The Sesquiterpene Biosynthesis and Vessel-Occlusion Formation in Stems of Aquilaria sinensis (Lour.) Gilg Trees Induced by Wounding Treatments without Variation of Microbial Communities
Abstract
:1. Introduction
2. Results
2.1. Fungal Diversity Did not Vary in the Stem of A. sinensis Treated by BCD and Agar-Wit Methods
2.2. Bacterial Diversity Did not Vary in the Stem of A. sinensis Treated by BCD and Agar-Wit Methods
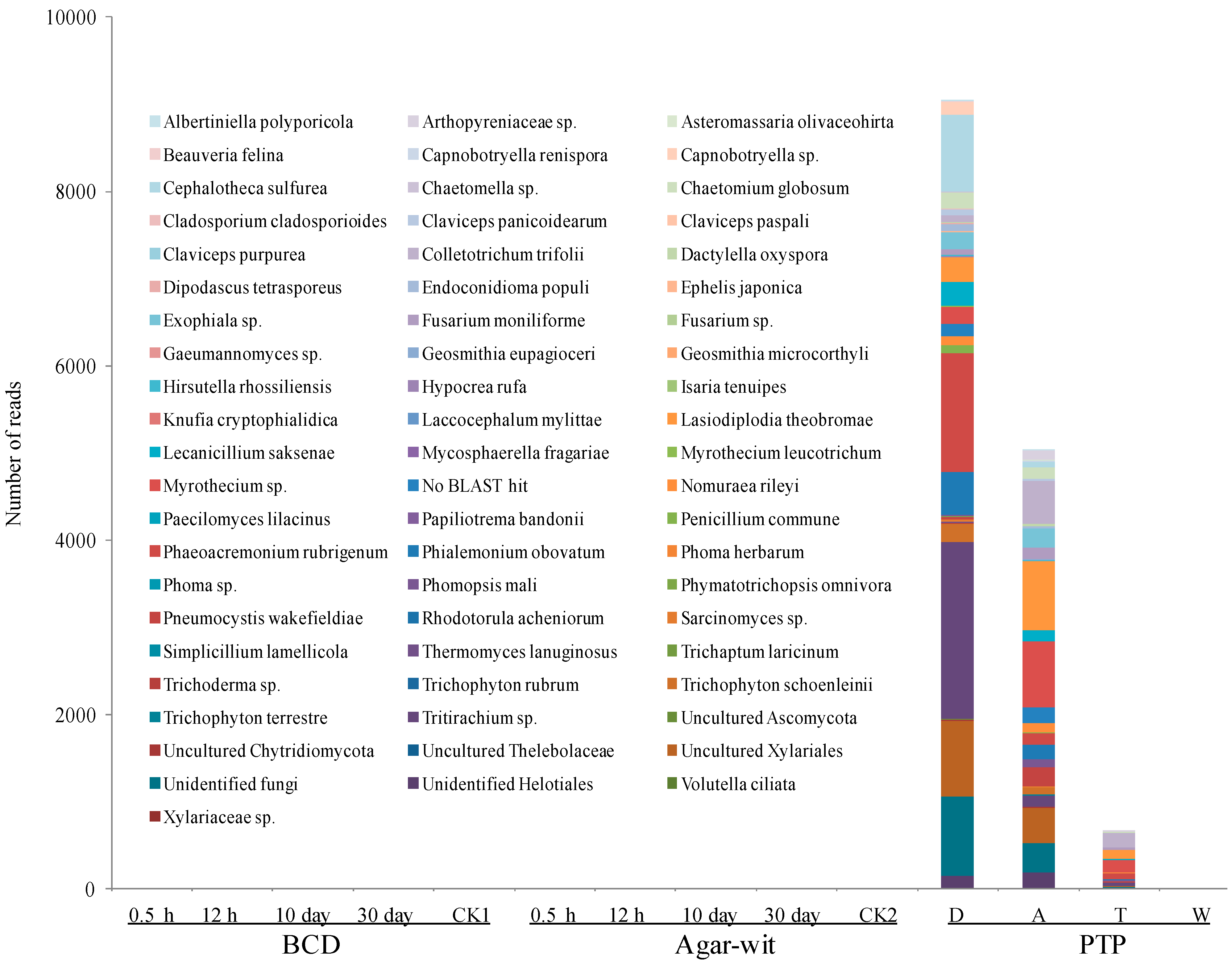

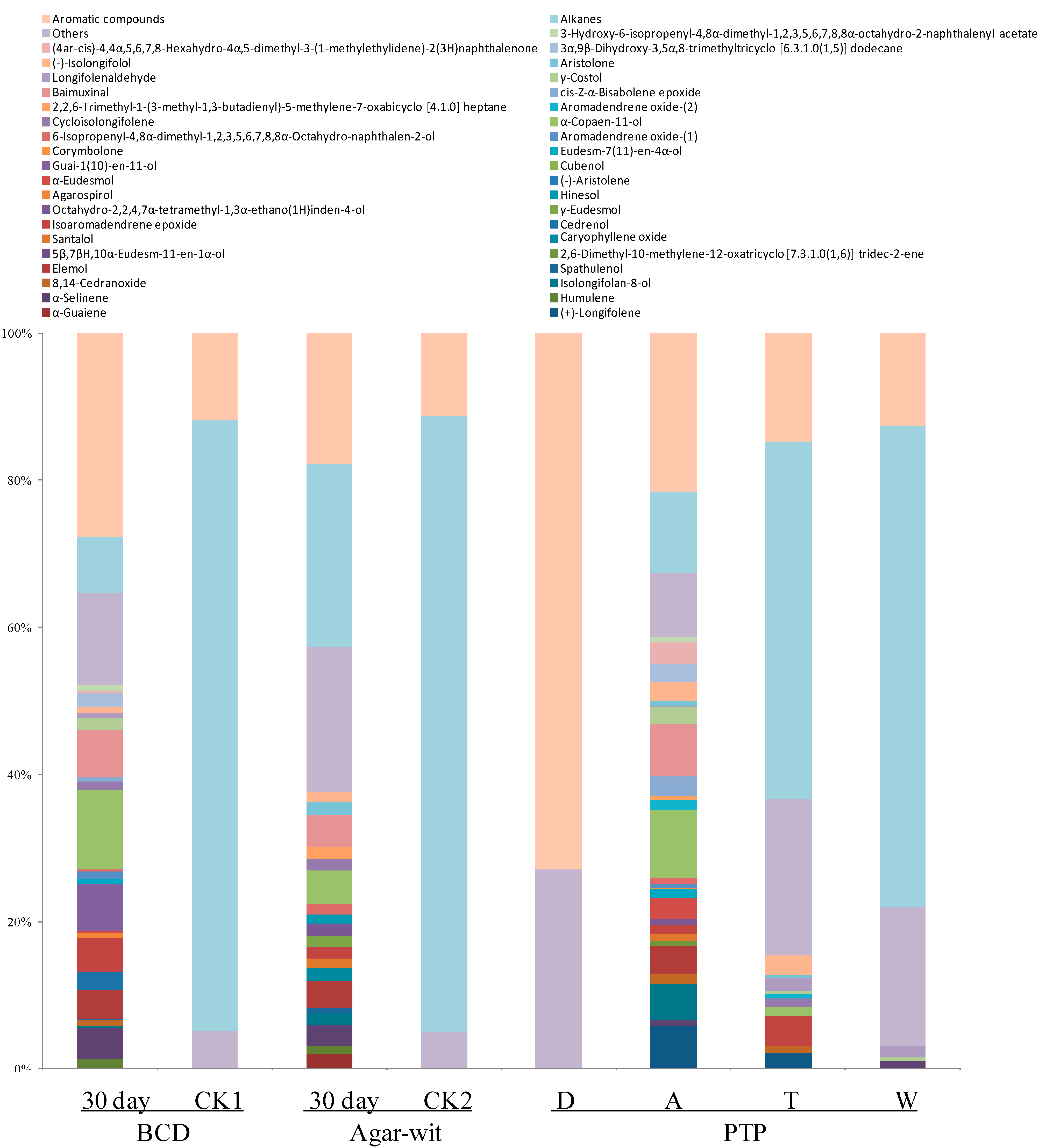
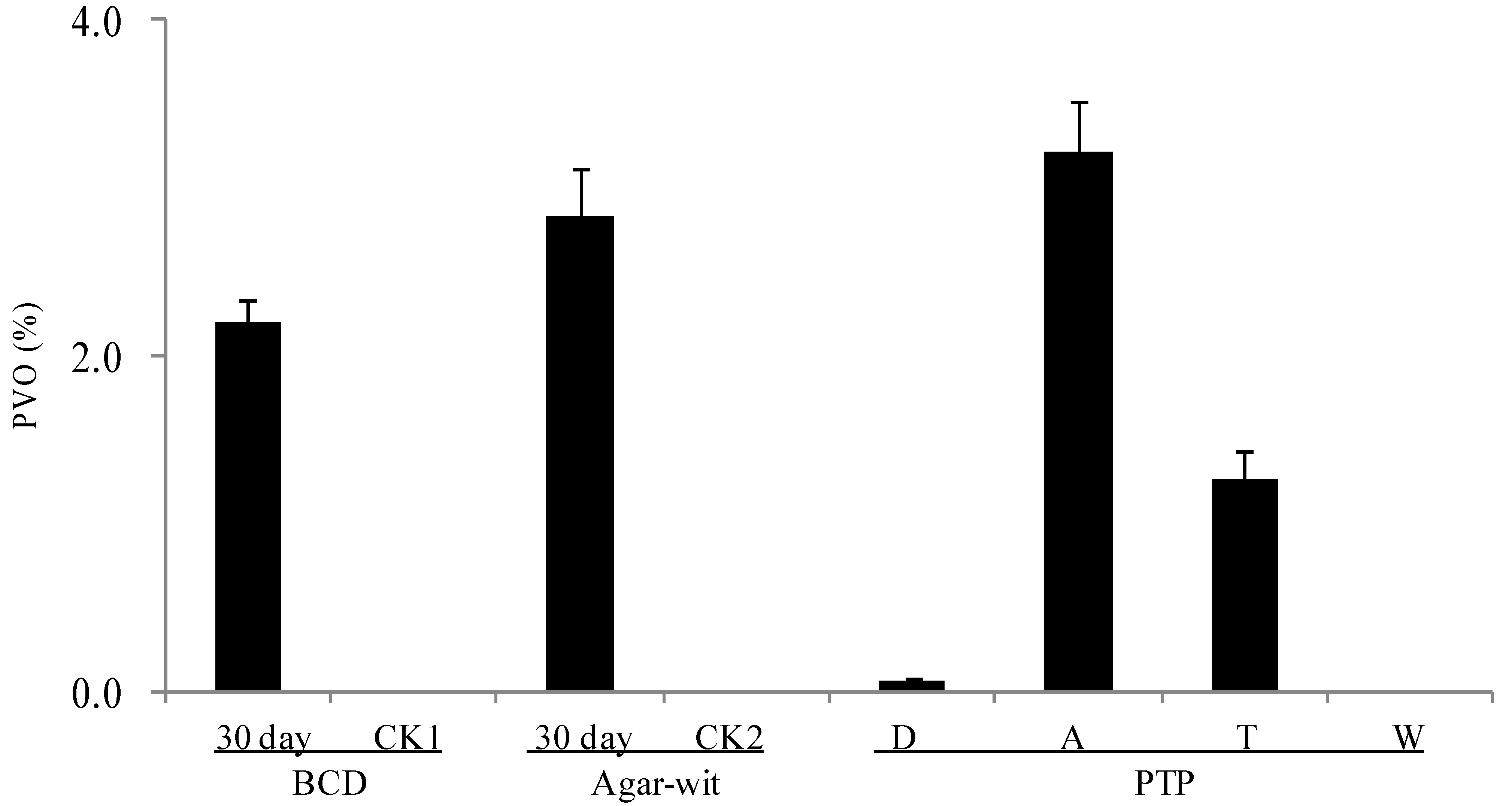
2.3. Three Different Types of Wounding All Induced Vessel-Occlusion Formation and Sesquiterpene Biosynthesis
3. Discussion
4. Experimental Section
4.1. Plant Material
4.1.1. Agarwood Induced by BCD Method
4.1.2. Agarwood Induced by Agar-Wit Method
4.1.3. Agarwood Induced by Partial-Trunk-Pruning (PTP) Method
4.2. Genomic DNA Isolation
4.3. Polymerase Chain Reaction, Sequencing, and Database Searching
4.4. OTU-Based Sequence Analysis
4.5. Taxonomic Classification of Representative OTU Reads
4.6. Anatomical Examination and Quantification of Vessel Occlusions in Stems
4.7. Essential-Oil Extraction
4.8. Gas Chromatography-Mass Spectrometry Analysis
4.9. Data Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sharma, M.; Sharma, A.; Kumar, A.; Basu, S.K. Enhancement of secondary metabolites in cultured plant cells through stress stimulus. Am. J. Plant Physiol. 2011, 6, 50–71. [Google Scholar] [CrossRef]
- Zhao, J.L.; Davis, C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biot. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef]
- Naef, R. The volatile and semi-volatile constituents of agarwood, the infected heartwood of Aquilaria species: A review. Flavour. Fragr. J. 2011, 26, 73–78. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.L.; Yang, Y.; Wei, J.H.; Meng, H.; Gao, Z.H.; Xu, Y.H. Hydrogen peroxide induces vessel occlusions and stimulates sesquiterpenes accumulation in stems of Aquilaria sinensis. Plant Growth Regul. 2014, 72, 81–87. [Google Scholar] [CrossRef]
- Qi, S.Y.; Lu, B.Y.; Zhu, L.F.; Li, B.L. Formation of oxoagarospirol in Aquilaria sinensis. Plant Physiol. Commun. 1992, 28, 336–339. [Google Scholar]
- Tamuli, P.; Baruah, P.; Samanta, R. Enzyme activities of agarwood (Aquilaria malaccensis Lamk.) stem under pathogenesis. J. Spices Arom. Crops 2011, 17, 240–243. [Google Scholar]
- Zhang, Z.; Han, X.M.; Wei, J.H.; Xue, J.; Yang, Y.; Liang, L.; Li, X.J.; Guo, Q.M.; Xu, Y.H.; Gao, Z.H. Compositions and antifungal activities of essential oils from agarwood of Aquilaria sinensis (Lour.) Gilg induced by Lasiodiplodia theobromae (Pat.) Griffon. & Maubl. J. Braz. Chem. Soc. 2014, 25, 20–26. [Google Scholar]
- Tamuli, P.; Boruah, P.; Nath, S.C.; Leclercq, P. Essential oil of eaglewood tree: A product of pathogenesis. J. Essent. Oil Res. 2005, 17, 601–604. [Google Scholar] [CrossRef]
- Gibson, I.A.S. The role of fungi in the origin of oleoresin deposits of agaru in the wood of Aquilaria agallocha Roxb. Bano Biggyan Patrika 1977, 6, 16–26. [Google Scholar]
- Bhattacharyya, B.; Datta, A.; Barauah, H.K. On the formation and development of agaru in Aquilaria agallocha. Sci. Cult. 1952, 18, 240–243. [Google Scholar]
- Jalaluddin, M. A useful pathological condition of wood. Econ. Bot. 1977, 31, 222–224. [Google Scholar] [CrossRef]
- Tamuli, P.; Boruah, P.; Nath, S.C.; Samanta, R. Fungi from diseased agarwood tree (Aquilaria agallocha Roxb.): Two new records. Adv. For. Res. India 2000, 22, 182–187. [Google Scholar]
- Mohamed, R.; Jong, P.L.; Kamziah, A.K. Fungal inoculation induces agarwood in young Aquilaria malaccensis trees in the nursery. J. For. Res. 2014, 25, 201–204. [Google Scholar] [CrossRef]
- Rahman, M.A.; Basak, A.C. Agar production in agar trees by artificial inoculation and wounding. Bano Biggyan Patrika 1980, 9, 87–93. [Google Scholar]
- Nobuchi, T.; Somkid, S. Preliminary observation of Aquliaria crassna wood associated with the formation of aloeswood. Kyoto Univ. For. 1991, 63, 226–235. [Google Scholar]
- Wong, M.T.; Siah, C.H.; Faridah, Q.Z.; Mohamed, R. Characterization of wound responsive genes in Aquilaria malaccensis. J. Plant. Biochem. Biotechnol. 2013, 22, 168–175. [Google Scholar] [CrossRef]
- Blanchette, R.A.; van Beek, H.H. Cultivated Agarwood. US Patent No. 6,848,211 B2, 1 Febuary 2005. [Google Scholar]
- Chen, H.Q.; Yang, Y.; Xue, J.; Wei, J.H.; Zhang, Z.; Chen, H.J. Comparison of compositions and antimicrobial activities of essential oils from chemically stimulated agarwood, wild agarwood and healthy Aquilaria sinensis (Lour.) Gilg trees. Molecules 2011, 16, 4884–4896. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, H.; Yang, Y.; Zhang, Z.; Wei, J.; Meng, H.; Chen, W.; Feng, J.; Gan, B.; Chen, X.; et al. Whole-tree agarwood-inducing technique: An efficient novel technique for producing high-quality agarwood in cultivated Aquilaria sinensis trees. Molecules 2013, 18, 3086–3106. [Google Scholar] [CrossRef] [PubMed]
- Ortmann, A.C.; Ortell, N. Changes in free-living bacterial community diversity reflect the magnitude of environmental variability. FEMS Microbiol. Ecol. 2014, 87, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.D.; Cottingham, S.D.; Billinge, J.; Cunliffe, M. Seasonal microbial community dynamics correlate with phytoplankton-derived polysaccharides in surface coastal waters. ISME J. 2014, 8, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Menchaca, A.C.; Visi, D.K.; Strey, O.F.; Teel, P.D.; Kalinowski, K.; Allen, M.S.; Williamson, P.C. Preliminary assessment of microbiome changes following blood-feeding and survivorship in the Amblyomma americanum nymph-to-adult transition using semiconductor sequencing. PLoS One 2013, 8, e67129. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.P.; Callaham, M.A.; Oliver, A.K.; Jumpponen, A. Deep Ion Torrent sequencing identifies soil fungal community shifts after frequent prescribed fires in a southeastern US forest ecosystem. FEMS Microbiol. Ecol. 2013, 86, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Kemler, M.; Garnas, J.; Wingfield, M.J.; Gryzenhout, M.; Pillay, K.A.; Slippers, B. Ion Torrent PGM as Tool for Fungal Community Analysis: A case study of endophytes in Eucalyptus grandis reveals high taxonomic diversity. PLoS One 2013, 8, e81718. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhou, X.F.; Yang, S.J.; Liu, W.H.; Hu, X.F. Design and application of specific 16S rDNA-targeted primers for assessing endophytic diversity in Dendrobium officinale using nested PCR-DGGE. Appl. Microbiol. Biotechnol. 2013, 97, 9825–9836. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.M.; Marina, M.; Pieckenstain, F.L. The communities of tomato (Solanum lycopersicum L.) leaf endophytic bacteria, analyzed by 16S-ribosomal RNA gene pyrosequencing. FEMS Microbiol. Lett. 2014, 351, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Vik, U.; Logares, R.; Blaalid, R.; Halvorsen, R.; Carlsen, T.; Bakke, I.; Kolstø, A.B.; Økstad, O.A.; Kauserud, H. Different bacterial communities in ectomycorrhizae and surrounding soil. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.T.; Chang, Y.S.; Kadir, A.A. A review on agar (gaharu) producing Aquilaria species. J. Trop. For. Prod. 1997, 2, 272–285. [Google Scholar]
- Cui, J.; Wang, C.; Guo, S.; Yang, L.; Xiao, P.; Wang, M. Evaluation of fungus-induced agilawood from Aquilaria sinensis in China. Symbiosis 2013, 60, 37–44. [Google Scholar] [CrossRef]
- Cui, J.S.; Guo, S.; Fu, S.; Xiao, P.; Wang, M. Effects of inoculating fungi on agilawood formation in Aquilaria sinensis. Chin. Sci. Bull. 2013, 58, 3280–3287. [Google Scholar] [CrossRef]
- Morrow, K.M.; Ritson-Williams, R.; Ross, C.; Liles, M.R.; Paul, V.J. Macroalgal extracts induce bacterial assemblage shifts and sublethal tissue stress in Caribbean corals. PLoS One 2012, 7, e44859. [Google Scholar] [CrossRef] [PubMed]
- Fouts, D.E.; Szpakowski, S.; Purushe, J.; Torralba, M.; Waterman, R.C.; MacNeil, M.D.; Alexander, L.J.; Nelson, K.E. Next generation sequencing to define prokaryotic and fungal diversity in the bovine rumen. PLoS One 2012, 7, e48289. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Rost, T.L.; Matthews, M.A. Pruning-induced tylose development in stems of current-year shoots of Vitis vinifera (Vitaceae). Am. J. Bot. 2006, 93, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Rost, T.L.; Matthews, M.A. Wound-induced vascular occlusions in Vitis vinifera (Vitaceae): Tyloses in summer and gels in winter. Am. J. Bot. 2008, 95, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Rost, T.L.; Reid, M.S.; Matthews, M.A. Ethylene is required for wound-induced tylose development in stems of grapevines (Vitis vinifera L.). Plant Physiol. 2007, 145, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Wei, J.; Han, X.; Liang, L.; Yang, Y.; Meng, H.; Xu, Y.; Gao, Z. The Sesquiterpene Biosynthesis and Vessel-Occlusion Formation in Stems of Aquilaria sinensis (Lour.) Gilg Trees Induced by Wounding Treatments without Variation of Microbial Communities. Int. J. Mol. Sci. 2014, 15, 23589-23603. https://doi.org/10.3390/ijms151223589
Zhang Z, Wei J, Han X, Liang L, Yang Y, Meng H, Xu Y, Gao Z. The Sesquiterpene Biosynthesis and Vessel-Occlusion Formation in Stems of Aquilaria sinensis (Lour.) Gilg Trees Induced by Wounding Treatments without Variation of Microbial Communities. International Journal of Molecular Sciences. 2014; 15(12):23589-23603. https://doi.org/10.3390/ijms151223589
Chicago/Turabian StyleZhang, Zheng, Jianhe Wei, Xiaomin Han, Liang Liang, Yun Yang, Hui Meng, Yanhong Xu, and Zhihui Gao. 2014. "The Sesquiterpene Biosynthesis and Vessel-Occlusion Formation in Stems of Aquilaria sinensis (Lour.) Gilg Trees Induced by Wounding Treatments without Variation of Microbial Communities" International Journal of Molecular Sciences 15, no. 12: 23589-23603. https://doi.org/10.3390/ijms151223589
APA StyleZhang, Z., Wei, J., Han, X., Liang, L., Yang, Y., Meng, H., Xu, Y., & Gao, Z. (2014). The Sesquiterpene Biosynthesis and Vessel-Occlusion Formation in Stems of Aquilaria sinensis (Lour.) Gilg Trees Induced by Wounding Treatments without Variation of Microbial Communities. International Journal of Molecular Sciences, 15(12), 23589-23603. https://doi.org/10.3390/ijms151223589



