Quorum Quenching Enzymes and Their Application in Degrading Signal Molecules to Block Quorum Sensing-Dependent Infection
Abstract
:1. Introduction
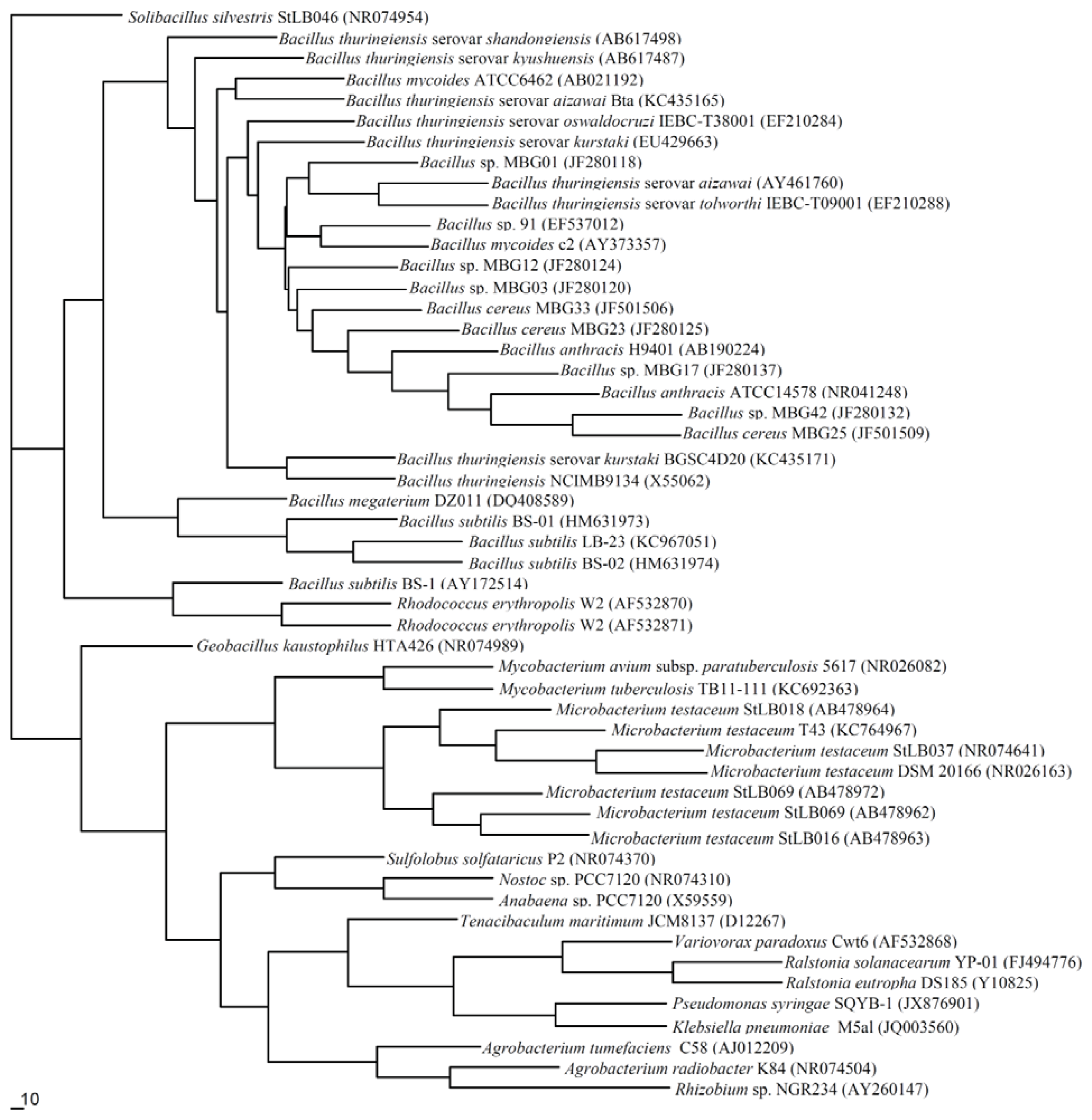
2. Biodiversity of Organisms with Potential to Quench QS Signals
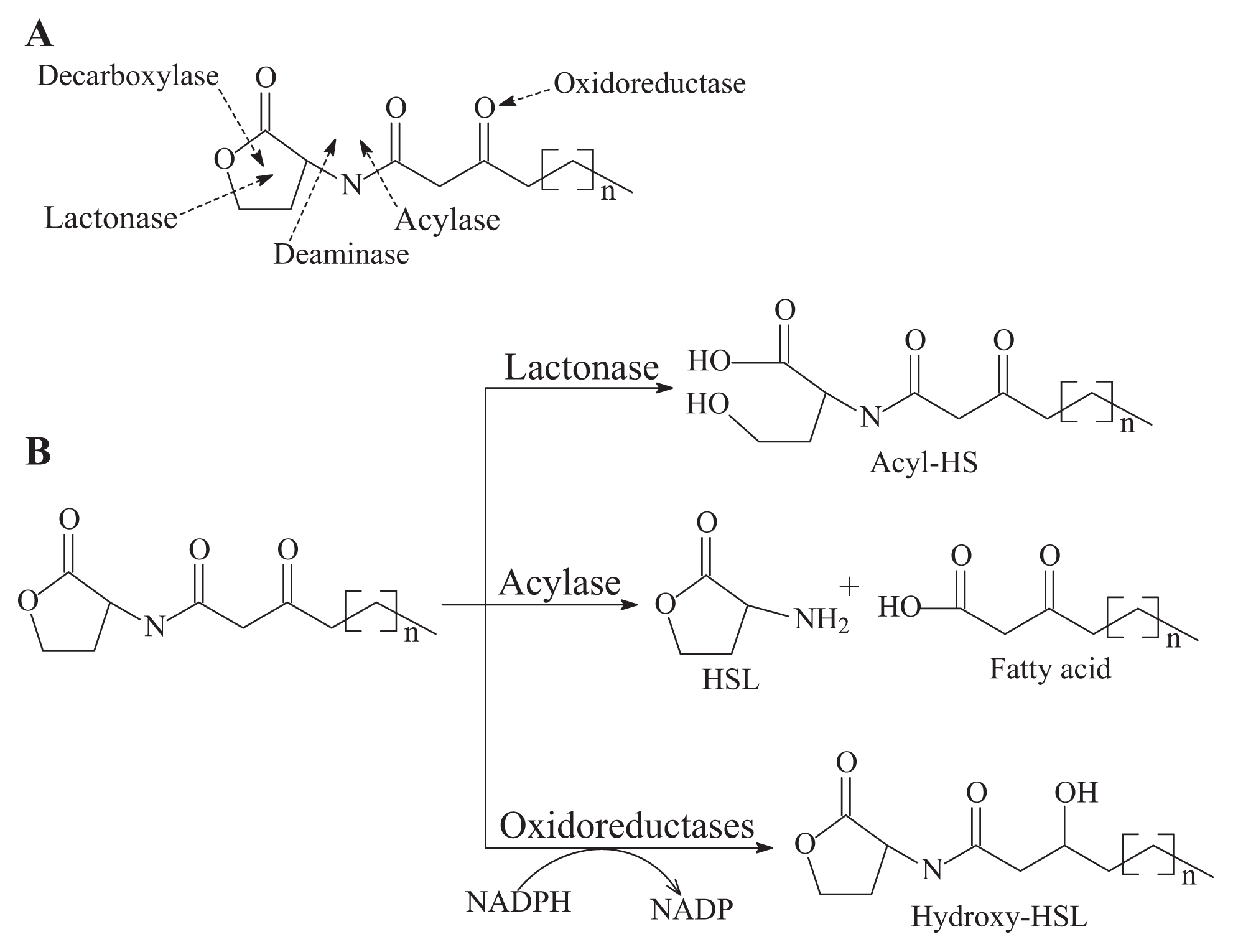
3. Enzymatic Degradation of QS Signals by QQ Enzymes
- (i)
- AHL-lactonase cleaves the homoserine lactone ring of molecule AHLs in a hydrolytic and reversible manner to open the homoserine lactone ring, as shown in Figure 2B, which renders the QS molecule incapable of binding to the target transcriptional regulator and attenuates the effectiveness of the signal molecule [2]. Such hydrolysis is identical to pH-mediated lactonolysis and can be reversed by acidification. Two families of lactonases have been identified in prokaryotes according to their overall similarity and the original microbes. One of the well-studied families is represented by the AiiA lactonase metallohydrolase, which requires two Zn2+ ions for full functionality [50,51]. The AiiA-like lactonase activity is not affected by differences in the acyl chain length and substitution in the AHLs. The second type of AHL-lactonase is represented by the QsdA lactonase from the Rh. erythropolis strain W2, which is not related to the AiiA lactonase family, although both are Zn2+-dependent metalloproteins [33]. QsdA belongs to the phosphotriesterase family that harbors phosphotriesterase, lactonase or amidohydrolase activities [33] and is more closely related to the phosphotriesterase-like lactonases, such as SsoPox from Sulfolobus solfataricus, which has a perfectly fitting pocket where the lactone ring and acyl chain interact [52].The first known cluster was designated as AiiA in Bacillus, followed by AttM in Agrobacterium [2,15]. Recently, other types of lactonases, such as BpiB, AiiM and AidH, have been identified and extend the diversity of the lactonase family proteins [30,31,53]. All of these lactonases are Zn2+-dependent lactonases that occurred in the bacterial genera Bacillus [13], Agrobacterium [15], Rhodococcus [16], Streptomyces [17], Arthrobacter [18], Pseudomonas [19] and Klebsiella [18], except the lactonase derived from Rhodococcus, which forms a new family within the metal-dependent lactonases [33].
- (ii)
- AHL-acylase irreversibly hydrolyzes the amide linkage between the acyl chain and homoserine moiety of AHL molecules. As shown in Figure 2B, this process releases homoserine lactone and the corresponding fatty acid, which do not exhibit any residual signaling activity [10]. The AHL-acylase was first described in the V. paradoxus strain VAI-C, which showed a wide range of AHL degradation capacity [9]. Subsequently, AHL-acylases from various groups of bacteria have been reported, predominantly including AiiD in Ralstonia sp XJ12B [10]; AhlM in Streptomyces sp. M664 [17]; PvdQ and QuiP in P. aeruginosa PAO1 [19,20,40]; and AiiC in Anabaena sp. PCC7120 [54]. Recently, an aac gene homologous to the AiiD acylase with undemonstrated function was identified from R. solanacearum GMI1000 [38]. Novel AHL-degrading genes have been isolated from the metagenomic libraries constructed from soil samples [50,53].
- (iii)
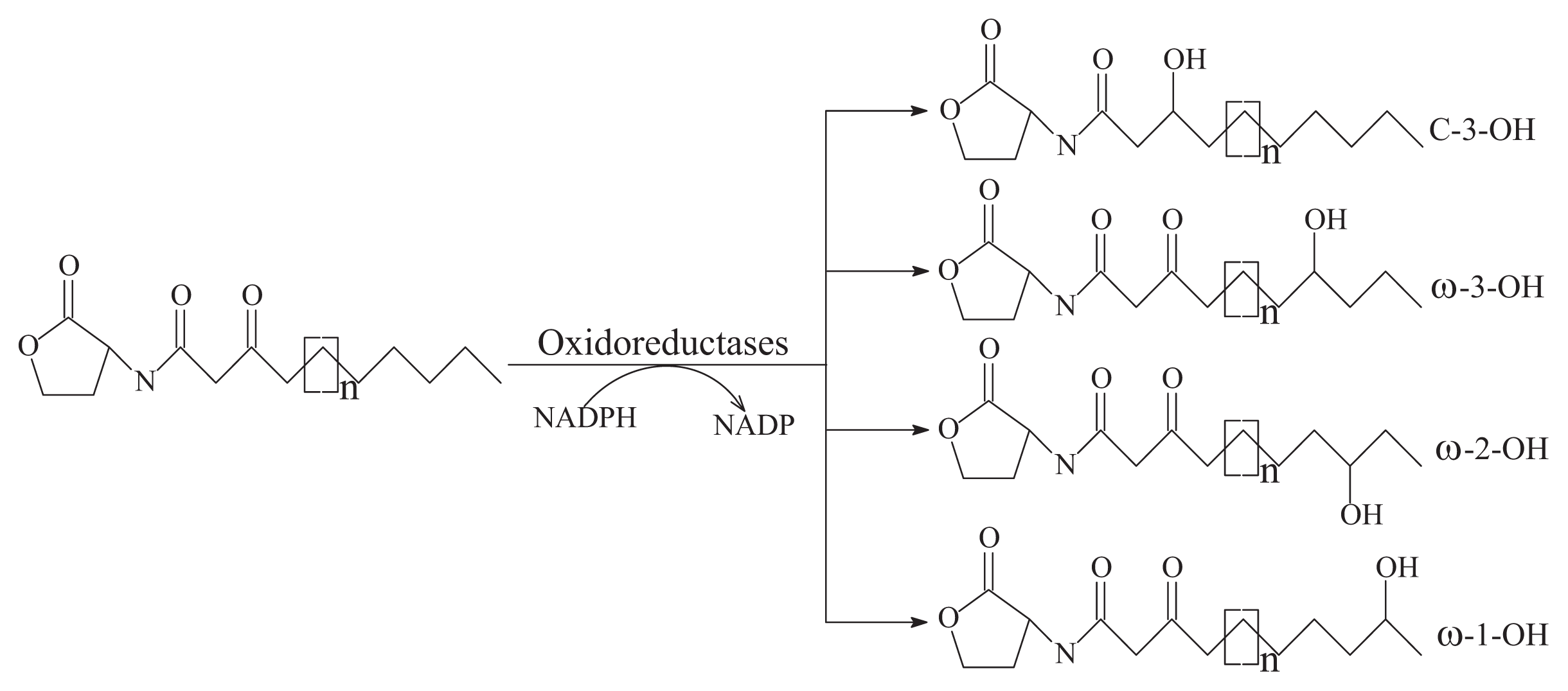
- Oxidoreductase targets the acyl side chain by oxidative or reducing activities and thus catalyzes a modification of the chemical structure of the signal but not degradation, as shown in Figure 2B. Such modification might affect the specificity and recognition of the AHL signal, thus disturbing the activation of the QS-mediated genes regulated by a particular AHL [41]. Long-chained AHLs and fatty acids with varying chain lengths at various positions could be oxidized. Two types of oxidoreductases have been discovered. The P-450/NADPH-P450 reductase, a previously known enzyme with fatty acids as the substrate, has been isolated from B. megaterium CYP102A1 and characterized in detail [55]. This substrate is capable of the efficient oxidation of AHLs at the ω-1, ω-2 and ω-3 carbons of the acyl chain to eliminate their quorum sensing activity (Figure 3). This oxidation of AHLs represents an important and different QQ mechanism: breaking AHL molecules [2,10]. Uroz et al. [22] reported the presence of another enzyme in Rh. erythropolis W in which the 3-oxo substituent of 3-oxo-C14-HSL was reduced to yield the corresponding derivative 3-hydroxy-C14-HSL and the QS system was inactivated. Recently, a novel oxidoreductase BpiB09 derived from a metagenomic library was found to be capable of inactivating 3-oxo-C12-HSL [56]. Its expression in P. aeruginosa PAO1 resulted in significantly reduced pyocyanin production, decreased motility and poor biofilm formation, although AHLs are likely not the native substrate of this metagenome-derived enzyme.
- (iv)
- The AHL-like-lactonases (paraoxonase) from mammalian sera have been described as AHL-lactonase-like enzymes and are involved in the hydrolysis of organophosphates [57].
4. Function and Characteristics of Enzymes with QQ Activity
5. Molecular Phylogenesis of QQ Enzymes in the QS System
6. Enzymatic Degradation of QS Signal Molecules in the Cell-Cell Signal Transduction Pathway
7. Application of Enzymatic Protection in Controlling Microbial Disease by Interfering with the QS System
8. Future Works
9. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| B. | Bacillus |
| E. | Erwinia |
| Pec. | Pectobacterium |
| V. | Variovorax |
| R. | Ralstonia |
| A. | Agrobacterium |
| Rh. | Rhodococcus |
| P. | Pseudomonas |
| K. | Klebsiella |
| M. | Microbacterium |
| S. | Solibacillus |
| C. | Chromobacterium |
| Bur. | Burkholderia |
| Sul. | Sulfolobus |
| Aer. | Aeromonas |
| Pic. | Pichia |
| Sta. | Staphylococcus |
| C4-HSL | N-butanoyl-l-homoserine lactone |
| C6-HSL | N-hexanoyl-l-homoserine lactone |
| C7-HSL | N-heptanoyl-l-homoserine lactone |
| C8-HSL | N-octanoyl-l-homoserine lactone |
| C10-HSL | N-decanoyl-l-homoserine lactone |
| C12-HSL | N-dodecanoyl-l-homoserine lactone |
| C14-HSL | N-tetradecanoyl-l-homoserine lactone |
| 3-oxo-C6-HSL | N-(3-oxohexanoyl)-l-homoserine lactone |
| 3-oxo-C8-HSL | N-(3-oxooctanoyl)-l-homoserine lactone |
| 3-oxo-C10-HSL | N-(3-oxodecanoyl)-l-homoserine lactone |
| 3-oxo-C12-HSL | N-(3-oxododecanoyl)-l-homoserine lactone |
| 3-oxo-C14-HSL | N-(3-oxotetradecanoyl)-l-homoserine lactone |
References
- De Kievit, T.R.; Iglewski, B.H. Bacterial quorum sensing in pathogenic relationships. Infect. Immun 2000, 68, 4839–4849. [Google Scholar]
- Dong, Y.H.; Xu, J.L.; Li, X.Z.; Zhang, L.H. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl. Acad. Sci. USA 2000, 97, 3526–3531. [Google Scholar]
- Rasmussen, T.B.; Givskov, M. Quorum-sensing inhibitors as antipathogenic drugs. Int. J. Med. Microbiol 2006, 296, 149–161. [Google Scholar]
- Li, X.; Du, G.; Chen, J. Use of enzymatic biodegradation for protection of plant against microbial disease. Curr. Top. Biotechnol 2008, 4, 1–12. [Google Scholar]
- Marshall, J. Quorum sensing. Proc. Natl. Acad. Sci. USA 2013, 110, 2690. [Google Scholar]
- Zhang, L.H. Quorum quenching and proactive host defense. Trends Plant Sci 2003, 8, 238–244. [Google Scholar]
- Dong, Y.H.; Wang, L.H.; Zhang, L.H. Quorum-quenching microbial infections: mechanisms and implications. Philos. T. Roy. Soc. B 2007, 362, 1201–1211. [Google Scholar]
- Czajkowski, R.; Jafra, S. Quenching of acyl-homoserine lactone dependent quorum sensing by enzymatic disruption of signal molecules. Acta Biochimica. Polonicol 2009, 56, 1–16. [Google Scholar]
- Leadbetter, J.R.; Greenberg, E.P. Metabolism of acyl-homoserine lactone quorum-sensing signals by Variovorax paradoxus. J. Bacteriol 2000, 182, 6921–6926. [Google Scholar]
- Lin, Y.H.; Xu, J.L.; Hu, J.; Wang, L.H.; Ong, S.L.; Leadbetter, J.R.; Zhang, L.H. Acyl-homoserine lactone acylase from Ralstonia strain XJ12B represents a novel and potent class of quorum-quenching enzymes. Mol. Microbiol 2003, 47, 849–860. [Google Scholar]
- Han, Y.; Chen, F.; Li, N.; Zhu, B.; Li, X. Bacillus marcorestinctum sp. nov., a novel bacterium quenching acylhomoserine lactone quorum-sensing signal from soil. Int. J. Mol. Sci 2010, 11, 507–520. [Google Scholar]
- Dong, Y.H.; Gusti, A.R.; Zhang, Q.; Xu, J.L.; Zhang, L.H. Identification of quorum-quenching N-acyl homoserine lactonases from Bacillus species. Appl. Environ. Microbiol 2002, 68, 1754–1759. [Google Scholar]
- Lee, S.J.; Park, S.Y.; Lee, J.J.; Yum, D.Y.; Koo, B.T.; Lee, J.K. Genes encoding the N-acyl homoserine lactone-degrading enzyme are widespread in many subspecies of Bacillus thuringiensis. Appl. Environ. Microbiol 2002, 68, 3919–3924. [Google Scholar]
- Ulrich, R.L. Quorum quenching: Enzymatic disruption of N-acylhomoserine lactone-mediated bacterial communication in Burkholderia thailandensis. Appl. Environ. Microbiol 2004, 70, 6173–6180. [Google Scholar]
- Carlier, A.; Uroz, S.; Smadja, B.; Fray, R.; Latour, X.; Dessaux, Y.; Faure, D. The Ti plasmid of Agrobacterium tumefaciens harbors an attM-paralogous gene, aiiB, also encoding N-acyl homoserine lactonase activity. Appl. Environ. Microbiol 2003, 69, 4989–4993. [Google Scholar]
- Park, S.Y.; Hwang, B.J.; Shin, M.H.; Kim, J.A.; Kim, H.K.; Lee, J.K. N-acylhomoserine lactonase-producing Rhodococcus spp. with different AHL-degrading activities. FEMS Microbiol. Lett 2006, 261, 102–108. [Google Scholar]
- Park, S.Y.; Kang, H.O.; Jang, H.S.; Lee, J.K.; Koo, B.T.; Yum, D.Y. Identification of extracellular N-acylhomoserine lactone acylase from a Streptomyces sp. and its application to quorum quenching. Appl. Environ. Microbiol 2005, 71, 2632–2641. [Google Scholar]
- Park, S.Y.; Lee, S.J.; Oh, T.K.; Oh, J.W.; Koo, B.T.; Yum, D.Y.; Lee, J.K. AhlD, an N-acylhomoserine lactonase in Arthrobacter sp., and predicted homologues in other bacteria. Microbiology 2003, 149, 1541–1550. [Google Scholar]
- Sio, C.F.; Otten, L.G.; Cool, R.H.; Diggle, S.P.; Braun, P.G.; Bos, R.; Daykin, M.; Camara, M.; Williams, P.; Quax, W.J. Quorum quenching by an N-acyl-homoserine lactone acylase from Pseudomonas aeruginosa PAO1. Infect. Immun 2006, 74, 1673–1682. [Google Scholar]
- Huang, J.J.; Han, J.I.; Zhang, L.H.; Leadbetter, J.R. Utilization of acyl-homoserine lactone quorum signals for growth by a soil pseudomonad and Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol 2003, 69, 5941–5949. [Google Scholar]
- Shepherd, R.W.; Lindow, S.E. Two dissimilar N-acyl-homoserine lactone acylases of Pseudomonas syringae influence colony and biofilm morphology. Appl. Environ. Microbiol 2009, 75, 45–53. [Google Scholar]
- Uroz, S.; Chhabra, S.R.; Cámara, M.; Williams, P.; Oger, P.; Dessaux, Y. N-acylhomoserine lactone quorum-sensing molecules are modified and degraded by Rhodococcus erythropolis W2 by both amidolytic and novel oxidoreductase activities. Microbiology 2005, 151, 3313–3322. [Google Scholar]
- Uroz, S.; Oger, P.; Chhabra, S.R.; Cámara, M.; Williams, P.; Dessaux, Y. N-Acyl homoserine lactones are degraded via an amidolytic activity in Comamonas sp. strain D1. Arch. Microbiol 2007, 187, 249–256. [Google Scholar]
- Morohoshi, T.; Nakazawa, S.; Ebata, A.; Kato, N.; Ikeda, T. Identification and characterization of N-acylhomoserine lactone-acylase from the fish intestinal Shewanella sp. strain MIB015. Biosci. Biotechnol. Biochem 2008, 72, 1887–1893. [Google Scholar]
- Pan, J.; Huang, T.; Yao, F.; Huang, Z.; Powell, C.A.; Qiu, S.; Guan, X. Expression and characterization of aiiA gene from Bacillus subtilis BS-1. Microbiol. Res 2008, 163, 711–716. [Google Scholar]
- Yin, X.T.; Xu, L.; Fan, S.S.; Xu, L.N.; Li, D.C.; Liu, Z.Y. Isolation and characterization of an AHL lactonase gene from Bacillus amyloliquefaciens. World J. Microbiol. Biotechnol 2010, 26, 1361–1367. [Google Scholar]
- Yin, W.F.; Tung, H.J.; Sam, C.K.; Koh, C.L.; Chan, K.G. Quorum quenching Bacillus sonorensis isolated from soya sauce fermentation brine. Sensors 2012, 12, 4065–4073. [Google Scholar]
- Zhang, H.B.; Wang, L.H.; Zhang, L.H. Genetic control of quorum-sensing signal turnover in Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. USA 2002, 99, 4638–4643. [Google Scholar]
- Haudecoeur, E.; Tannières, M.; Cirou, A.; Raffoux, A.; Dessaux, Y.; Faure, D. Different regulation and roles of lactonases AiiB and AttM in Agrobacterium tumefaciens C58. Mol. Plant Microbe. Interact 2009, 22, 529–537. [Google Scholar]
- Mei, G.Y.; Yan, X.X.; Turak, A.; Luo, Z.Q.; Zhang, L.Q. AidH, an alpha/beta-hydrolase fold family member from an Ochrobactrum sp. strain, is a novel N-acylhomoserine lactonase. Appl. Environ. Microbiol 2010, 76, 4933–4942. [Google Scholar]
- Wang, W.Z.; Morohoshi, T.; Ikenoya, M.; Someya, N.; Ikeda, T. AiiM, a novel class of N-acylhomoserine lactonase from the leaf-associated bacterium Microbacterium testaceum. Appl. Environ. Microbiol 2010, 76, 2524–2530. [Google Scholar]
- Morohoshi, T.; Tominaga, Y.; Someya, N.; Ikeda, T. Complete genome sequence and characterization of the N-acylhomoserine lactone-degrading gene of the potato leaf-associated Solibacillus silvestris. J. Biosci. Bioeng 2012, 113, 20–25. [Google Scholar]
- Uroz, S.; Oger, P.M.; Chapelle, E.; Adeline, M.T.; Faure, D.; Dessaux, Y. A Rhodococcus qsdA-encoded enzyme defines a novel class of large-spectrum quorum-quenching lactonases. Appl. Environ. Microbiol 2008, 74, 1357–1366. [Google Scholar]
- Rashid, R.; Morohoshi, T.; Someya, N.; Ikeda, T. Degradation of N-acylhomoserine lactone quorum sensing signaling molecules by potato root surface-associated Chryseobacterium strains. Microbes Environ 2011, 26, 144–148. [Google Scholar]
- Yoon, J.H.; Lee, J.K.; Jung, S.Y.; Kim, J.A.; Kim, H.K.; Oh, T.K. Nocardioides kongjuensis sp. nov., an N-acylhomoserine lactone-degrading bacterium. Int. J. Syst. Evol. Microbiol 2006, 56, 1783–1787. [Google Scholar]
- Kang, B.R.; Lee, J.H.; Ko, S.J.; Lee, Y.H.; Cha, J.S.; Cho, B.H.; Kim, Y.C. Degradation of acyl-homoserine lactone molecules by Acinetobacter sp. strain C1010. Can. J. Microbiol 2004, 50, 935–941. [Google Scholar]
- Morohoshi, T.; Wang, W.Z.; Someya, N.; Ikeda, T. Genome sequence of Microbacterium testaceum StLB037, an N-acylhomoserine lactone-degrading bacterium isolated from potato leaves. J. Bacteriol 2011, 193, 2072–2073. [Google Scholar]
- Chen, C.N.; Chen, C.J.; Liao, C.T.; Lee, C.Y. A probable aculeacin A acylase from the Ralstonia solanacearum GMI1000 is N-acyl-homoserine lactone acylase with quorum-quenching activity. BMC Microbiol 2009, 9, 89. [Google Scholar]
- Wong, C.S.; Yin, W.F.; Sam, C.K.; Koh, C.L.; Chan, K.G. Characterization of wetland quorum quenching Pseudomonas aeruginosa strain 2SW8 and its 2-heptyl-3-hydroxy-4-quinolone production. New Microbiol 2012, 35, 43–51. [Google Scholar]
- Huang, J.J.; Petersen, A.; Whiteley, M.; Leadbetter, J.R. Identification of QuiP, the product of gene PA1032, as the second acyl-homoserine lactone acylase of Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol 2006, 72, 1190–1197. [Google Scholar]
- Chan, K.G.; Atkinson, S.; Mathee, K.; Sam, C.K.; Chhabra, S.R.; Cámara, M.; Koh, C.L.; Williams, P. Characterization of N-acylhomoserine lactone-degrading bacteria associated with the Zingiber officinale (ginger) rhizosphere: Co-existence of quorum quenching and quorum sensing in Acinetobacter and Burkholderia. BMC Microbiol 2011, 11, 51. [Google Scholar]
- Uroz, S.; D’Angelo-Picard, C.; Carlier, A.; Elasri, M.; Sicot, C.; Petit, A.; Oger, P.; Faure, D.; Dessaux, Y. Novel bacteria degrading N-acylhomoserine lactones and their use as quenchers of quorum-sensing-regulated functions of plant-pathogenic bacteria. Microbiology 2003, 149, 1981–1989. [Google Scholar]
- Jafra, S.; Przysowa, J.; Czajkowski, R.; Michta, A.; Garbeva, P.; van der Wolf, J.M. Detection and characterization of N-acyl homoserine lactone-degrading bacteria from the potato rhizosphere. Can. J. Microbiol 2006, 52, 1006–1015. [Google Scholar]
- Dong, Y.H.; Zhang, L.H. Quorum sensing and quorum-quenching enzymes. J. Microbiol 2005, 43, 101–109. [Google Scholar]
- Fray, R.G. Altering plant-microbe interaction through artificially manipulating bacterial quorum sensing. Ann. Bot 2002, 89, 245–253. [Google Scholar]
- Barber, C.E.; Tang, J.L.; Feng, J.X.; Pan, M.Q.; Wilson, T.J.; Slater, H.; Dow, J.M.; Williams, P.; Daniels, M.J. A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol. Microbiol 1997, 24, 555–566. [Google Scholar]
- Holden, M.T.; McGowan, S.J.; Bycroft, B.W.; Stewart, G.S.; Williams, P.; Salmond, G.P. Cryptic carbapenem antibiotic production genes are widespread in Erwinia carotovora: Facile trans activation by the carR transcriptional regulator. Microbiology 1998, 144, 1495–1508. [Google Scholar]
- Wong, C.S.; Yin, W.F.; Choo, Y.M.; Sam, C.K.; Koh, C.L.; Chan, K.G. Coexistence of quorum-quenching and quorum-sensing in tropical marine Pseudomonas aeruginosa strain MW3A. World J. Microbiol. Biotechnol 2011, 28, 453–461. [Google Scholar]
- Krysciak, D.; Schmeisser, C.; Preuß, S.; Riethausen, J.; Quitschau, M.; Grond, S.; Streit, W.R. Involvement of multiple loci in quorum quenching of autoinducer I molecules in the nitrogen-fixing symbiont Rhizobium (Sinorhizobium) sp. strain NGR234. Appl. Environ. Microbiol 2011, 77, 5089–5099. [Google Scholar]
- Riaz, K.; Elmerich, C.; Moreira, D.; Raffoux, A.; Dessaux, Y.; Faure, D. A metagenomic analysis of soil bacteria extends the diversity of quorum-quenching lactonases. Environ. Microbiol 2008, 10, 560–570. [Google Scholar]
- Kim, M.H.; Choi, W.C.; Kang, H.O.; Lee, J.S.; Kang, B.S.; Kim, K.J.; Derewenda, Z.S.; Oh, T.K.; Lee, C.H.; Lee, J.K. The molecular structure and catalytic mechanism of a quorum-quenching N-acyl-l-homoserine lactone hydrolase. Proc. Natl. Acad. Sci. USA 2005, 102, 17606–17611. [Google Scholar]
- Elias, M.; Dupuy, J.; Merone, L.; Mandrich, L.; Porzio, E.; Moniot, S.; Rochu, D.; Lecomte, C.; Rossi, M.; Masson, P.; et al. Structural basis for natural lactonase and promiscuous phosphotriesterase activities. J. Mol. Biol 2008, 379, 1017–1028. [Google Scholar]
- Schipper, C.; Hornung, C.; Bijtenhoorn, P.; Quitschau, M.; Grond, S.; Streit, W.R. Metagenome-derived clones encoding two novel lactonase family proteins involved in biofilm inhibition in Pseudomonas aeruginosa. Appl. Environ. Microbiol 2009, 75, 224–233. [Google Scholar]
- Romero, M.; Diggle, S.P.; Heeb, S.; Camara, M.; Otero, A. Quorum quenching activity in Anabaena sp PCC 7120: Identification of AiiC, a novel AHL-acylase. FEMS Microbiol. Lett 2008, 280, 73–80. [Google Scholar]
- Chowdhary, P.K.; Keshavan, N.; Nguyen, H.Q.; Peterson, J.A.; González, J.E.; Haines, D.C. Bacillus megaterium CYP102A1 oxidation of acyl homoserine lactones and acyl homoserines. Biochemistry 2007, 46, 14429–14437. [Google Scholar]
- Bijtenhoorn, P.; Mayerhofe, H.; Müller-Dieckman, J.; Utpatel, C.; Schippe, C.; Hornung, C.; Szesny, M.; Grond, S.; Thürmer, A.; Brzuszkiewicz, E.; et al. A novel metagenomic short-chain dehydrogenase/reductase attenuates Pseudomonas aeruginosa biofilm formation and virulence on Caenorhabditis elegans. PLoS One 2011, 6, e26278. [Google Scholar]
- Yang, F.; Wang, L.H.; Wang, J.; Dong, Y.H.; Hu, J.Y.; Zhang, L.H. Quorum quenching activity is widely conserved in the sera of mammalian species. FEBS Lett 2005, 579, 3713–3717. [Google Scholar]
- Uroz, S.; Dessaux, Y.; Oger, P. Quorum sensing and quorum quenching: the Yin and Yang of bacterial communication. ChemBiochem 2009, 10, 205–216. [Google Scholar]
- Riaz, K.; Elmerich, C.; Raffoux, A.; Moreira, D.; Dessaux, Y.; Faure, D. Metagenomics revealed a quorum quenching lactonase QlcA from yet unculturable soil bacteria. Commun. Agric. Appl. Biol. Sci 2008, 73, 3–6. [Google Scholar]
- Merone, L.; Mandrich, L.; Rossi, M.; Manco, G. A thermostable phosphotriesterase from the archaeon Sulfolobus solfataricus: Cloning, overexpression and properties. Extremophiles 2005, 9, 297–305. [Google Scholar]
- Chow, J.Y.; Xue, B.; Lee, K.H.; Tung, A.; Wu, L.; Robinson, R.C.; Yew, W.S. Directed evolution of a thermostable quorum-quenching lactonase from the amidohydrolase superfamily. J. Biol. Chem 2010, 285, 40911–40920. [Google Scholar]
- Afriat, L.; Roodveldt, C.; Manco, G.; Tawfik, D.S. The latent promiscuity of newly identified microbial lactonases is linked to a recently diverged phosphotriesterase. Biochemistry 2006, 45, 13677–13686. [Google Scholar]
- Chow, J.Y.; Wu, L.; Yew, W.S. Directed evolution of a quorum-quenching lactonase from Mycobacterium avium subsp. paratuberculosis K-10 in the amidohydrolase superfamily. Biochemistry 2009, 48, 4344–4353. [Google Scholar]
- Bijtenhoorn, P.; Schipper, C.; Hornung, C.; Quitschau, M.; Grond, S.; Weiland, N.; Streit, W.R. BpiB05, a novel metagenome-derived hydrolase acting on N-acylhomoserine lactones. J. Biotechnol 2011, 155, 86–94. [Google Scholar]
- Morohoshi, T.; Ebata, A.; Nakazawa, S.; Kato, N.; Ikeda, T. N-acyl homoserine lactone-producing or -degrading bacteria isolated from the intestinal microbial flora of Ayu fish (Plecoglossus altivelis). Microbes Environ 2005, 20, 264–268. [Google Scholar]
- Romero, M.; Avendaño-Herrera, R.; Magariños, B.; Cámara, M.; Otero, A. Acyl homoserine lactone production and degradation by the fish pathogen Tenacibaculum maritimum, a member of the Cytophaga-Flavobacterium-Bacteroides (CFB) group. FEMS Microbiol. Lett 2010, 304, 131–139. [Google Scholar]
- Wahjudi, M.; Papaioannou, E.; Hendrawati, O.; van Assen, A.H.G.; van Merkerk, R.; Cool, R.H.; Poelarends, G.J.; Quax, W.J. PA0305 of Pseudomonas aeruginosa is a quorum quenching acylhomoserine lactone acylase belonging to the Ntn hydrolase superfamily. Microbiology 2011, 157, 2042–2055. [Google Scholar]
- Chai, Y.; Tsai, C.S.; Cho, H.; Winans, S.C. Reconstitution of the biochemical activities of the AttJ repressor and the AttK, AttL, and AttM catabolic enzymes of Agrobacterium tumefaciens. J. Bacteriol 2007, 189, 3674–3679. [Google Scholar]
- Wang, L.H.; Weng, L.X.; Dong, Y.H.; Zhang, L.H. Specificity and enzyme kinetics of the quorum-quenching N-acyl homoserine lactone lactonase (AHL lactonase). J. Biol. Chem 2004, 279, 13645–13651. [Google Scholar]
- Chen, R.; Zhou, Z.; Cao, Y.; Bai, Y.; Yao, B. High yield expression of an AHL lactonase from Bacillus sp. B546 in Pichia pastoris and its application to reduce Aeromonas hydrophila mortality in aquaculture. Microb. Cell Fact 2010, 9, 39. [Google Scholar]
- Cao, Y.; He, S.; Zhou, Z.; Zhang, M.; Mao, W.; Zhang, H.; Yao, B. Administered thermostable N-acyl homoserine lactonase from Bacillus sp. strain AI96 attenuates Aeromonas hydrophila infection in zebrafish. Appl. Environ. Microbiol 2012, 78, 1899–1908. [Google Scholar]
- Ng, F.S.W.; Wright, D.M.; Seah, S.Y.K. Characterization of a phosphotriesterase-like lactonase from Sulfolobus solfataricus and its immobilization for disruption of quorum sensing. Appl. Environ. Micobiol 2011, 77, 1181–1186. [Google Scholar]
- Schmeisser, C.; Steele, H.; Streit, W.R. Metagenomics, biotechnology with non culturable microbes. Appl. Microbiol. Biotechnol 2007, 75, 955–962. [Google Scholar]
- Williamson, L.L.; Borlee, B.R.; Schloss, P.D.; Guan, C.; Allen, H.K.; Handelsman, J. Intracellular screen to identify metagenomic clones that induce or inhibit a quorum-sensing biosensor. Appl. Environ. Microbiol 2005, 71, 6335–6344. [Google Scholar]
- Fan, X.; Liu, X.; Liu, Y. The cloning and characterization of one novel metagenome-derived thermostable esterase acting on N-acylhomoserine lactones. J. Mol. Catal. B 2012, 83, 29–37. [Google Scholar]
- Liao, R.Z.; Yu, J.G.; Himo, F. Reaction mechanism of the dinuclear zinc enzyme N-acyl-l-homoserine lactone hydrolase: A quantum chemical study. Inorg. Chem 2009, 48, 1442–1448. [Google Scholar]
- Momb, J.; Wang, C.; Liu, D.; Thomas, P.W.; Petsko, G.A.; Guo, H.; Ringe, D.; Fast, W. Mechanism of the quorum-quenching lactonase (AiiA) from Bacillus thuringiensis. 2. Substrate modeling and active site mutations. Biochemistry 2008, 47, 7715–7725. [Google Scholar]
- Liu, D.; Thomas, P.W.; Momb, J.; Hoang, Q.Q.; Petsko, G.A.; Ringe, D.; Fast, W. Structure and specificity of a quorum-quenching lactonase (AiiB) from Agrobacterium tumefaciens. Biochemistry 2007, 46, 11789–11799. [Google Scholar]
- Molina, L.; Rezzonico, F.; Défago, G.; Duffy, B. Autoinduction in Erwinia amylovora: Evidence of an acyl-homoserine lactone signal in the fire blight pathogen. J. Bacteriol 2005, 187, 3206–3213. [Google Scholar]
- Tinh, N.T.; Asanka, N.; Gunasekara, R.A.Y.S.; Boon, N.; Dierckens, K.; Sorgeloos, P.; Bossier, P. N-Acylhomoserine lactone degrading microbial enrichment cultures isolated from Penaues vannamei shrimp gut and their probiotic properties in Brachionus plicatilis cultures. FEMS Microbiol. Ecol 2007, 62, 45–53. [Google Scholar]
- Dong, Y.H.; Zhang, X.F.; Xu, J.L.; Zhang, L.H. Insecticidal Bacillus thuringiensis silences Erwinia carotovora virulence by a new form of microbial antagonism, signal interference. Appl. Environ. Microbiol 2004, 70, 954–960. [Google Scholar]
- Mole, B.M.; Baltrus, D.A.; Dangl, J.L.; Grant, S.R. Global virulence regulation networks in phytopathogenic bacteria. Trends Microbiol 2007, 15, 363–371. [Google Scholar]
- Park, J.Y.; Lee, Y.H.; Yang, K.Y.; Kim, Y.C. AiiA-mediated quorum quenching does not affect virulence or toxoflavin expression in Burkholderia glumae SL2376. Lett. Appl. Microbiol 2010, 51, 619–624. [Google Scholar]
- Lyczak, J.B.; Cannon, C.L.; Pier, G.B. Establishment of Pseudomonas aeruginosa infection: Lessons from a versatile opportunist. Microbe. Infect 2000, 2, 1051–1060. [Google Scholar]
- Reimmann, C.; Ginet, N.; Michel, L.; Keel, C.; Michaux, P.; Krishnapillai, V.; Zala, M.; Heurlier, K.; Triandafillu, K.; Harms, H.; et al. Genetically programmed autoinducer destruction reduces virulence gene expression and swarming motility in Pseudomonas aeruginosa PAO1. Microbiology 2002, 148, 923–932. [Google Scholar]
- Papaioannou, E.; Wahjudi, M.; Nadal-Jimenez, P.; Koch, G.; Setroikromo, R.; Quax, W.J. Quorum-quenching acylase reduces the virulence of Pseudomonas aeruginosa in a Caenorhabditis elegans infection model. Antimicrob. Agents Chemother 2009, 53, 4891–4897. [Google Scholar]
- Wopperer, J.; Cardona, S.T.; Huber, B.; Jacobi, C.A.; Valvano, M.A.; Eberl, L. A quorum-quenching approach to investigate the conservation of quorum-sensing-regulated functions within the Burkholderia cepacia complex. Appl. Environ. Microbiol 2006, 72, 1579–1587. [Google Scholar]
- Dong, Y.H.; Wang, L.H.; Xu, J.L.; Zhang, H.B.; Zhang, X.F.; Zhang, L.H. Quenching quorum sensing dependent bacterial infection by an N-acyl homoserine lactonase. Nature 2001, 411, 813–817. [Google Scholar]
- Estrela, A.B.; Heck, M.G.; Abraham, W.R. Novel approaches to control biofilm infections. Curr. Med. Chem 2009, 16, 1512–1530. [Google Scholar]
- Estrela, A.B.; Abraham, W.R. Combining biofilm-controlling compounds and antibiotics as a promising new way to control biofilm infections. Pharmaceuticals 2010, 3, 1374–1393. [Google Scholar]
- Paul, D.; Kim, Y.S.; Ponnusamy, K.; Kweon, J.H. Application of quorum quenching to inhibit biofilm formation. Environ. Engineer. Sci 2009, 26, 1319–1324. [Google Scholar]
- Drews, A. Membrane fouling in membrane bioreactors, characterisation, contradictions, cause and cures. J. Membrane Sci 2010, 363, 1–28. [Google Scholar]
- Oh, H.S.; Yeon, K.M.; Yang, C.S.; Kim, S.R.; Lee, C.H.; Park, S.Y.; Han, J.Y.; Lee, J.K. Control of membrane biofouling in MBR for wastewater treatment by quorum quenching bacteria encapsulated in microporous membrane. Environ. Sci. Technol 2012, 46, 4877–4884. [Google Scholar]
- Yeon, K.M.; Lee, C.H.; Kim, J. Magnetic enzyme carrier for effective biofouling control in the membrane bioreactor based on enzymatic quorum quenching. Environ. Sci. Technol 2009, 43, 7403–7409. [Google Scholar]
- Kim, J.H.; Choi, D.C.; Yeon, K.M.; Kim, S.R.; Lee, C.H. Enzyme-immobilized nanofiltration membrane to mitigate biofouling based on quorum quenching. Environ. Sci. Technol 2011, 45, 1601–1607. [Google Scholar]
- Xiong, Y.H.; Liu, Y. Biological control of microbial attachment: A promising alternative for mitigating membrane biofouling. Appl. Microbiol. Biotechnol 2010, 86, 825–837. [Google Scholar]
- Pang, Y.; Liu, X.; Ma, Y.; Chernin, L.; Berg, G.; Gao, K. Induction of systemic resistance, root colonization and control activities of the rhizospheric strain of Serratia plymuthica are dependent on N-acyl homoserine lactones. Eur. J. Plant Pathol 2009, 124, 261–268. [Google Scholar]
- Park, S.J.; Park, S.Y.; Ryu, C.M.; Park, S.H.; Lee, J.K. The role of AiiA, a quorum-quenching enzyme from Bacillus thuringiensis on the rhizosphere competence. J. Microbiol. Biotechnol 2008, 18, 1518–1521. [Google Scholar]
- Gao, M.; Chen, H.; Eberhard, A.; Gronquist, M.R.; Robinson, J.B.; Connolly, M.; Teplitski, M.; Rolfe, B.G.; Bauer, W.D. Effects of AiiA-mediated quorum quenching in Sinorhizobium meliloti on quorum-sensing signals, proteome patterns, and symbiotic interactions. Mol. Plant Microbe. Interact 2007, 20, 843–856. [Google Scholar]
- Kiran, S.; Sharma, P.; Harjai, K.; Capalash, N. Enzymatic quorum quenching increases antibiotic susceptibility of multidrug resistant Pseudomonas aeruginosa. Iran J. Microbiol 2011, 3, 1–12. [Google Scholar]
- Zamani, M.; Behboudi, K.; Ahmadzadeh, M. Quorum quenching by Bacillus cereus U92: A double-edged sword in biological control of plant diseases. Biocontrol Sci. Technol 2013, 23, 555–573. [Google Scholar]
- Liu, X.; Jia, J.; Popat, R.; Ortori, C.A.; Li, J.; Diggle, S.P.; Gao, K.; Cámara, M. Characterization of two quorum sensing systems in the endophytic Serratia plymuthica strain G3: Differential control of motility and biofilm formation according to life-style. BMC Microbiol 2011, 11, 26. [Google Scholar]
- Case, R.J.; Labbate, M.; Kjelleberg, S. AHL-driven quorum-sensing circuits: Their frequency and function among the Proteobacteria. ISME J 2008, 2, 345–349. [Google Scholar]

| Enzyme | Host | Substrate | References |
|---|---|---|---|
| AHL lactonase | |||
| Bacillus sp. 240B1 | C6-10-HSL | [2] | |
| Bacillus cereus A24 | AHL | [12] | |
| AiiA | Bacillus mycoides | AHL | [12] |
| Bacillus thuringiensis | AHL | [13] | |
| Bacillus anthracis | C6, C8, C10-HSL | [14] | |
| AttM | Agrobacterium tumefaciens | 3-oxo-C8-HSL, C6-HSL | [28] |
| AiiB | Agrobacterium tumefaciens C58 | Broad | [15] |
| AiiS | Agrobacterium radiobacter K84 | Broad | [58] |
| AhlD | Arthrobacter sp. IBN110 | Broad | [18] |
| AhlK | Klebsiella pneumoniae | C6-8-HSL | [18] |
| QlcA | Acidobacteria | C6-8-HSL | [59] |
| AiiM | Microbacterium testaceum StLB037 | C6-10-HSL | [31] |
| QsdA | Rhodococcus erythropolis W2 | C6-14-HSL with or without C3-substitution | [33] |
| AidH | Ochrobactrum sp. T63 | C4-10-HSL | [30] |
| DlhR, QsdR1 | Rhizobium sp. NGR234 | nd. | [49] |
| AhlS | Solibacillus silvestris StLB046 | C6-HSL, C10-HSL | [32] |
| SsoPox | Sulfolobus solfataricus strain P2 | C8-12-HSL | [52,60] |
| Rhodococcus sp. | Broad | [16] | |
| GKL | Geobacillus kaustophilus strain HTA426 | C6-12-HSL | [61] |
| PPH | Mycobacterium tuberculosis | C4, C8, C10-HSL, | [62] |
| MCP | Mycobacterium avium subsp. paratuberculosis | C7-12-HSL | [63] |
| BpiB01, BpiB04, BpiB05, BpiB07 | Soil metagenome | 3-oxo-C8-HSL | [53,64] |
| QlcA | Soil metagenome | C6-10-HSL | [59] |
| AHL acylase | |||
| AiiD | Ralstonia eutropha | C8-12-HSL | [10] |
| PvdQ | Pseudomonas aeruginosa | C7-12-HSL with or without C3-substitution | [19,20] |
| QuiP | Pseudomonas aeruginosa | C7-14-HSL with or without C3-substitution | [40] |
| AiiC | Anabaena sp. PCC 7120 | Chain length more than C10 | [54] |
| AhlM | Streptomyces sp. M664 | Chain length more than C8 | [17] |
| Aac | Ralstonia solanacearum | Chain length more than C6 | [38] |
| Shewanella sp. MIB015 | Broad but prefer long chain | [65] | |
| HacA | Pseudomonas syringae | C8,C10, C12-HSL | [21] |
| HacB | Pseudomonas syringae | C6-12-HSL with or without C3-substitution | [21] |
| Variovorax sp. | Broad | [42] | |
| Variovorax paradoxus | Broad | [9] | |
| Tenacibaculum maritimum | C10-HSL | [66] | |
| Comomonas sp. D1 | C4-16-AHL with or without C3-substitution | [23] | |
| Rhodococcus erythropolis W2 | C10-HSL | [22] | |
| Oxidoreductase | |||
| P450BM-3 | Bacillus megaterium CYP102 A1 | C12-20-HSL(ω-1, ω-2, ω-3 hydroxylated) | [55] |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, F.; Gao, Y.; Chen, X.; Yu, Z.; Li, X. Quorum Quenching Enzymes and Their Application in Degrading Signal Molecules to Block Quorum Sensing-Dependent Infection. Int. J. Mol. Sci. 2013, 14, 17477-17500. https://doi.org/10.3390/ijms140917477
Chen F, Gao Y, Chen X, Yu Z, Li X. Quorum Quenching Enzymes and Their Application in Degrading Signal Molecules to Block Quorum Sensing-Dependent Infection. International Journal of Molecular Sciences. 2013; 14(9):17477-17500. https://doi.org/10.3390/ijms140917477
Chicago/Turabian StyleChen, Fang, Yuxin Gao, Xiaoyi Chen, Zhimin Yu, and Xianzhen Li. 2013. "Quorum Quenching Enzymes and Their Application in Degrading Signal Molecules to Block Quorum Sensing-Dependent Infection" International Journal of Molecular Sciences 14, no. 9: 17477-17500. https://doi.org/10.3390/ijms140917477
APA StyleChen, F., Gao, Y., Chen, X., Yu, Z., & Li, X. (2013). Quorum Quenching Enzymes and Their Application in Degrading Signal Molecules to Block Quorum Sensing-Dependent Infection. International Journal of Molecular Sciences, 14(9), 17477-17500. https://doi.org/10.3390/ijms140917477




