google Scholar]
- Shevchenko, I.; Karakhanova, S.; Soltek, S.; Link, J.; Bayry, J.; Werner, J.; Umansky, V.; Bazhin, A.V. Low-dose gemcitabine depletes regulatory T cells and improves survival in the orthotopic panc02 model of pancreatic cancer. Int. J. Cancer 2013, 133, 98–107. [Google Scholar]
- Bazhin, A.V.; Bayry, J.; Umansky, V.; Werner, J.; Karakhanova, S. Overcoming immunosuppression as a new immunotherapeutic approach against pancreatic cancer. Oncoimmunology 2013, 2, e25736. [Google Scholar]
- Bazhin, A.V.; Shevchenko, I.; Umansky, V.; Werner, J.; Karakhanova, S. Two immune faces of pancreatic adenocarcinoma: Possible implication for immunotherapy. Cancer Immunol. Immunother. 2014, 63, 59–65. [Google Scholar]
- Corbett, T.H.; Roberts, B.J.; Leopold, W.R.; Peckham, J.C.; Wilkoff, L.J.; Griswold, D.P., Jr; Schabel, F.M., Jr. Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in c57bl/6 mice. Cancer Res. 1984, 44, 717–726. [Google Scholar]
- Schmidt, J.; Jager, D.; Hoffmann, K.; Buchler, M.W.; Marten, A. Impact of interferon-alpha in combined chemoradioimmunotherapy for pancreatic adenocarcinoma (capri): First data from the immunomonitoring. J. Immunother. 2007, 30, 108–115. [Google Scholar]
- Yang, Y.; Karakhanova, S.; Soltek, S.; Werner, J.; Philippov, P.P.; Bazhin, A.V. In vivo immunoregulatory properties of the novel mitochondria-targeted antioxidant skq1. Mol. Immunol. 2012, 52, 19–29. [Google Scholar]
- GraphPad Prism Software; version 5.01. Available Online: http://graphpad.com/scientific-software/prism/ (accessed on 21 February 2014).
| Arm/Treatment | Patient | Gender | Age | Resection Type | Tumor Grade | TNM | Relapse/Location | DFS *, Month | Status | OS **, Month |
|---|---|---|---|---|---|---|---|---|---|---|
| A/ | A1 | M | 52 | R1 | G2 | pT3, pN1 (7/32), pMx | Liver | 15 | Dead | 16 |
| IFN | A2 | F | 47 | R1 | G2 | pT3, pN1 (2/24), pMx | 0 | 20 | Alive | 20 |
| 5FU | A3 | M | 58 | R1 | G2 | pT3, pN1(8/49), pMx | Liver | 4 | Dead | 12 |
| EBR | A4 | M | 50 | R1 | G2 | pT2, pN1(1/6), M0 | Peritonealcarcinosis | 18 | Dead | 23 |
| Cis | A5 | M | 67 | R1 | G2 | pT3, pN1(1/19), M0 | 0 | 51 | Alive | 51 |
| B/ | B1 | M | 64 | R1 | G2 | pT3, pN1 (6/22), pMx | Liver | 3 | Alive | 63 |
| IFN | B2 | F | 69 | R0 | G2 | pT3, pN1 (1/37), pMx | 0 | 63 | Alive | 63 |
| 5FU | B3 | F | 60 | R1 | G2 | pT3, pN1 (3/28), pMx | Liver | 15 | Dead | 21 |
| EBR | B4 | M | 65 | R0 | n.d. *** | pT3 pN1 (1/17), cM0 | 0 | 53 | Alive | 53 |
| B5 | M | 54 | R1 | G2 | pT3 pN1 (18/58), cM0 | Peritonealcarcinosis | 17 | Dead | 48 | |
| B6 | M | 62 | R1 | G3 | pT3, pN1(6(35), pMx | Peritonealcarcinosis | 4 | Dead | 7 | |
| C/ | C1 | F | 67 | R1 | G2 | pT3, pN1(3/24), M0 | Liver | 27 | Dead | 48 |
| IFN | C2 | M | 63 | R1 | G2 | pT3, pN1 (10/29), Mx | 0 | 47 | Alive | 47 |
| 5FU | C3 | M | 59 | R1 | G2 | pT3, pN1 (5/29), pMx | Liver | 12 | Dead | 20 |
| C4 | F | 69 | R1 | G2 | pT3, pN1(5/21), pMx | Liver | 3 | Dead | 7 | |
| C5 | M | 62 | R1 | G2 | pT3 pN1 (13/27), M0 | Liver | 19 | Dead | 38 | |
| C6 | F | 65 | R1 | G3 | pT3, pN0 (0/43), M0 | Peritonealcarcinosis | 4 | Dead | 7 | |
| CD4+ Cells | MDSC | ||||
|---|---|---|---|---|---|
| r | p | r | p | ||
| Tumor growth | co | −0.94 | 0.017 | 0.83 | 0.058 |
| IFN | −0.7 | 0.233 | 0.54 | 0.297 | |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Karakhanova, S.; Mosl, B.; Harig, S.; Ahn, K.V.; Fritz, J.; Schmidt, J.; Jäger, D.; Werner, J.; Bazhin, A.V. Influence of Interferon-Alpha Combined with Chemo (Radio) Therapy on Immunological Parameters in Pancreatic Adenocarcinoma. Int. J. Mol. Sci. 2014, 15, 4104-4125. https://doi.org/10.3390/ijms15034104
Karakhanova S, Mosl B, Harig S, Ahn KV, Fritz J, Schmidt J, Jäger D, Werner J, Bazhin AV. Influence of Interferon-Alpha Combined with Chemo (Radio) Therapy on Immunological Parameters in Pancreatic Adenocarcinoma. International Journal of Molecular Sciences. 2014; 15(3):4104-4125. https://doi.org/10.3390/ijms15034104
Chicago/Turabian StyleKarakhanova, Svetlana, Beate Mosl, Sabine Harig, Katharina Von Ahn, Jasmin Fritz, Jan Schmidt, Dirk Jäger, Jens Werner, and Alexandr V. Bazhin. 2014. "Influence of Interferon-Alpha Combined with Chemo (Radio) Therapy on Immunological Parameters in Pancreatic Adenocarcinoma" International Journal of Molecular Sciences 15, no. 3: 4104-4125. https://doi.org/10.3390/ijms15034104
APA StyleKarakhanova, S., Mosl, B., Harig, S., Ahn, K. V., Fritz, J., Schmidt, J., Jäger, D., Werner, J., & Bazhin, A. V. (2014). Influence of Interferon-Alpha Combined with Chemo (Radio) Therapy on Immunological Parameters in Pancreatic Adenocarcinoma. International Journal of Molecular Sciences, 15(3), 4104-4125. https://doi.org/10.3390/ijms15034104




