Catalpol Suppresses Proliferation and Facilitates Apoptosis of OVCAR-3 Ovarian Cancer Cells through Upregulating MicroRNA-200 and Downregulating MMP-2 Expression
Abstract
:1. Introduction
2. Results
2.1. MTT Analysis and Caspase-3 Activity Measurement
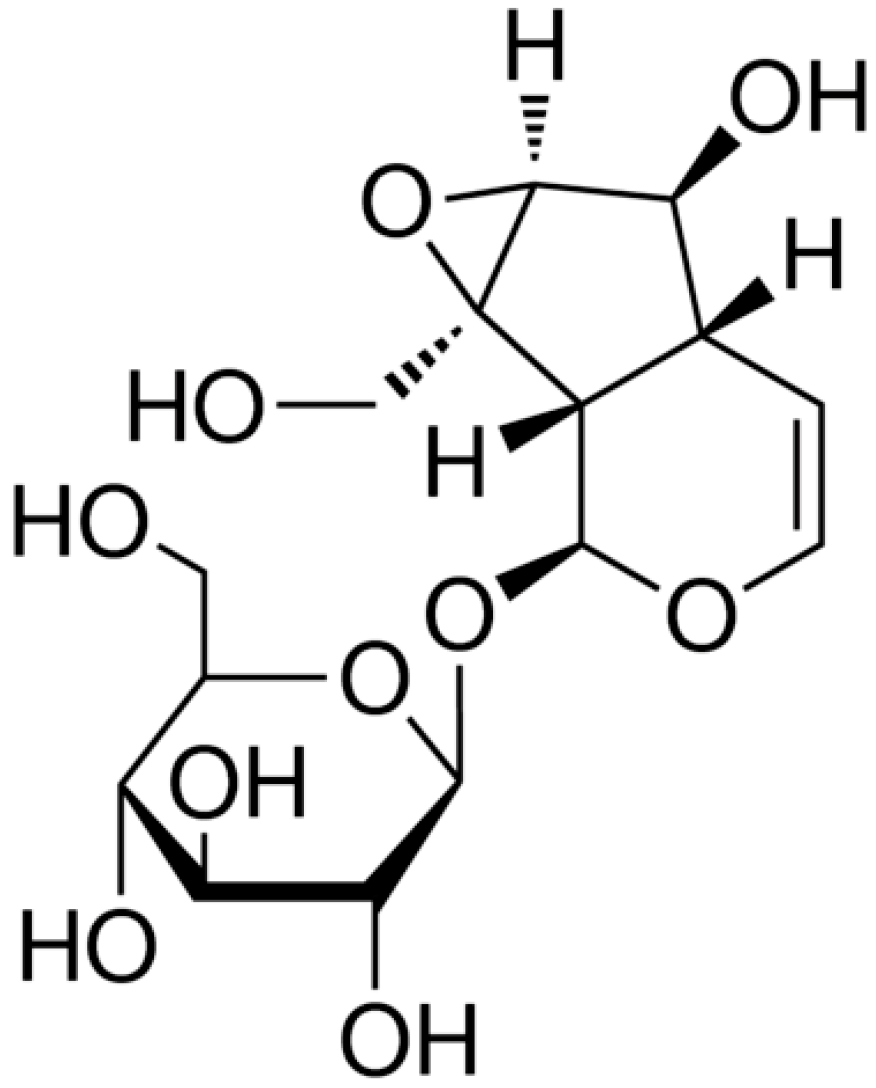
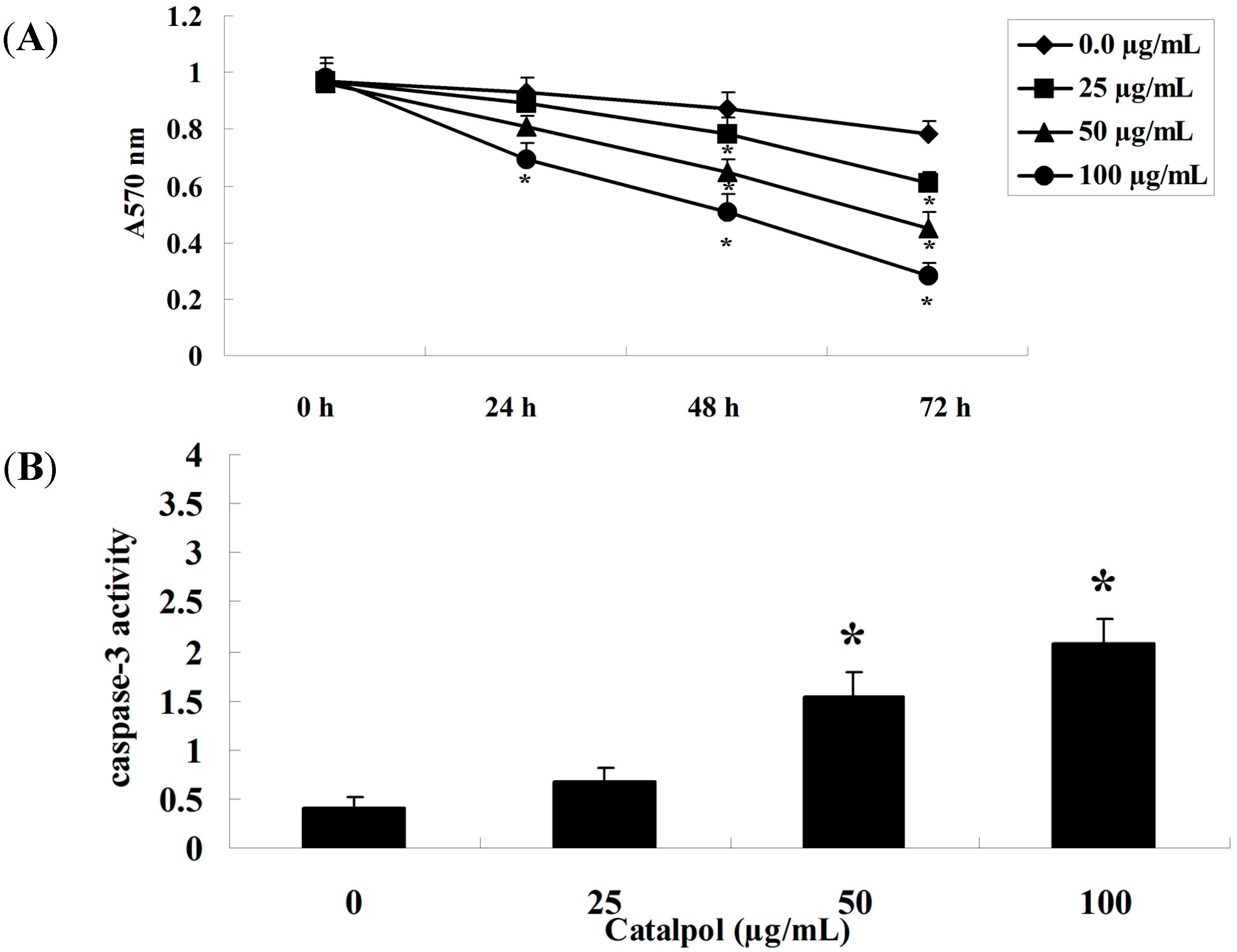
2.2. Flow-Cytometric Analysis for Detecting Cellular Apoptosis
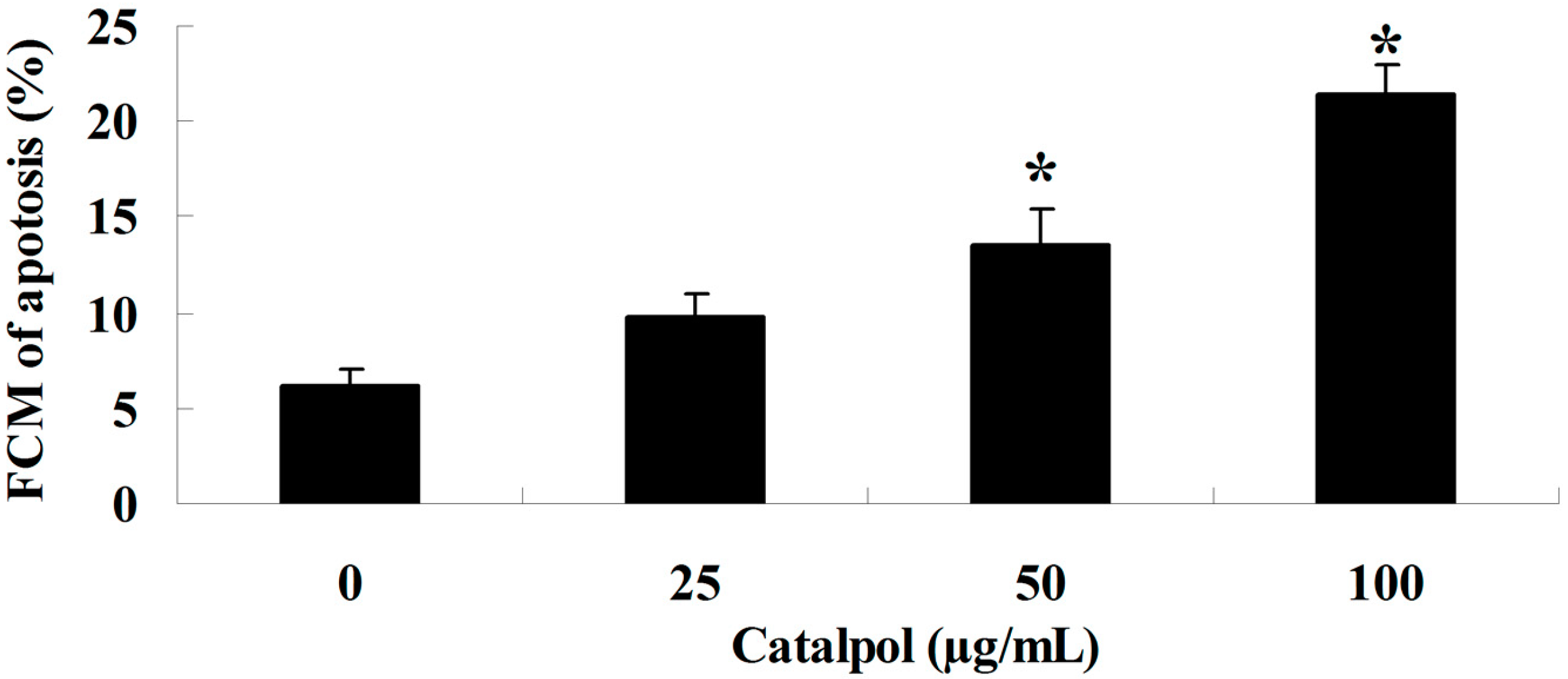
2.3. Inhibition of Catalpol on MMP-2
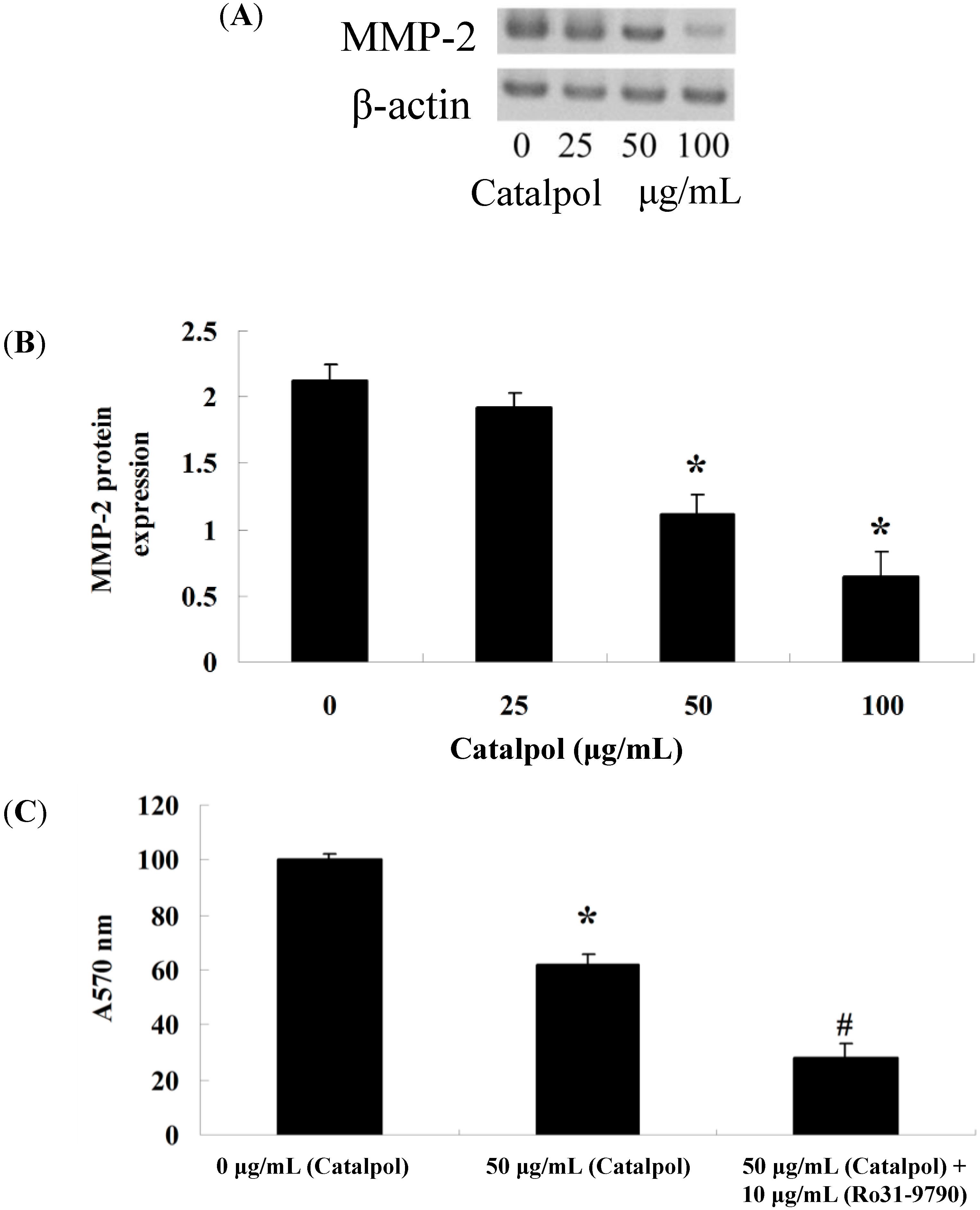
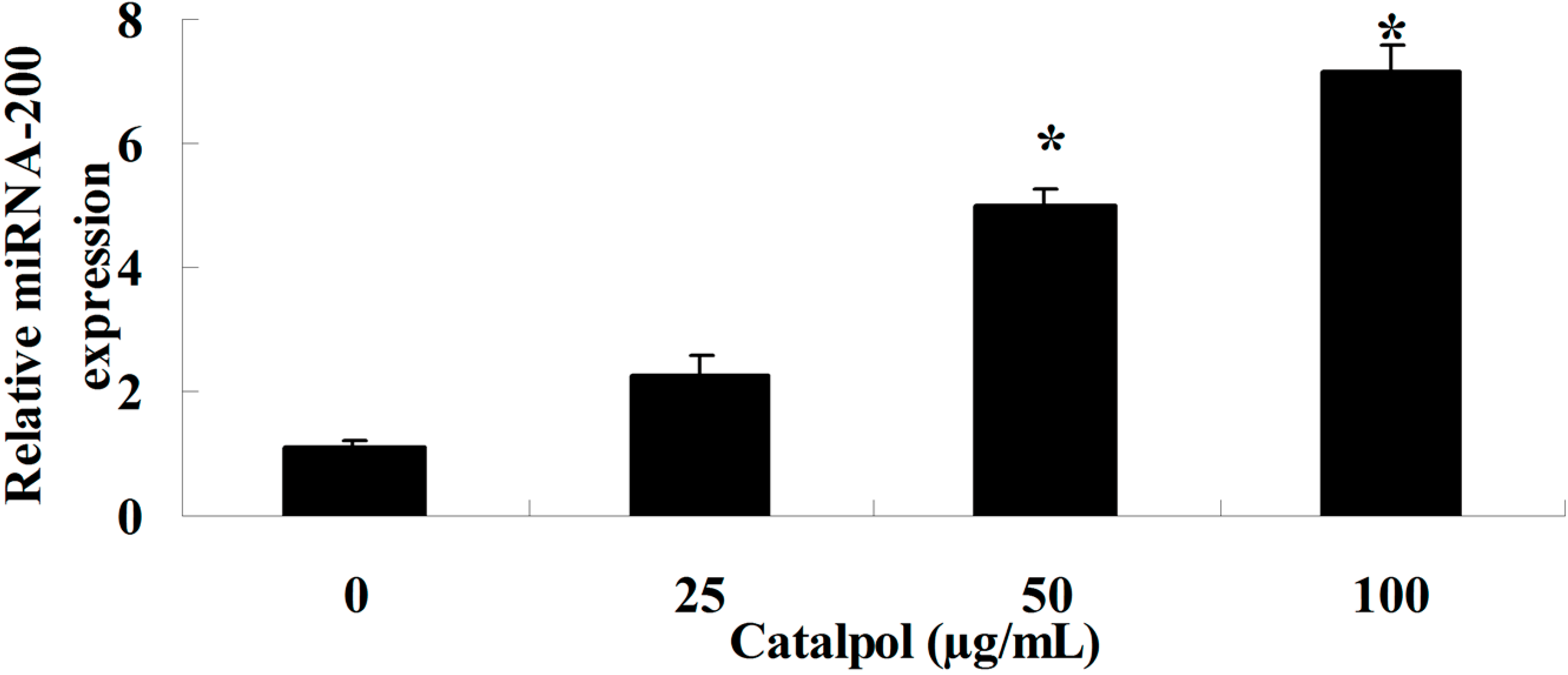
2.4. Catalpol Activates miR-200 Expression
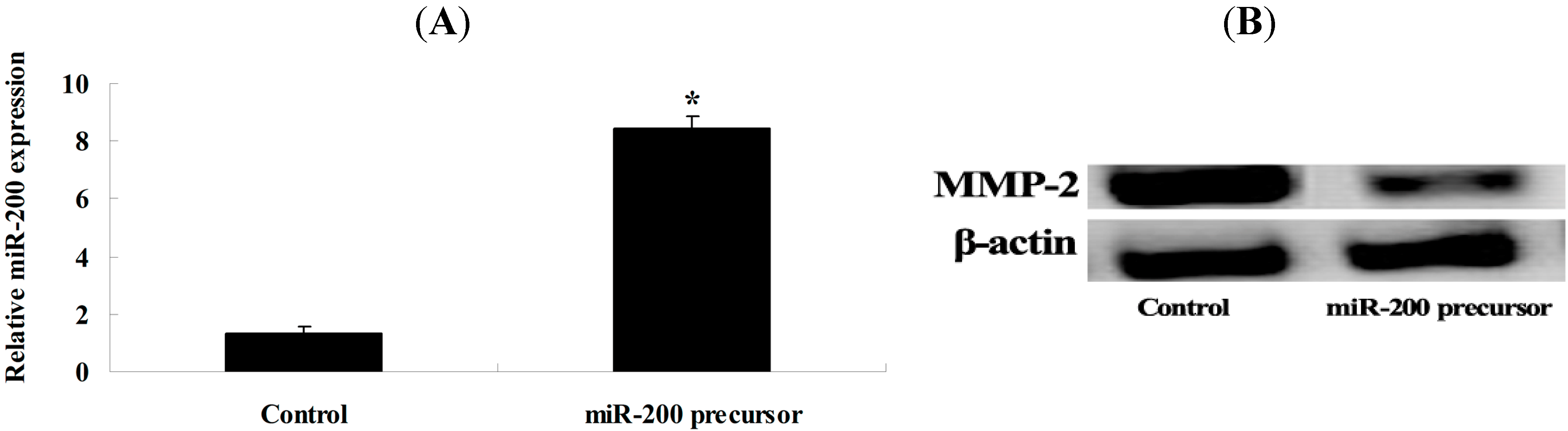
2.5. Overexpression of miR-200 and MMP-2 Expression
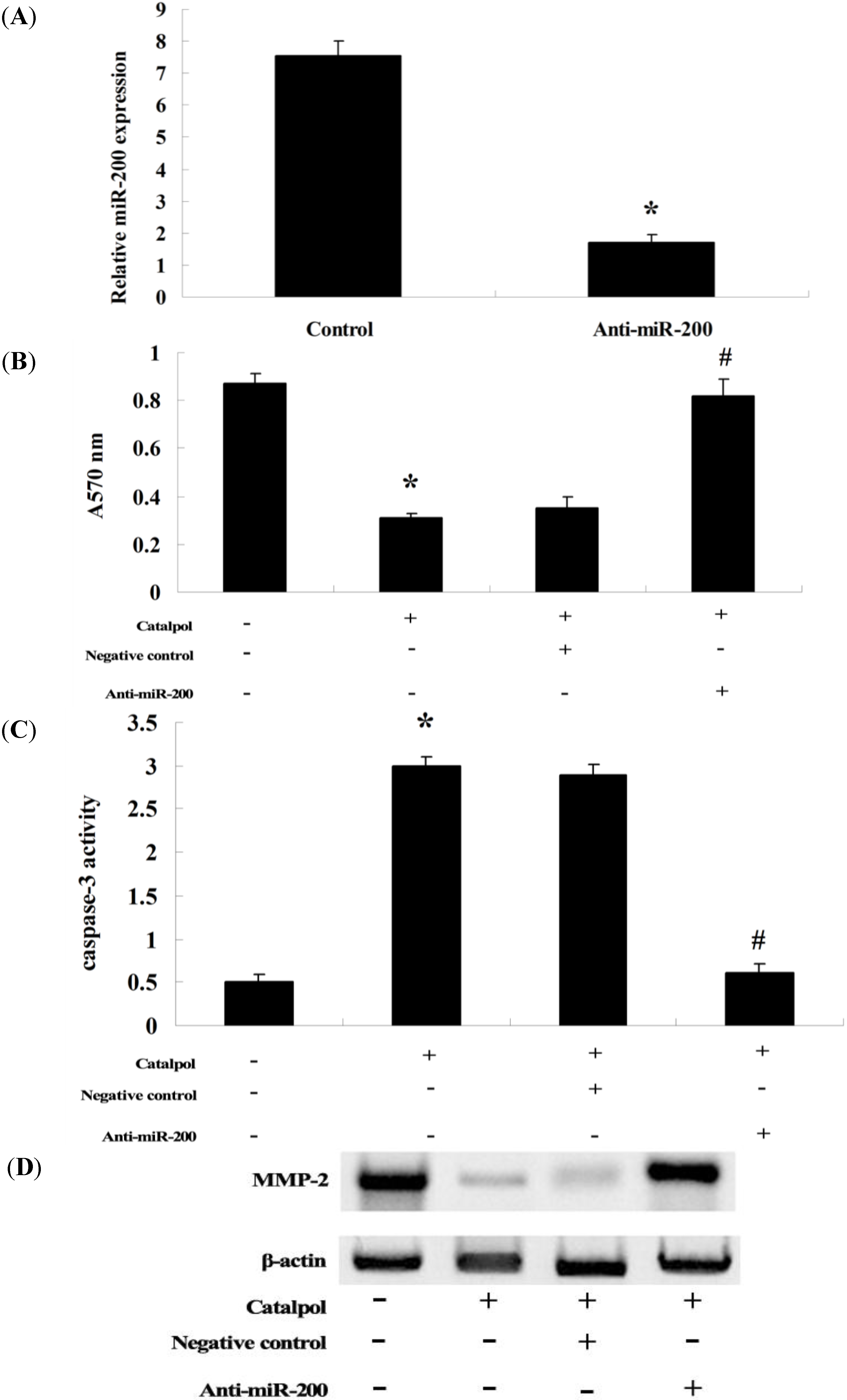
2.6. Anti-miR-200 Can Reverse the Effect of Catalpol
3. Discussion
4. Experimental Section
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Caspase-3 Activity Assays
4.5. Flow Cytometry
4.6. Gelatin Zymography Assays of MMP-2
4.7. Q-PCR Analysis of miR-200 Expression
4.8. Transfection of miR-200 and Anti-miR-200
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jiang, L.; Wang, H.; Li, J.; Fang, X.; Pan, H.; Yuan, X.; Zhang, P. Up-regulated FASN expression promotes transcoelomic metastasis of ovarian cancer cell through epithelial-mesenchymal transition. Int. J. Mol. Sci. 2014, 15, 11539–11554. [Google Scholar] [CrossRef] [PubMed]
- Mehtala, J.G.; Torregrosa-Allen, S.; Elzey, B.D.; Jeon, M.; Kim, C.; Wei, A. Synergistic effects of cisplatin chemotherapy and gold nanorod-mediated hyperthermia on ovarian cancer cells and tumors. Nanomedicine (Lond.) 2014, in press. [Google Scholar]
- Ayyagari, V.N.; Brard, L. Bithionol inhibits ovarian cancer cell growth in vitro—studies on mechanism(s) of action. BMC Cancer 2014, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Rafii, A.; Halabi, N.M.; Malek, J.A. High-prevalence and broad spectrum of Cell Adhesion and Extracellular Matrix gene pathway mutations in epithelial ovarian cancer. J. Clin. Bioinforma. 2012, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J. The complex interaction of matrix metalloproteinases in the migration of cancer cells through breast tissue stroma. Int. J. Breast Cancer 2014, 2014, 839094. [Google Scholar] [PubMed]
- Seo, J.M.; Park, S.; Kim, J.H. Leukotriene B4 receptor-2 promotes invasiveness and metastasis of ovarian cancer cells through signal transducer and activator of transcription 3 (STAT3)-dependent up-regulation of matrix metalloproteinase 2. J. Biol. Chem. 2012, 287, 13840–13849. [Google Scholar] [CrossRef] [PubMed]
- Salido-Guadarrama, I.; Romero-Cordoba, S.; Peralta-Zaragoza, O.; Hidalgo-Miranda, A.; Rodriguez-Dorantes, M. MicroRNAs transported by exosomes in body fluids as mediators of intercellular communication in cancer. Onco Targets Ther. 2014, 7, 1327–1338. [Google Scholar] [PubMed]
- Wen, K.C.; Sung, P.L.; Yen, M.S.; Chuang, C.M.; Liou, W.S.; Wang, P.H. MicroRNAs regulate several functions of normal tissues and malignancies. Taiwan J. Obstet. Gynecol. 2013, 52, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Koutsaki, M.; Spandidos, D.A.; Zaravinos, A. Epithelial-mesenchymal transition-associated miRNAs in ovarian carcinoma, with highlight on the miR-200 family: Prognostic value and prospective role in ovarian cancer therapeutics. Cancer Lett. 2014, 351, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Salomon, C.; Tapia, J.; Illanes, S.E.; Mitchell, M.D.; Rice, G.E. Ovarian cancer cell invasiveness is associated with discordant exosomal sequestration of Let-7 miRNA and miR-200. J. Transl. Med. 2014, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jin, C.; Li, Y.; Guan, S.; Han, F.; Zhang, S. Catalpol improves cholinergic function and reduces inflammatory cytokines in the senescent mice induced by D-galactose. Food Chem. Toxicol. 2013, 58, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Piao, T.; Wang, M.; Zhang, J.; Jiang, J.; Wang, X.; Liu, H. Protective effect of catalpol on lipopolysaccharide-induced acute lung injury in mice. Int. Immunopharmacol. 2014, 14, S1567–S5769. [Google Scholar]
- Liu, Y.R.; Li, P.W.; Suo, J.J.; Sun, Y.; Zhang, B.A.; Lu, H.; Zhu, H.C.; Zhang, G.B. Catalpol provides protective effects against cerebral ischaemia/reperfusion injury in gerbils. J. Pharm. Pharmacol. 2014, 66, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.Y.; An, L.J.; Jiang, L.; Duan, Y.L.; Chen, J.; Jiang, B. Catalpol protects dopaminergic neurons from LPS-induced neurotoxicity in mesencephalic neuron-glia cultures. Life Sci. 2006, 80, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Pungitore, C.R.; Leon, L.G.; Garcia, C.; Martin, V.S.; Tonn, C.E.; Padron, J.M. Novel antiproliferative analogs of the Taq DNA polymerase inhibitor catalpol. Bioorg. Med. Chem. Lett. 2007, 17, 1332–1335. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Ren, C.; Han, J.; Ding, Y.; Du, J.; Dai, N.; Dai, J.; Ma, H.; Hu, Z.; Shen, H.; et al. A five-microRNA panel in plasma was identified as potential biomarker for early detection of gastric cancer. Br. J. Cancer 2014, 110, 2291–2299. [Google Scholar] [CrossRef] [PubMed]
- Ween, M.P.; Oehler, M.K.; Ricciardelli, C. Transforming Growth Factor-Beta-Induced Protein (TGFBI)/(betaig-H3): A Matrix Protein with Dual Functions in Ovarian Cancer. Int. J. Mol. Sci. 2012, 13, 10461–10477. [Google Scholar] [CrossRef] [PubMed]
- Chekerov, R.; Braicu, I.; Castillo-Tong, D.C.; Richter, R.; Cadron, I.; Mahner, S.; Woelber, L.; Marth, C.; van Gorp, T.; et al. Outcome and clinical management of 275 patients with advanced ovarian cancer International Federation of Obstetrics and Gynecology II to IV inside the European Ovarian Cancer Translational Research Consortium-OVCAD. Int. J. Gynecol. Cancer 2013, 23, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Pungitore, C.R.; Ayub, M.J.; Borkowski, E.J.; Tonn, C.E.; Ciuffo, G.M. Inhibition of Taq DNA polymerase by catalpol. Cell Mol. Biol. 2004, 50, 767–772. [Google Scholar] [PubMed]
- Martin, O.A.; Garro, H.A.; Kurina Sanz, M.B.; Pungitore, C.R.; Tonn, C.E. In silico study of the inhibition of DNA polymerase by a novel catalpol derivative. J. Mol. Model. 2011, 17, 2717–2723. [Google Scholar] [CrossRef] [PubMed]
- Grelewski, P.G.; Bar, J.K. The role of p53 protein and MMP-2 tumor/stromal cells expression on progressive growth of ovarian neoplasms. Cancer Investig. 2013, 31, 472–479. [Google Scholar] [CrossRef]
- Poon, S.L.; Klausen, C.; Hammond, G.L.; Leung, P.C. 37-kDa laminin receptor precursor mediates GnRH-II-induced MMP-2 expression and invasiveness in ovarian cancer cells. Mol. Endocrinol. 2011, 25, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Lu, K.; Dai, S.; Hu, Y.; Fan, W. Clinicopathological and prognostic implications of the miR-200 family in patients with epithelial ovarian cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 2392–2401. [Google Scholar] [PubMed]
- Soubani, O.; Ali, A.S.; Logna, F.; Ali, S.; Philip, P.A.; Sarkar, F.H. Re-expression of miR-200 by novel approaches regulates the expression of PTEN and MT1-MMP in pancreatic cancer. Carcinogenesis 2012, 33, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, N.; Tian, J.-X.; Shang, Y.-H.; Zhao, D.-Y.; Wu, T. Catalpol Suppresses Proliferation and Facilitates Apoptosis of OVCAR-3 Ovarian Cancer Cells through Upregulating MicroRNA-200 and Downregulating MMP-2 Expression. Int. J. Mol. Sci. 2014, 15, 19394-19405. https://doi.org/10.3390/ijms151119394
Gao N, Tian J-X, Shang Y-H, Zhao D-Y, Wu T. Catalpol Suppresses Proliferation and Facilitates Apoptosis of OVCAR-3 Ovarian Cancer Cells through Upregulating MicroRNA-200 and Downregulating MMP-2 Expression. International Journal of Molecular Sciences. 2014; 15(11):19394-19405. https://doi.org/10.3390/ijms151119394
Chicago/Turabian StyleGao, Na, Jian-Xin Tian, Yu-Hong Shang, Dan-Yi Zhao, and Tao Wu. 2014. "Catalpol Suppresses Proliferation and Facilitates Apoptosis of OVCAR-3 Ovarian Cancer Cells through Upregulating MicroRNA-200 and Downregulating MMP-2 Expression" International Journal of Molecular Sciences 15, no. 11: 19394-19405. https://doi.org/10.3390/ijms151119394
APA StyleGao, N., Tian, J.-X., Shang, Y.-H., Zhao, D.-Y., & Wu, T. (2014). Catalpol Suppresses Proliferation and Facilitates Apoptosis of OVCAR-3 Ovarian Cancer Cells through Upregulating MicroRNA-200 and Downregulating MMP-2 Expression. International Journal of Molecular Sciences, 15(11), 19394-19405. https://doi.org/10.3390/ijms151119394



