Synthesis and Antimicrobial Evaluation of Some Novel Thiazole, Pyridone, Pyrazole, Chromene, Hydrazone Derivatives Bearing a Biologically Active Sulfonamide Moiety
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening for Antimicrobial Activity
3. Experimental Section
3.1. General Experimental Procedures
3.2. Synthetic Procedures
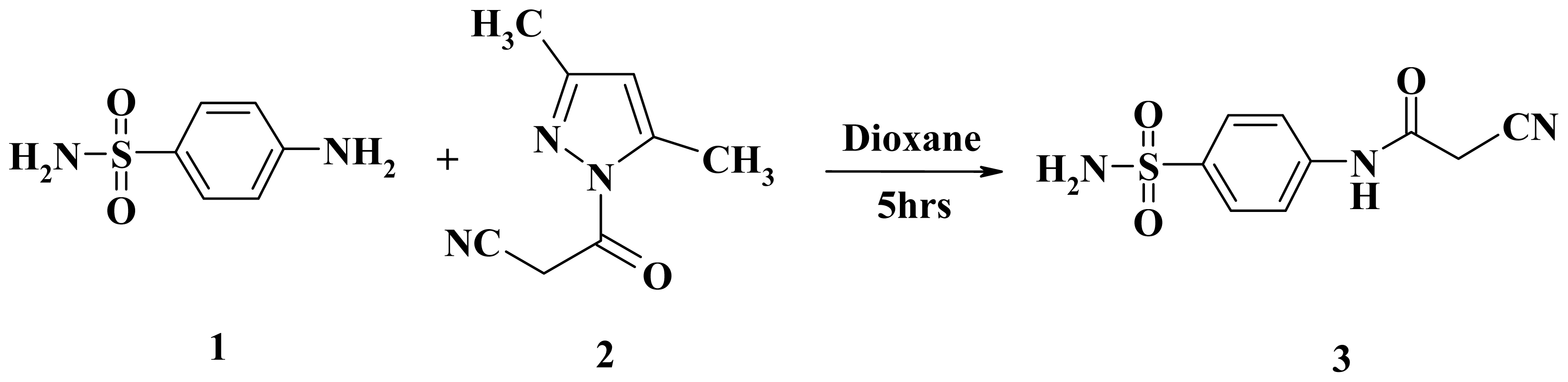
3.2.1. 2-Cyano-N-(4-sulfamoylphenyl)acetamide (3)
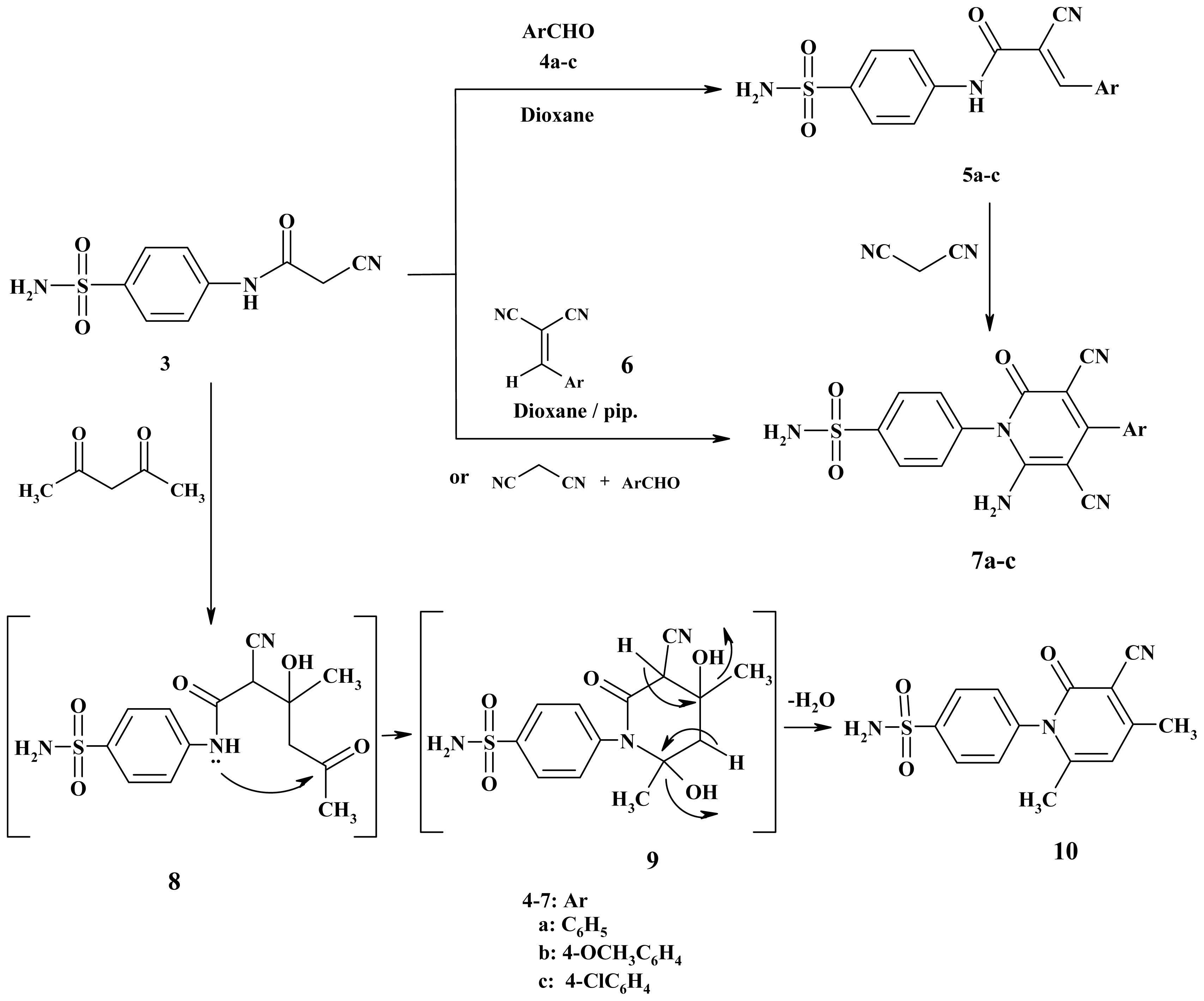
3.2.2. N-[4-(Aminosulfonyl)phenyl]-3-aryl-2-cyanoacrylamide (5a–c)
3.2.3. 2-Cyano-3-phenyl-N-(4-sulfamoylphenyl)prop-2-enamide (5a)
3.2.4. 2-Cyano-3-(4-methoxyphenyl)-N-(4-sulfamoylphenyl)prop-2-enamide (5b)
3.2.5. 2-Cyano-3-(4-chlorophenyl)-N-(4-sulfamoylphenyl)prop-2-enamide (5c)
3.2.6. Synthesis of Pyridines 7a–c
3.2.7. 4-(6-Amino-3,5-dicyano-2-oxo-4-phenylpyridin-1(2H)-yl)benzenesulfonamide (7a)
3.2.8. 4-[6-Amino-3,5-dicyano-4-(4-methoxyphenyl)-2-oxopyridin-1(2H)-yl]benzenesulfonamide (7b)
3.2.9. 4-[6-Amino-4-(4-chlorophenyl)-3,5-dicyano-2-oxopyridin-1(2H)-yl]benzenesulfonamide (7c)
3.2.10. 4-(3-Cyano-4,6-dimethyl-2-oxopyridin-1(2H)-yl)benzenesulfonamide (10)
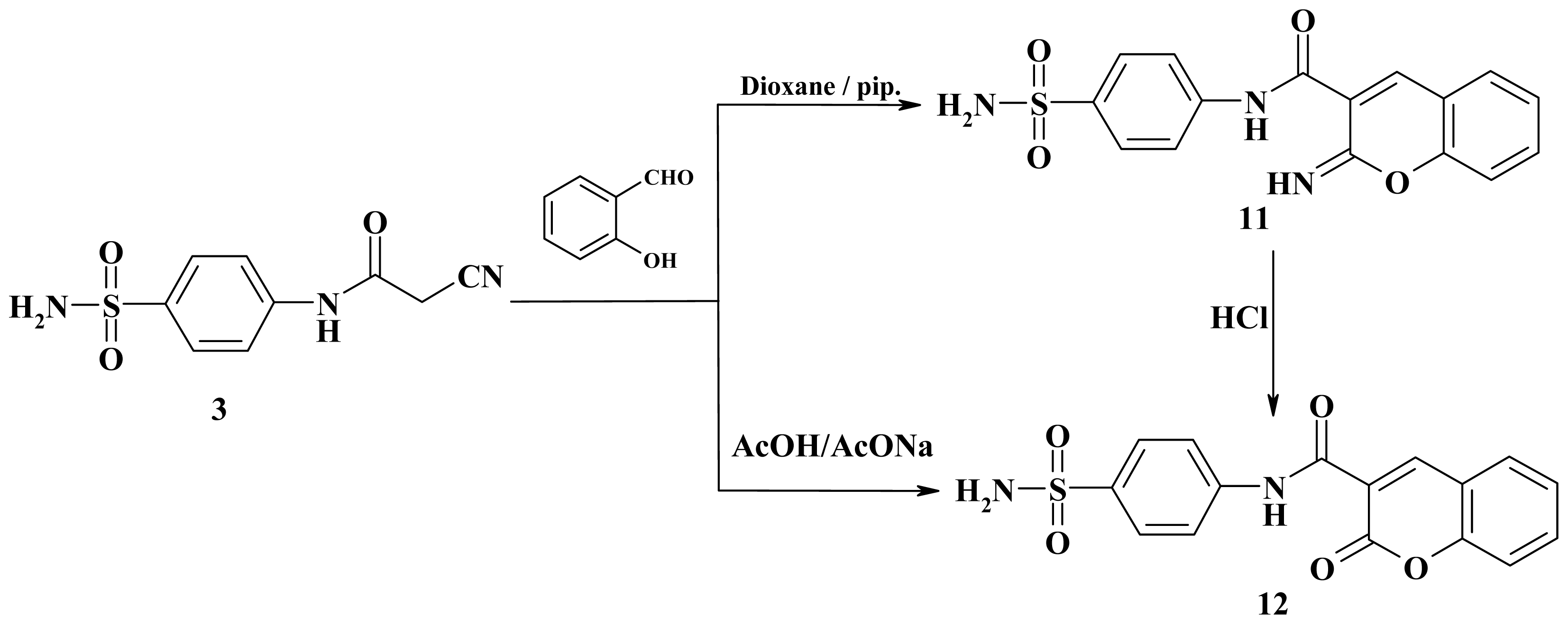
3.2.11. 2-Imino-N-(4-sulfamoylphenyl)-2H-chromene-3-carboxamide (11)
3.2.12. 2-Oxo-N-(4-sulfamoylphenyl)-2H-chromene-3-carboxamide (12)
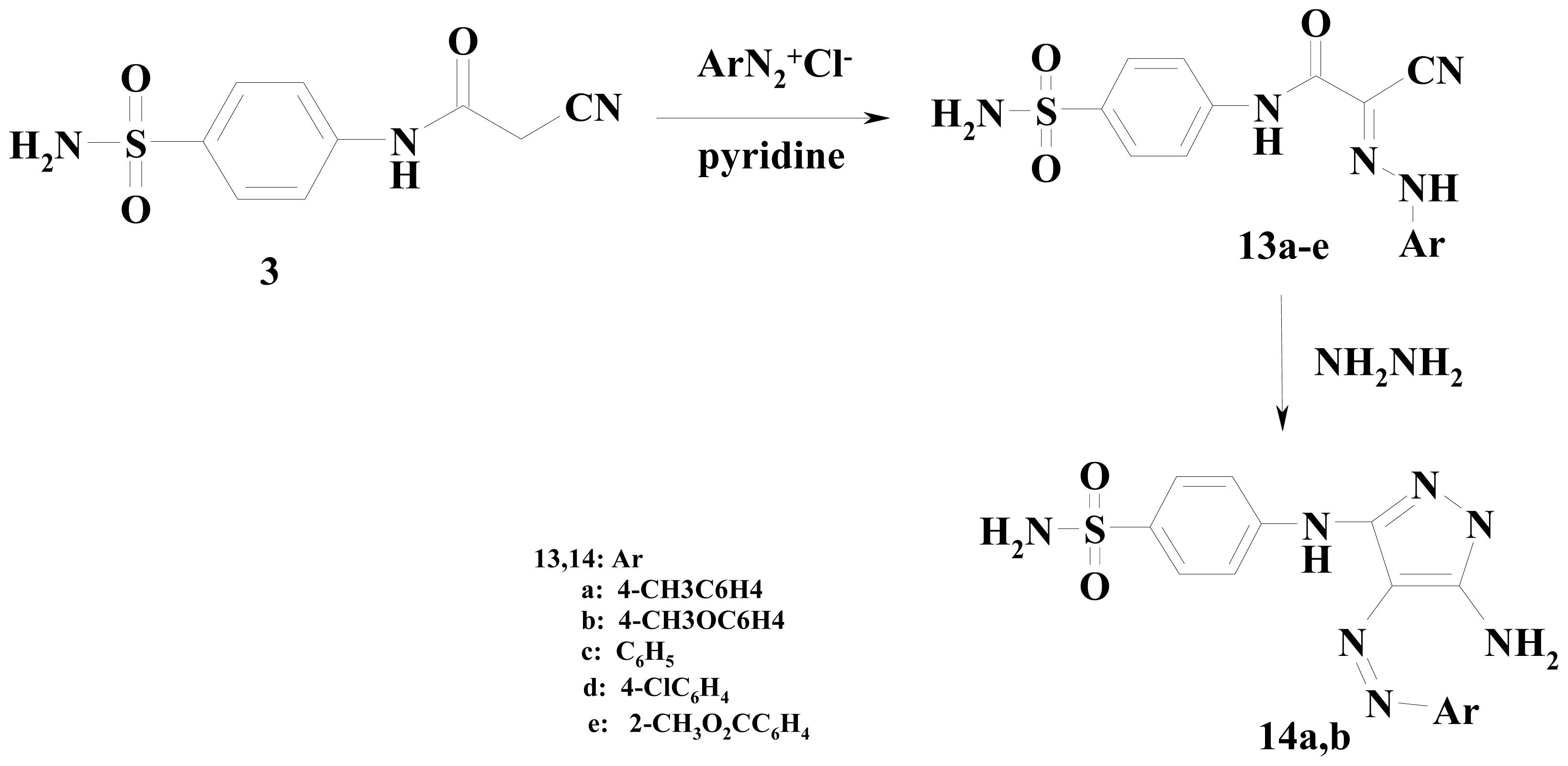
3.2.13. Coupling of N-[4-(Aminosulfonyl)phenyl]-2-cyanoacetamide (3) with the Appropriate Diazonium Salt of Aromatic Amines
3.2.14. 2-Cyano-2-[2-(4-methylphenyl)hydrazinylidene]-N-(4-sulfamoylphenyl)ethanamide (13a)
3.2.15. 2-Cyano-2-[2-(4-methoxyphenyl)hydrazinylidene]-N-(4-sulfamoylphenyl)ethanamide (13b)
3.2.16. 2-Cyano-2-(2-phenylhydrazinylidene)-N-(4-sulfamoylphenyl)ethanamide (13c)
3.2.17. 2-[2-(4-Chlorophenyl)hydrazinylidene]-2-cyano-N-(4-sulfamoylphenyl)ethanamide (13d)
3.2.18. Methyl 4-[2-(1-Cyano-2-oxo-2-{(4-sulfamoylphenyl)amino}ethylidene)hydrazinyl]benzoate (13e)
3.2.19. Synthesis of Aminopyrazoles 14a,b
3.2.20. 4-({5-Amino-4-[(4-methylphenyl)diazenyl]-1H-pyrazol-3-yl}amino)benzenesulfonamide (14a)
3.2.21. 4-({5-Amino-4-[(4-methoxyphenyl)diazenyl]-1H-pyrazol-3-yl}amino)benzenesulfonamide (14b)
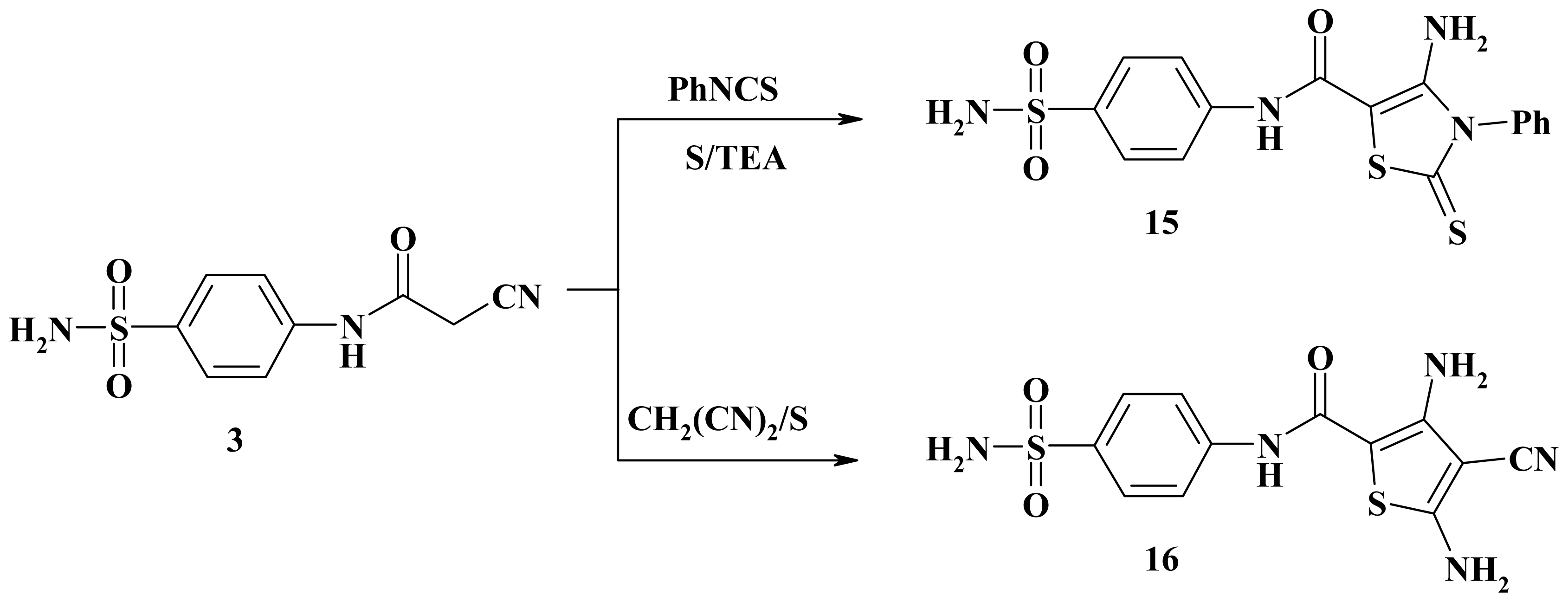
3.2.22. 4-Amino-3-phenyl-N-(4-sulfamoylphenyl)-2-thioxo-2,3-dihydro-1,3-thiazole-5-carboxamide (15)
3.2.23. 3,5-Diamino-4-cyano-N-(4-sulfamoylphenyl)thiophene-2-carboxamide (16)
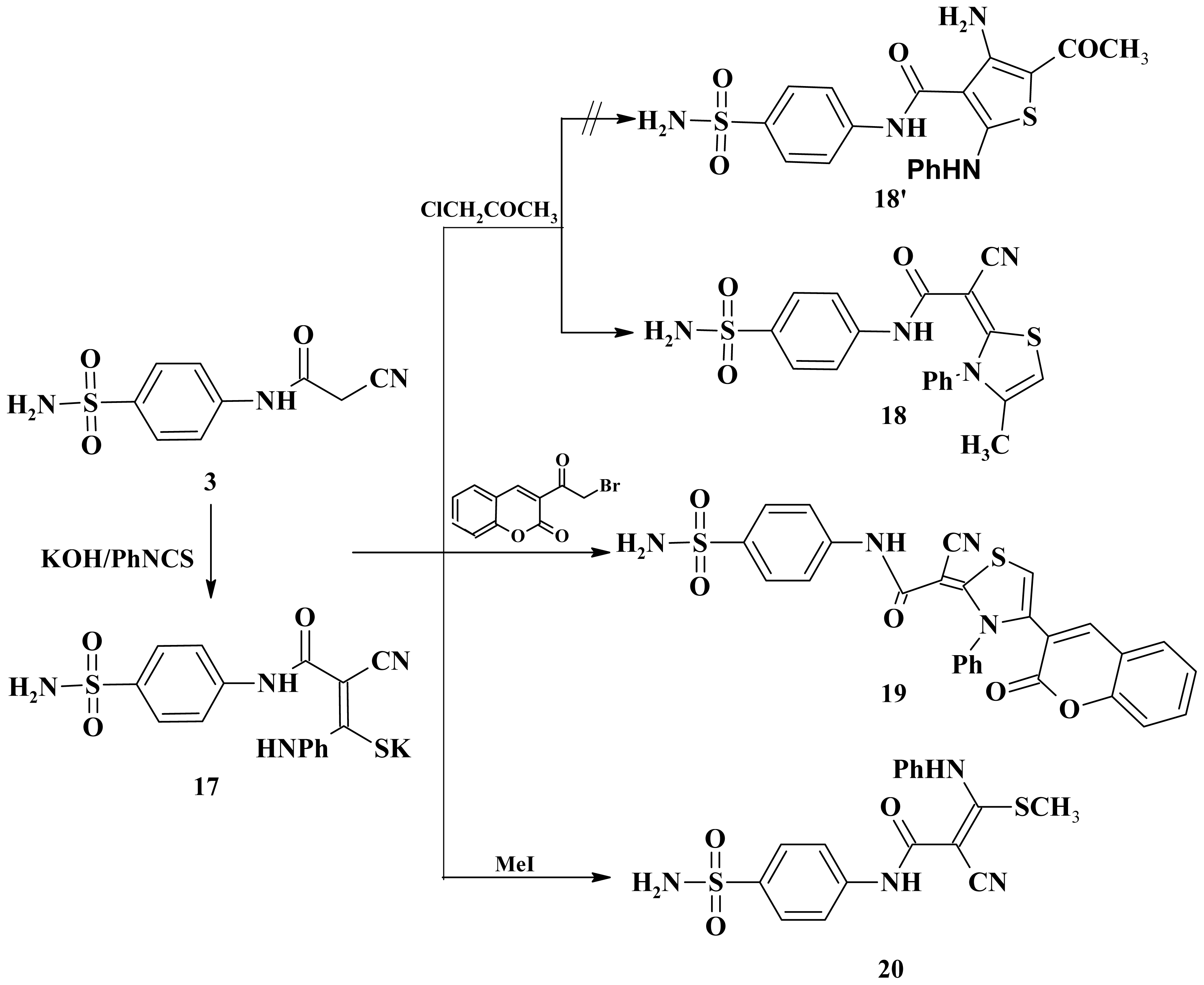
3.2.24. Synthesis of 18, 19 and 20
3.2.25. N-[4-(Aminosulfonyl)phenyl]-2-cyano-2-(4-methyl-3-phenyl-1,3-thiazol-2(3H)-ylidene)acet- amide (18)
3.2.26. 2-Cyano-2-[4-(2-oxo-2H-chromen-4-yl]-3-phenyl-1,3-thiazol-2(3H)-ylidene)-N-(4-sulfamoyl- phenyl)ethanamide (19)
3.2.27. 2-Cyano-3-(methylsulfanyl)-3-(phenylamino)-N-(4-sulfamoylphenyl)prop-2-enamide (20)
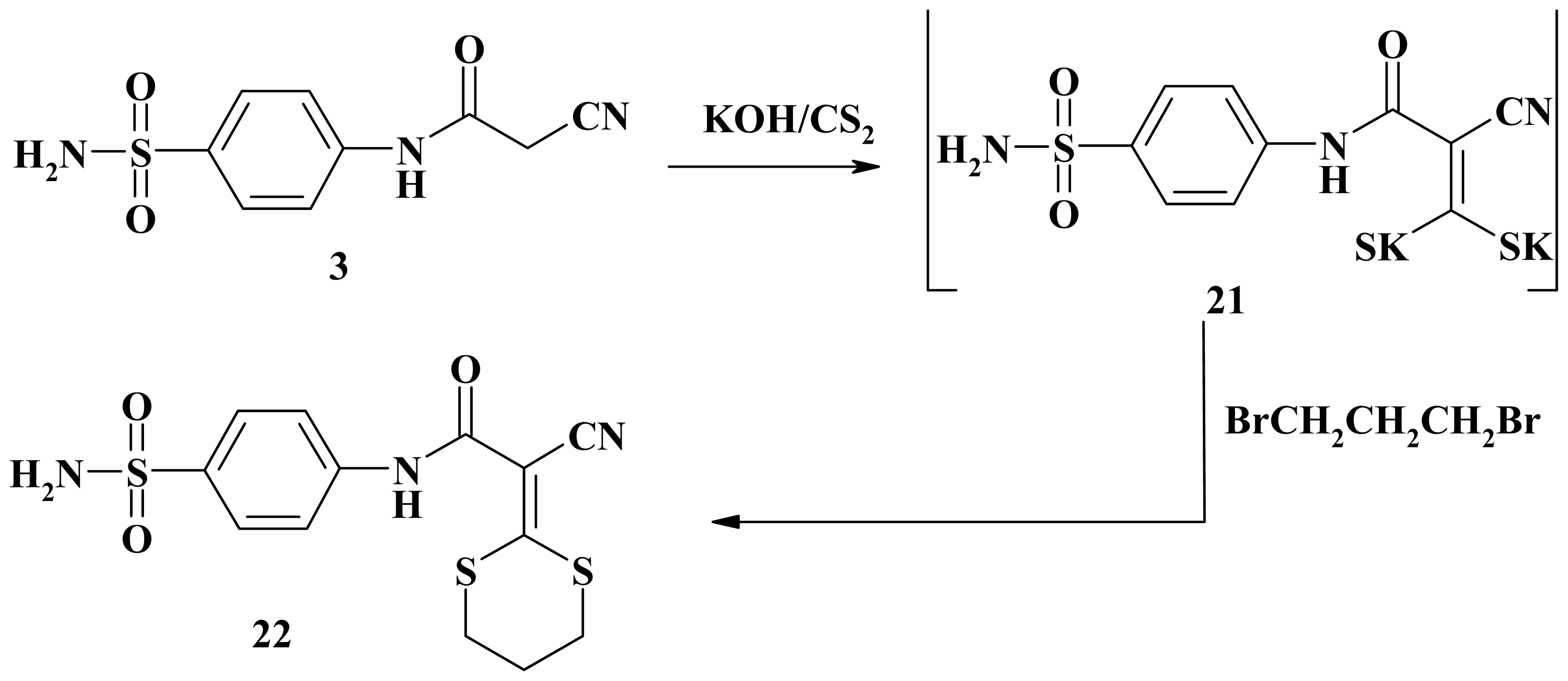
3.2.28. 2-Cyano-2-(1,3-dithian-2-ylidene)-N-(4-sulfamoylphenyl)acetamide (22)
3.3. Antimicrobial Evaluation
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- El-Gaby, M.S.A.; Atalla, A.A.; Gaber, A.M.; Abd Al-Wahab, K.A. Studies on aminopyrazoles: Antibacterial activity of some novel pyrazolo[1,5-a]pyrimidines containing sulfonamido moieties. Il Farm 2000, 55, 596–602. [Google Scholar]
- El-Gaby, M.S.A.; Taha, N.M.; Micky, J.A.; El-Sharief, M.A.M.Sh. Preparation of some novel 3, 5-diaminopyrazole, pyrazolo [1,5-a][1,3,5]triazine and pyrazolo [1,5-a]pyrimidine derivatives containing sulfonamido moieties as antimicrobial agents. Acta Chim. Slov 2002, 49, 159–171. [Google Scholar]
- El-Gaby, M.S.A.; Gaber, A.M.; Atalla, A.A.; Abd Al-Wahab, K.A. Novel synthesis and antifungal activity of pyrrole and pyrrolo[2,3-d]pyrimidine derivatives containing sulfonamido moieties. Il Farm 2002, 57, 613–617. [Google Scholar]
- Maren, T.H. Relations between structure and biological activity of sulfonamides. Annu. Rev. Pharmacol. Toxicol 1976, 16, 309–327. [Google Scholar]
- Alafeefy, A.M.; Isik, S.; Abdel-Aziz, H.A.; Ashour, A.E.; Vullo, D.; Al-Jaber, N.A.; Supuran, C.T. Carbonic anhydrase inhibitors: Benzenesulfonamides incorporating cyanoacrylamide moieties are low nanomolar/subnanomolar inhibitors of the tumor-associated isoforms IX and XII. Bioorg. Med. Chem 2013, 21, 1396–1403. [Google Scholar]
- Roifman, C.M.; Aviv, G.; Alexander, L. Preparation of N-benzyl-3-aryl-2-cyanoacrylamides activity for treatment of neoplastic disorders. PCT. Int. Appl. WO Patent 0055, 128, 2000. [Google Scholar]Chem. Abstr. 133, 237695h.
- Fadda, A.A.; Mukhtar, M.M.; Refat, H.M. Utility of activated nitriles in the synthesis of some new heterocyclic compounds. Am. J. Org. Chem 2012, 2, 32–40. [Google Scholar]
- Labro, M.T. Immunomodulation by antibacterial agents: Is it clinically relevant. Drugs 1993, 45, 319–328. [Google Scholar]
- Azab, M.E.; Youssef, M.M.; El-Bordany, E.A. Synthesis and antibacterial evaluation of novel heterocyclic compounds containing a sulfonamido moiety. Molecules 2013, 18, 832–844. [Google Scholar]
- Abu-Hashem, A.A.; Gouda, M.A.; Badria, F.A. Synthesis of some new pyrimido [2′,1′:2,3] thiazolo[4,5-b]quinoxaline derivatives as anti-inflammatory and analgesic agents. Eur. J. Med. Chem 2010, 45, 1976–1981. [Google Scholar]
- Gouda, M.A.; Berghot, M.A.; Abd El-Ghani, G.E.; Khalil, A.M. Synthesis and antimicrobial activities of some new thiazole and pyrazole derivatives based on 4,5,6,7-tetrahydrobenzo thiophene moiety. Eur. J. Med. Chem 2010, 45, 1338–1345. [Google Scholar]
- Khalil, A.M.; Berghot, M.A.; Gouda, M.A. Synthesis and antibacterial activity of some new thiazole and thiophene derivatives. Eur. J. Med. Chem. 2009a, 44, 4434–4440. [Google Scholar]
- Khalil, A.M.; Berghot, M.A.; Gouda, M.A.; Abd El-Ghani, G.E. Synthesis and antimicrobial evaluation of some new thiophene derivatives. Synth. Commun. 2010a, 40, 1658–1669. [Google Scholar]
- Khalil, A.M.; Berghot, M.A.; Gouda, M.A.; Shoeib, A.I. Synthesis and antimicrobial of certain new thiazolidinone, thiazoline, and thiophene derivatives. Phosphorus Sulfur Silicon 2010b, 185, 1455–1462. [Google Scholar]
- Wilby, M.J.; Hutchinson, P.J. The pharmacology of chlormethiazole: A potential neuroprotective agent? CNS Drug Rev 2004, 10, 281–294. [Google Scholar]
- Harnett, J.J.; Roubert, V.; Dolo, C.; Charnet, C.; Spinnewyn, B.; Cornet, S.; Rolland, A.; Marin, J.G.; Bigg, D.; Chabrier, P.E. Phenolic thiazoles as novel orally-active neuroprotective agents. Bioorg. Med. Chem. Lett 2004, 14, 157–160. [Google Scholar]
- Anderson, W.K.; Dean, D.C.; Endo, T. Synthesis, chemistry, and antineoplastic activity of α-halopyridinium salts: Potential pyridone prodrugs of acylated vinylogous carbinolamine tumor inhibitors. J. Med. Chem 1990, 33, 1667–1675. [Google Scholar]
- Li, Q.; Mitscher, L.A.; Shen, L.L. The 2-pyridone antibacterial agents: Bacterial topoisomerase inhibitors. Med. Res. Rev 2000, 20, 231–293. [Google Scholar]
- Dragovich, P.S.; Prins, T.J.; Zhou, R.; Brown, E.L.; Maldonado, F.C.; Fuhrman, S.A.; Zalman, L.S.; Tuntland, T.; Lee, C.A.; Patick, A.K.; et al. Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 6: Structure–activity studies of orally bioavailable, 2-pyridone-containing peptidomimetics. J. Med. Chem 2002, 45, 1607–1623. [Google Scholar]
- Dragovich, P.S.; Prins, T.J.; Zhou, R.; Johnson, T.O.; Brown, E.L.; Maldonado, F.C.; Fuhrman, S.A.; Zalman, L.S.; Patick, A.K.; Matthews, D.A.; et al. Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. Part 7: Structure–activity studies of bicyclic 2-pyridone-containing peptidomimetics. Bioorg. Med. Chem. Lett 2002, 12, 733–738. [Google Scholar]
- Darwish, E.S.; Kheder, N.A.; Farag, A.M. Synthesis and antimicrobial evaluation of some new pyridine based heterocycles. Heterocycles 2010, 81, 2247–2256. [Google Scholar]
- Darwish, E.S.; Mahmoud, F.F.; Hussein, A.M.; Altalbawy, F.M. β-Oxoanilides in heterocyclic: Synthesis of som new pyridazine and polyfunctionally substituted heterocyclic compounds. Arab. J. Chem 2013, in press. [Google Scholar]
- Ammar, Y.A.; Saleh, N.M.; Micky, J.A.; Abas, H.S.; El-Gaby, M.S.A. Activated nitriles in heterocyclic chemistry: Facile synthesis and antimicrobial activity of some pyrimidine, pyrazolopyrimidine and pyrazolotriazinederivatives containing sulfonamido moiety. Indian J. Chem 2004, 43B, 2203–2211. [Google Scholar]
- El-Bayouki, K.A.M.; Basyouni, W.M.; Aly, Y.A.M.M.; Abbas, S.Y. Novel 4(3H)-quinazolinones containing biologically active thiazole, pyridinone and chromene of expected antitumor and antifungal activities. Eur. J. Chem 2011, 2, 455–462. [Google Scholar]
- Gorobets, Y.N.; Yousefi, B.H.; Belaj, F.; Kappe, C.O. Rapid microwave-assisted solution phase synthesis of substituted 2-pyridone libraries. Tetrahedron 2004, 60, 8633–8644. [Google Scholar]
- Esmail, R.; Kurzer, F. Heterocyclic compounds from urea derivatives. Part XXIII. Thiobenzoylated thiocarbonohydrazides and their cyclization. J. Chem. Soc 1975, 18, 1787–1791. [Google Scholar]
- Muanz, D.N.; Kim, B.W.; Euler, K.L.; Williams, L. Antibacterial and antifungal activities of nine medicinal plants from Zaire. Int. J. Pharmacogn 1994, 32, 337–345. [Google Scholar]
- Harborne, J.B.; Williams, C.A. A survey of antifungal compounds from higher plants, 1982–1993. Phytochemistry 1994, 37, 19–42. [Google Scholar]
- Koelsch, C.F. Bromination of acetocoumarin. J. Am. Chem. Soc 1950, 72, 2993–2995. [Google Scholar]
- Czerney, P.; Hartman, H. 3-α-Bromoacetyl-coumarines as synthones for heterocyclic substituted coumarines. J. Prakt. Chem 1983, 325, 551–560. [Google Scholar]
- Ammar, Y.A.; Aly, M.M.; Al-Sehem, A.G.; Mohamed, Y.A.; Salem, M.A.; El-Gaby, M.S.A. Cyanoacetanilides intermediates in heterocyclic synthesis. Part 4: Preparation of some hitherto unknown thiazolidine and bisthiazolidine derivatives. Phosphorus Sulfur Silicon 2008, 183, 1710–1721. [Google Scholar]
- Butler, R.N. The diazotization of heterocyclic primary amines. Chem. Rev 1975, 75, 241–257. [Google Scholar]
- Ammar, Y.A.; Aly, M.M.; Al-Sehemi, A.G.; Salem, M.A.; El-Gaby, M.S.A. Cyanoacetanilides intermediates in heterocyclic synthesis. Part 5: Preparation of hitherto unknown 5-aminopyrazole and pyrazolo[1,5-a]pyrimidine derivatives containing sulfamoyl moiety. J. Chin. Chem. Soc 2009, 56, 1064–1071. [Google Scholar]
| Comp. | Inhibition zone diameter (cm) | |||||||
|---|---|---|---|---|---|---|---|---|
| Gram (+) | Gram (−) | Fungi | ||||||
| Standard values | (SP) | (BS) | (PA) | (EC) | (AF) | (SR) | (GC) | (CA) |
| 23.8 ± 0.2 | 32.4 ± 0.3 | 17.3 ± 0.1 | 19.9 ± 0.3 | 23.7 ± 0.2 | 19.7 ± 0.2 | 28.7 ± 0.2 | 25.4 ± 0.1 | |
| 5a | 18.9 ± 0.44 | 21.7 ± 0.25 | 11.6 ± 0.19 | 15.4 ± 0.39 | 20.2 ± 0.55 | 16.3 ± 0.25 | 22.4 ± 0.58 | 19.6 ± 0.33 |
| 5c | 16.3 ± 0.55 | 18.3 ± 0.25 | NA | NA | 17.3 ± 0.44 | 12.6 ± 0.25 | 19.0 ± 0.58 | 16.9 ± 0.25 |
| 7b | 16.9 ± 0.58 | 18.2 ± 0.44 | NA | 11.9 ± 0.63 | 15.7 ± 0.33 | 13.8 ± 0.25 | 18.3 ± 0.34 | NA |
| 7c | 18.3 ± 0.25 | 22.6 ± 0.44 | 13.1 ± 0.32 | 20.3 ± 0.09 | 20.6 ± 0.58 | 16.7 ± 0.33 | 22.4 ± 0.36 | 17.6 ± 0.58 |
| 10 | 16.7 ± 0.36 | 19.2 ± 0.27 | NA | 13.6 ± 0.36 | 16.8 ± 0.39 | 13.4 ± 0.58 | 19.6 ± 0.19 | 15.9 ± 0.44 |
| 11 | NA | NA | NA | NA | 15.7 ± 0.36 | 11.2 ± 0.33 | 17.3 ± 0.44 | 13.3 ± 0.36 |
| 13b | 12.3 ± 0.58 | 12.7 ± 0.37 | NA | 8.5 ± 0.37 | 17.6 ± 0.58 | 15.4 ± 0.25 | 12.6 ± 0.38 | NA |
| 14a | 17.5 ± 0.44 | 19.8 ± 0.63 | NA | 18.9 ± 0.25 | 15.3 ± 0.55 | 13.4 ± 0.35 | 11.5 ± 0.58 | NA |
| 15 | 15.0 ± 0.43 | 17.4 ± 0.53 | 12.3 ± 0.25 | 17.8 ± 0.03 | 11.3 ± 0.34 | 12.1 ± 0.25 | 15.3 ± 0.38 | NA |
| 16 | NA | NA | NA | NA | NA | NA | NA | NA |
| 18 | 16.9 ± 0.58 | 18.2 ± 0.44 | NA | 11.9 ± 0.63 | 16.2 ± 0.36 | 15.0 ± 0.44 | 17.6 ± 0.58 | NA |
| 19 | 12.9 ± 0.63 | 13.2 ± 0.58 | NA | 10.8 ± 0.44 | 18.7 ± 0.36 | 16.9 ± 0.27 | 13.4 ± 0.65 | NA |
| 22 | 12.3 ± 0.58 | 12.7 ± 0.37 | NA | 10.8 ± 0.44 | 17.6 ± 0.58 | 15.4 ± 0.25 | 12.6 ± 0.38 | NA |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Darwish, E.S.; Fattah, A.M.A.; Attaby, F.A.; Al-Shayea, O.N. Synthesis and Antimicrobial Evaluation of Some Novel Thiazole, Pyridone, Pyrazole, Chromene, Hydrazone Derivatives Bearing a Biologically Active Sulfonamide Moiety. Int. J. Mol. Sci. 2014, 15, 1237-1254. https://doi.org/10.3390/ijms15011237
Darwish ES, Fattah AMA, Attaby FA, Al-Shayea ON. Synthesis and Antimicrobial Evaluation of Some Novel Thiazole, Pyridone, Pyrazole, Chromene, Hydrazone Derivatives Bearing a Biologically Active Sulfonamide Moiety. International Journal of Molecular Sciences. 2014; 15(1):1237-1254. https://doi.org/10.3390/ijms15011237
Chicago/Turabian StyleDarwish, Elham S., Azza M. Abdel Fattah, Fawzy A. Attaby, and Oqba N. Al-Shayea. 2014. "Synthesis and Antimicrobial Evaluation of Some Novel Thiazole, Pyridone, Pyrazole, Chromene, Hydrazone Derivatives Bearing a Biologically Active Sulfonamide Moiety" International Journal of Molecular Sciences 15, no. 1: 1237-1254. https://doi.org/10.3390/ijms15011237
APA StyleDarwish, E. S., Fattah, A. M. A., Attaby, F. A., & Al-Shayea, O. N. (2014). Synthesis and Antimicrobial Evaluation of Some Novel Thiazole, Pyridone, Pyrazole, Chromene, Hydrazone Derivatives Bearing a Biologically Active Sulfonamide Moiety. International Journal of Molecular Sciences, 15(1), 1237-1254. https://doi.org/10.3390/ijms15011237





