Effects of Icariside II on Corpus Cavernosum and Major Pelvic Ganglion Neuropathy in Streptozotocin-Induced Diabetic Rats
Abstract
:1. Introduction
2. Results
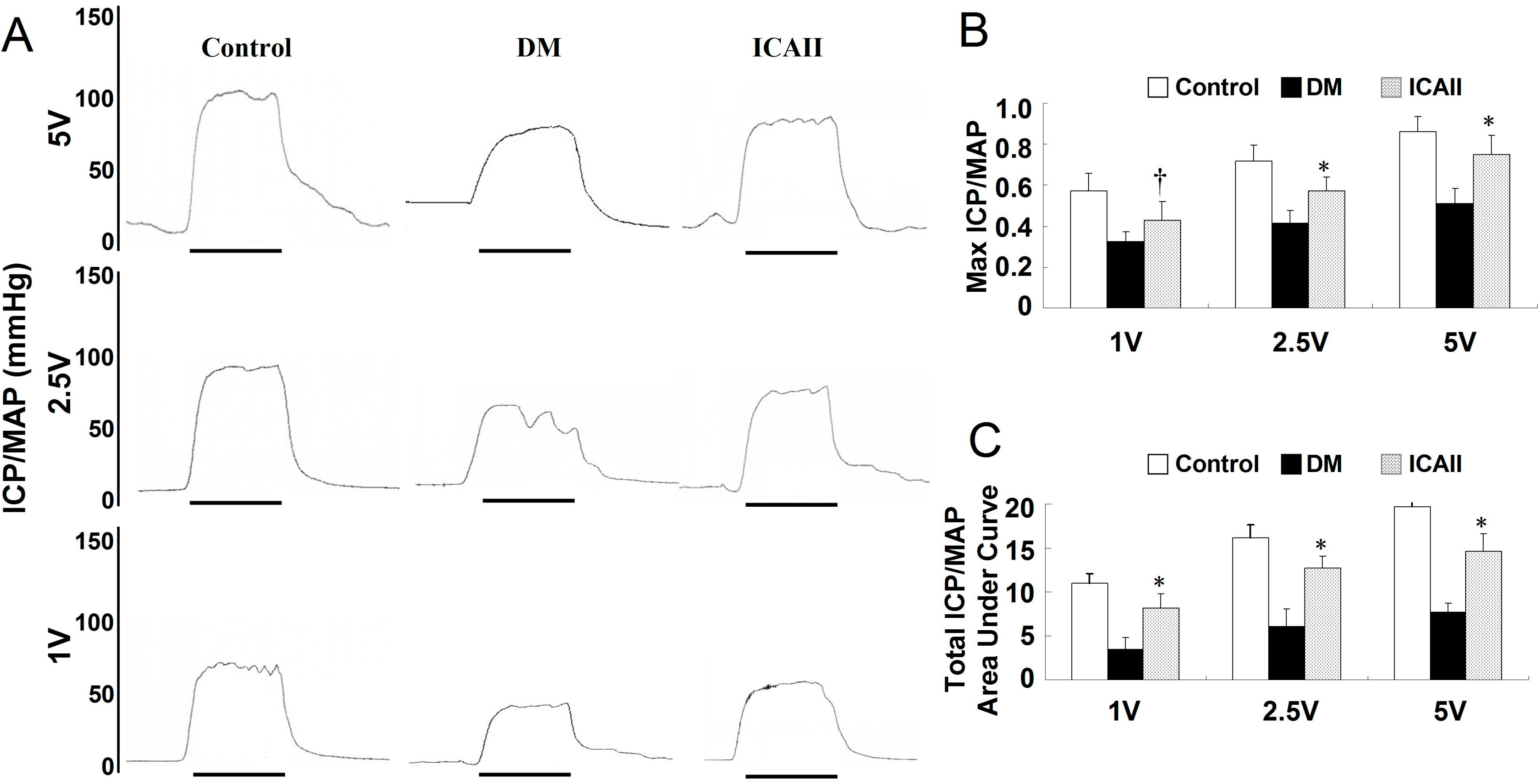
2.1. Metabolic Variables and ICP/MAP (Intracavernous Pressure/Mean Arterial Pressure) Assessment
| Variable | Control | Diabetic | ICA II |
|---|---|---|---|
| Initial weight (g) | 219.6 ± 25.1 | 221.2 ± 13.4 | 211.4 ± 17.7 |
| Final weight (g) | 602.1 ± 23.2 | 346.1 ± 32.6 | 397.5 ± 24.4 |
| Initial fasting glucose (mg/dL) | 109.2 ± 3.6 | 107.2 ± 3.2 | 105.7 ± 2.6 |
| Final fasting glucose (mg/dL) | 112.4 ± 11.5 | 493.6 ± 32.3 | 423.5 ± 22.5 |
| MAP (mm Hg) | 109.27 ± 7.15 | 96.22 ± 5.85 | 104.25 ± 4.20 |
| ICP (mm Hg) | 96.33 ± 8.30 | 41.32 ± 7.55 | 74.30 ± 9.33 # |
| AUC | 1980 ± 135 | 675 ± 156 | 1667 ± 142 # |
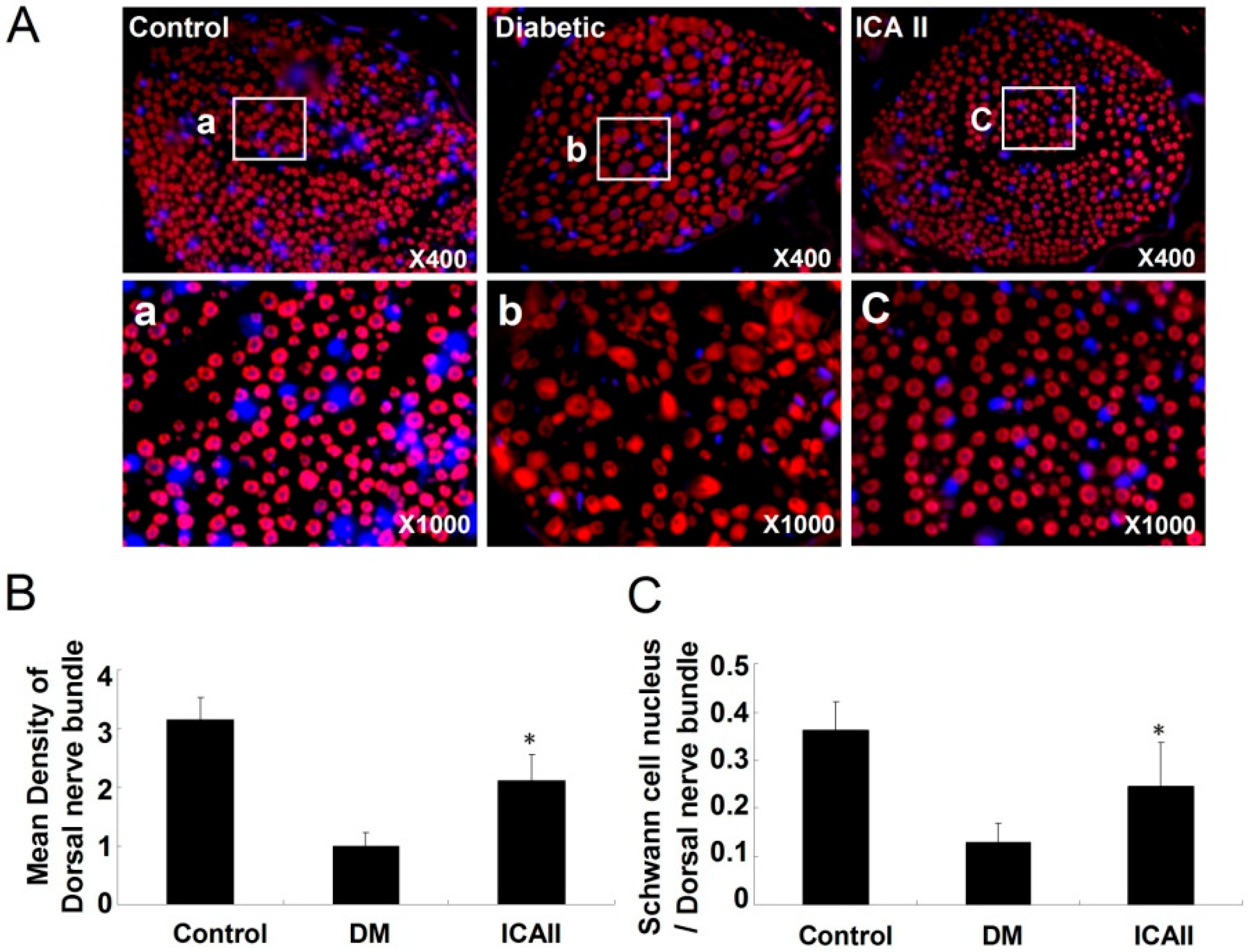
2.2. The Pathological Change of the Dorsal Nerve Bundle

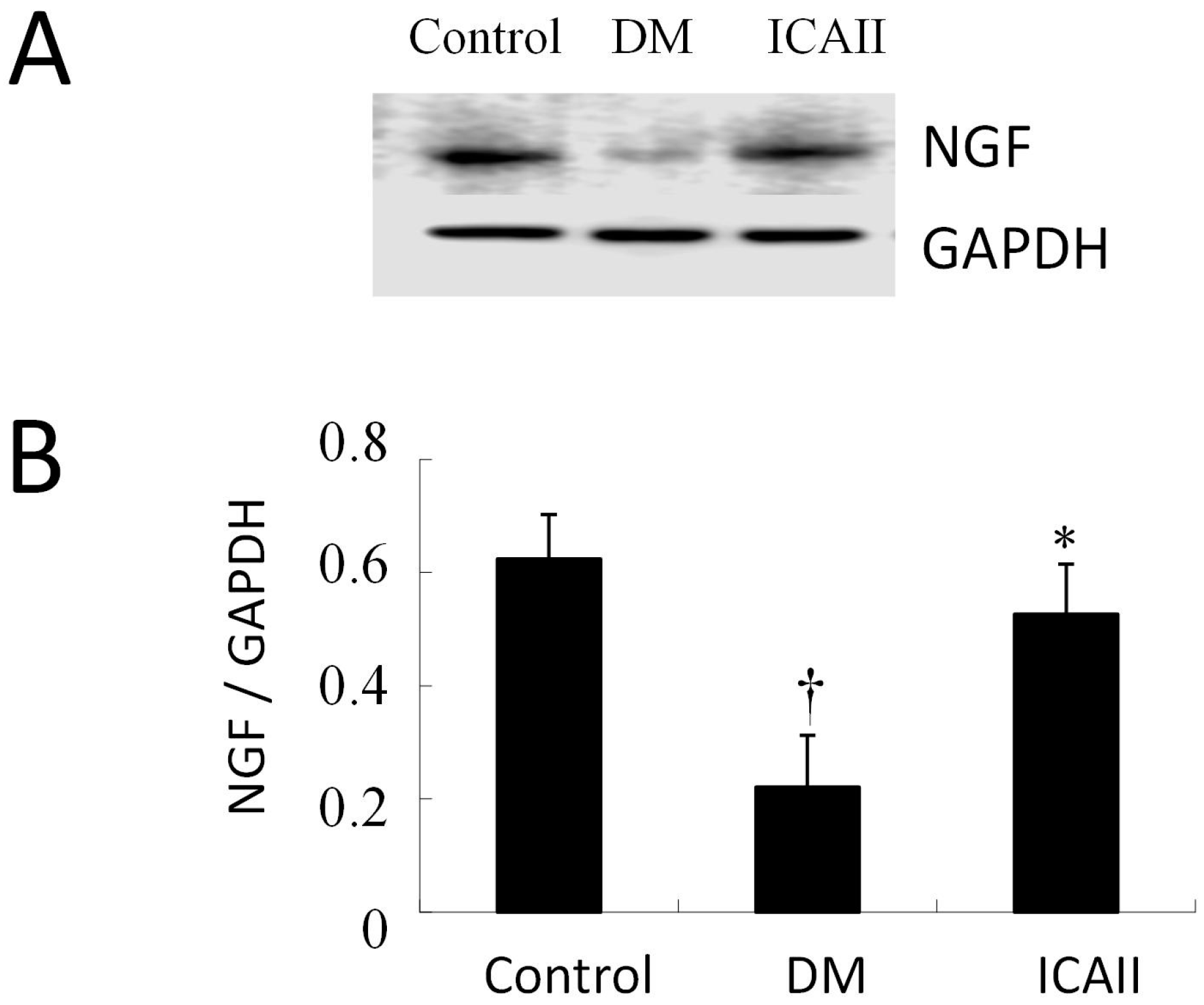
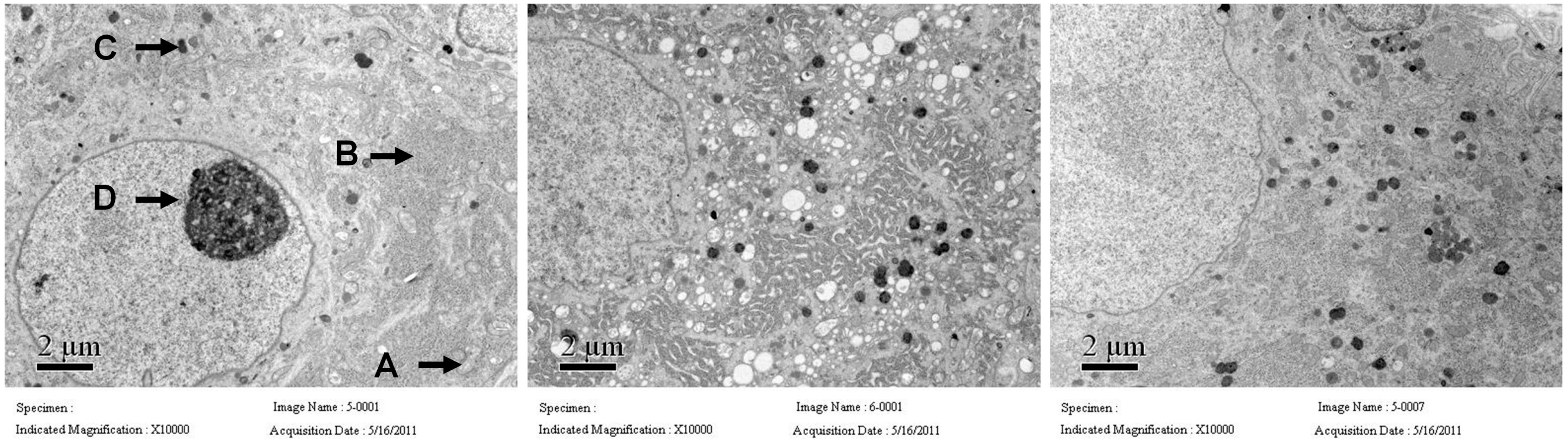
2.3. The Pathological Change of Major Pelvic Ganglion
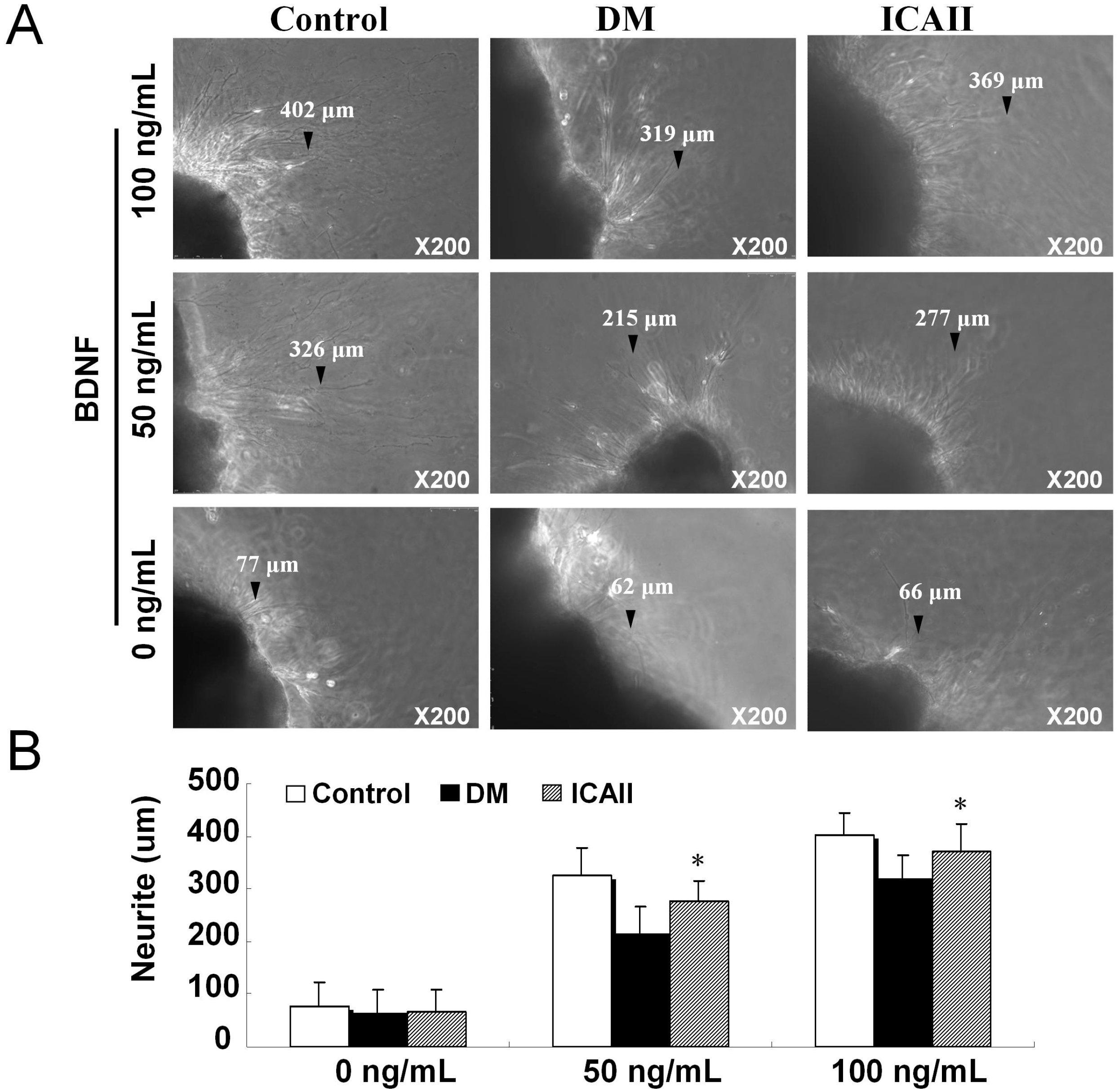
3. Discussion
4. Experimental Section
4.1. Preparation of ICA II
4.2. Animals
4.3. Measurement of Erectile Function
4.4. Immunohistochemistry and Immunofluorescence
4.5. Western Blot
4.6. Transmission Electron Microscopy
4.7. Major Pelvic Nerve Ganglion Tissue Culture in Vitro
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shamloul, R.; Ghanem, H. Erectile dysfunction. Lancet 2013, 381, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.L.; Guindalini, C.; Tufik, S. Genetics of erectile dysfunction: A review of the interface between sex and molecular biomarkers. J. Sex. Med. 2011, 8, 3030–3039. [Google Scholar] [CrossRef] [PubMed]
- Lopushnyan, N.A.; Chitaley, K. Genetics of erectile dysfunction. J. Urol. 2012, 188, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Fedele, D. Therapy Insight: Sexual and bladder dysfunction associated with diabetes mellitus. Nat. Clin. Pract. Urol. 2005, 2, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Adeniyi, A.F.; Adeleye, J.O.; Adeniyi, C.Y. Diabetes, sexual dysfunction and therapeutic exercise: A 20 year review. Curr. Diabetes Rev. 2010, 6, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, L.; Qiao, Y.; Zhou, X.; Wu, G.; Wang, L.; Peng, Y.; Dong, X.; Huang, H.; Si, L.; et al. Heme oxygenase-1 prevents cardiac dysfunction in streptozotocin-diabetic mice by reducing inflammation, oxidative stress, apoptosis and enhancing autophagy. PLoS One 2013, 8, e75927. [Google Scholar] [CrossRef]
- Angulo, J.; Wright, H.M.; Cuevas, P.; Gonzalez-Corrochano, R.; Fernandez, A.; Cuevas, B.; La Fuente, J.M.; Gupta, S.; Saenz de Tejada, I. Nebivolol dilates human penile arteries and reverses erectile dysfunction in diabetic rats through enhancement of nitric oxide signaling. J. Sex. Med. 2010, 7, 2681–2697. [Google Scholar] [CrossRef] [PubMed]
- Vickers, M.A.; Satyanarayana, R. Phosphodiesterase type 5 inhibitors for the treatment of erectile dysfunction in patients with diabetes mellitus. Int. J. Impot. Res. 2002, 14, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Harraz, A.; Shindel, A.W.; Lue, T.F. Emerging gene and stem cell therapies for the treatment of erectile dysfunction. Nat. Rev. Urol. 2010, 7, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.S.; Hawthorne, W.J.; Manolios, N. Gene therapy in diabetes. Self Nonself 2010, 1, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Albersen, M.; Lin, G.; Fandel, T.M.; Zhang, H.; Qiu, X.; Lin, C.S.; Lue, T.F. Functional, Metabolic, and Morphologic Characteristics of a Novel Rat Model of Type 2 Diabetes-associated Erectile Dysfunction. Urology 2011, 78, 476.e1–476.e8. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.H.; Kommu, S.S.; Siddiqui, E.J.; Thompson, C.S.; Morgan, R.J.; Mikhailidis, D.P.; Mumtaz, F.H. Gene therapy and erectile dysfunction: The current status. Asian J. Androl. 2007, 9, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Bahk, J.Y.; Jung, J.H.; Han, H.; Min, S.K.; Lee, Y.S. Treatment of diabetic impotence with umbilical cord blood stem cell intracavernosal transplant: preliminary report of 7 cases. Exp. Clin. Transplant. 2010, 8, 150–160. [Google Scholar] [PubMed]
- Jin, H.R.; Kim, W.J.; Song, J.S.; Piao, S.; Choi, M.J.; Tumurbaatar, M.; Shin, S.H.; Yin, G.N.; Koh, G.Y.; Ryu, J.K.; et al. Intracavernous delivery of a designed angiopoietin-1 variant rescues erectile function by enhancing endothelial regeneration in the streptozotocin-induced diabetic mouse. Diabetes 2011, 60, 969–980. [Google Scholar] [CrossRef]
- Xin, Z.C.; Kim, E.K.; Lin, C.S.; Liu, W.J.; Tian, L.; Yuan, Y.M.; Fu, J. Effects of icariin on cGMP-specific PDE5 and cAMP-specific PDE4 activities. Asian J. Androl. 2003, 5, 15–18. [Google Scholar] [PubMed]
- Liu, T.; Xin, H.; Li, W.R.; Zhou, F.; Li, G.Y.; Gong, Y.Q.; Gao, Z.Z.; Qin, X.C.; Cui, W.S.; Shindel, A.W.; et al. Effects of icariin on improving erectile function in streptozotocin-induced diabetic rats. J. Sex. Med. 2011, 8, 2761–2772. [Google Scholar] [CrossRef]
- Zhou, F.; Xin, H.; Liu, T.; Li, G.Y.; Gao, Z.Z.; Liu, J.; Li, W.R.; Cui, W.S.; Bai, G.Y.; Park, N.C.; et al. Effects of icariside II on improving erectile function in rats with streptozotocin-induced diabetes. J. Androl. 2012, 33, 832–844. [Google Scholar] [CrossRef]
- Musicki, B.; Ross, A.E.; Champion, H.C.; Burnett, A.L.; Bivalacqua, T.J. Posttranslational modification of constitutive nitric oxide synthase in the penis. J. Androl. 2009, 30, 352–362. [Google Scholar] [PubMed]
- Shindel, A.W.; Xin, Z.C.; Lin, G.; Fandel, T.M.; Huang, Y.C.; Banie, L.; Breyer, B.N.; Garcia, M.M.; Lin, C.S.; Lue, T.F. Erectogenic and neurotrophic effects of icariin, a purified extract of horny goat weed (Epimedium spp.) in vitro and in vivo. J. Sex. Med. 2010, 7, 1518–1528. [Google Scholar]
- Miner, M.; Seftel, A.D.; Nehra, A.; Ganz, P.; Kloner, R.A.; Montorsi, P.; Vlachopoulos, C.; Ramsey, M.; Sigman, M.; Tilkemeier, P.; et al. Prognostic utility of erectile dysfunction for cardiovascular disease in younger men and those with diabetes. Am. Heart J. 2012, 164, 21–28. [Google Scholar] [CrossRef]
- Nehra, A.; Jackson, G.; Miner, M.; Billups, K.L.; Burnett, A.L.; Buvat, J.; Carson, C.C.; Cunningham, G.R.; Goldstein, I.; Guay, A.T.; et al. Diagnosis and treatment of erectile dysfunction for reduction of cardiovascular risk. J. Urol. 2013, 189, 2031–2038. [Google Scholar] [CrossRef]
- Ning, H.; Xin, Z.C.; Lin, G.; Banie, L.; Lue, T.F.; Lin, C.S. Effects of icariin on phosphodiesterase-5 activity in vitro and cyclic guanosine monophosphate level in cavernous smooth muscle cells. Urology 2006, 68, 1350–1354. [Google Scholar]
- Dell’Agli, M.; Galli, G.V.; dal Cero, E.; Belluti, F.; Matera, R.; Zironi, E.; Pagliuca, G.; Bosisio, E. Potent inhibition of human phosphodiesterase-5 by icariin derivatives. J. Nat. Prod. 2008, 71, 1513–1517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.B.; Ma, C.G.; Liu, T.; Li, W.R.; Gong, Y.Q.; Xin, Z.C. Icarisid II, a PDE5 inhibitor from Epimedium wanshanense, increases cellular cGMP by enhancing NOS in diabetic ED rats corpus cavernosum tissue. Andrologia 2012, 44, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Chen, K.C.; Hsieh, P.S.; Yeh, C.H.; Lue, T.F.; Lin, C.S. Neurotrophic effects of vascular endothelial growth factor and neurotrophins on cultured major pelvic ganglia. BJU Int. 2003, 92, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Bella, A.J.; Lue, T.F.; Lin, C.S. Brain-derived neurotrophic factor (BDNF) acts primarily via the JAK/STAT pathway to promote neurite growth in the major pelvic ganglion of the rat: Part 2. J. Sex. Med. 2006, 3, 821–827. [Google Scholar] [PubMed]
- Xia, Q.; Xu, D.; Huang, Z.; Liu, J.; Wang, X.; Liu, S. Preparation of icariside II from icariin by enzymatic hydrolysis method. Fitoterapia 2010, 81, 437–442. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, G.-Y.; Zhou, F.; Hui, Y.; Xu, Y.-D.; Lei, H.-E.; Pu, J.-X.; Xin, Z.-C. Effects of Icariside II on Corpus Cavernosum and Major Pelvic Ganglion Neuropathy in Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2014, 15, 23294-23306. https://doi.org/10.3390/ijms151223294
Bai G-Y, Zhou F, Hui Y, Xu Y-D, Lei H-E, Pu J-X, Xin Z-C. Effects of Icariside II on Corpus Cavernosum and Major Pelvic Ganglion Neuropathy in Streptozotocin-Induced Diabetic Rats. International Journal of Molecular Sciences. 2014; 15(12):23294-23306. https://doi.org/10.3390/ijms151223294
Chicago/Turabian StyleBai, Guang-Yi, Feng Zhou, Yu Hui, Yong-De Xu, Hong-En Lei, Jin-Xian Pu, and Zhong-Cheng Xin. 2014. "Effects of Icariside II on Corpus Cavernosum and Major Pelvic Ganglion Neuropathy in Streptozotocin-Induced Diabetic Rats" International Journal of Molecular Sciences 15, no. 12: 23294-23306. https://doi.org/10.3390/ijms151223294
APA StyleBai, G.-Y., Zhou, F., Hui, Y., Xu, Y.-D., Lei, H.-E., Pu, J.-X., & Xin, Z.-C. (2014). Effects of Icariside II on Corpus Cavernosum and Major Pelvic Ganglion Neuropathy in Streptozotocin-Induced Diabetic Rats. International Journal of Molecular Sciences, 15(12), 23294-23306. https://doi.org/10.3390/ijms151223294




