Quorum Sensing in the Squid-Vibrio Symbiosis
Abstract
:1. Introduction
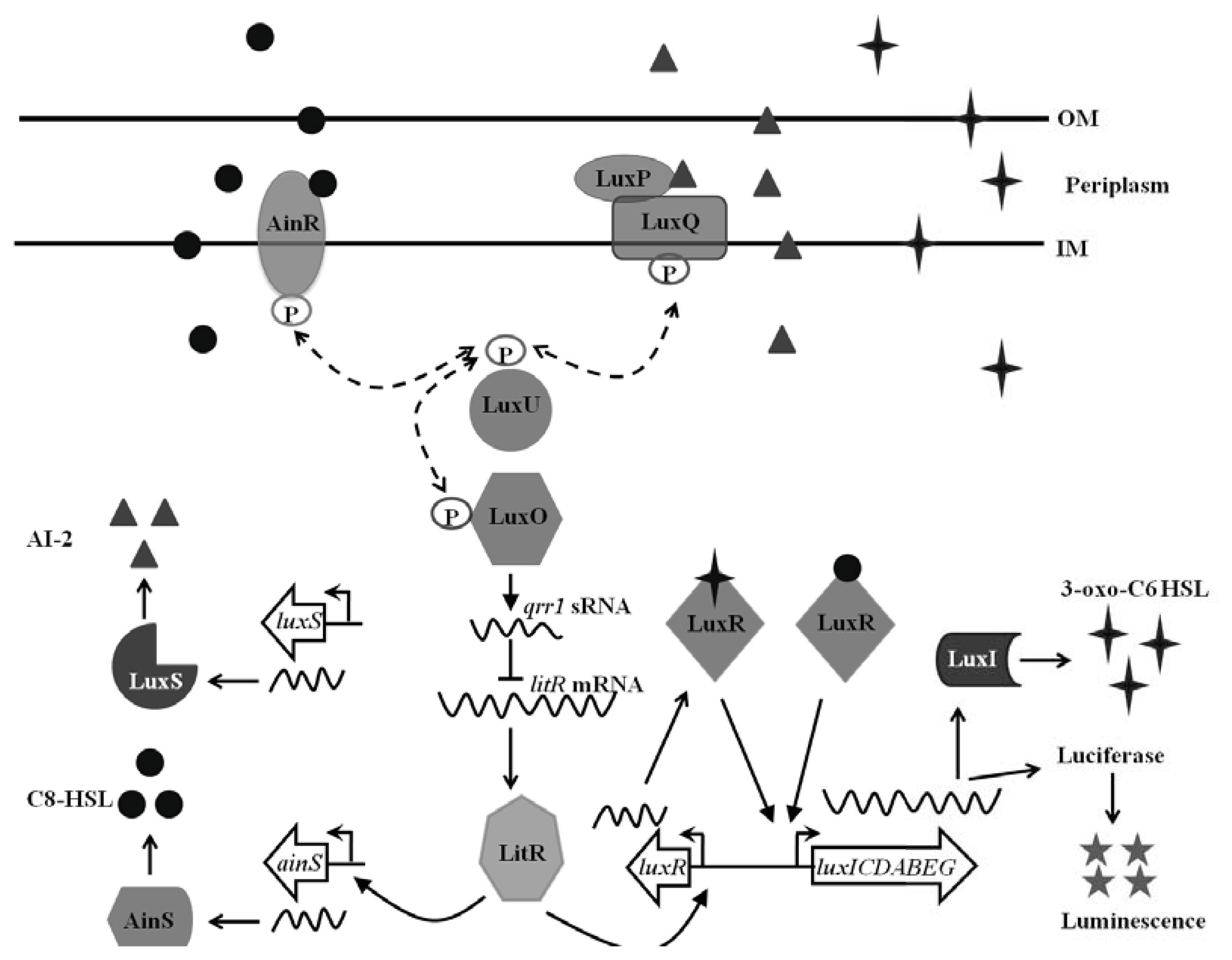
1.1. Quorum Sensing in Vibrio fischeri
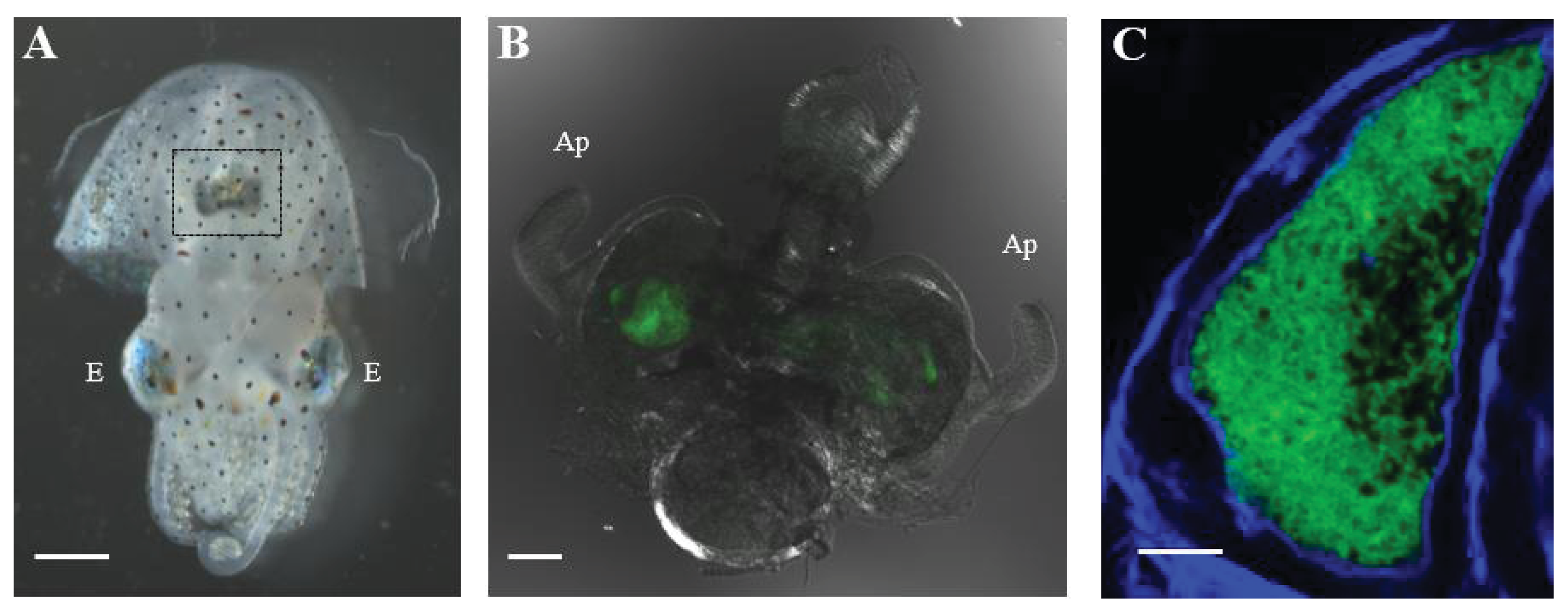
1.2. The Euprymna scolopes-Vibrio fischeri Symbiosis
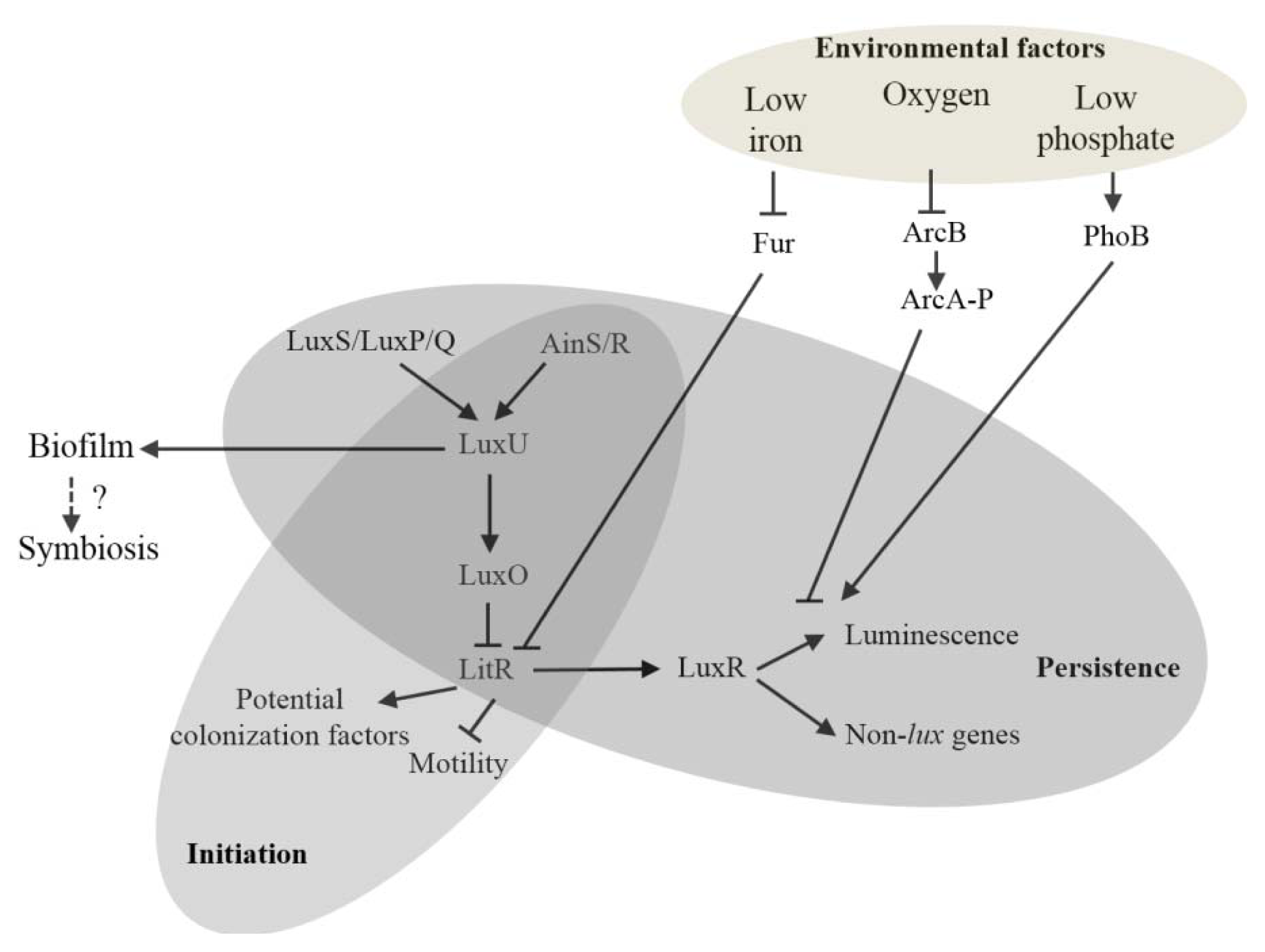
2. Impact of QS Network on the Euprymna scolopes-Vibrio fischeri Symbiosis
2.1. Impact of LuxI-LuxR Signaling
2.2. Impact of AinS-AinR Signaling
2.3. Impact of the LuxS/LuxP/Q Signaling
3. Influence of Abiotic Environment Cues on the QS Network
4. Concluding Remarks and Future Perspective
Acknowledgments
Conflict of Interest
References
- Schuster, M.; Sexton, D.J.; Diggle, S.P.; Greenberg, E.P. Acyl-homoserine lactone quorum sensing: From evolution to application. Annu. Rev. Microbiol 2013, 67, 43–63. [Google Scholar]
- Waters, C.M.; Bassler, B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol 2005, 21, 319–346. [Google Scholar]
- Ng, W.L.; Bassler, B.L. Bacterial quorum-sensing network architectures. Annu. Rev. Genet 2009, 43, 197–222. [Google Scholar]
- Nealson, K.H.; Platt, T.; Hastings, J.W. Cellular control of the synthesis and activity of the bacterial luminescent system. J. Bacteriol 1970, 104, 313–322. [Google Scholar]
- Urbanczyk, H.; Ast, J.C.; Higgins, M.J.; Carson, J.; Dunlap, P.V. Reclassification of Vibrio fischeri, Vibrio logei, Vibrio salmonicida and Vibrio wodanis as Aliivibrio fischeri gen. nov., comb. nov., Aliivibrio logei comb. nov., Aliivibrio salmonicida comb. nov. and Aliivibrio wodanis comb. nov. Int. J. Syst. Evol. Microbiol 2007, 57, 2823–2829. [Google Scholar]
- Engebrecht, J.; Nealson, K.; Silverman, M. Bacterial bioluminescence: Isolation and genetic analysis of functions from Vibrio fischeri. Cell 1983, 32, 773–781. [Google Scholar]
- Miyashiro, T.; Ruby, E.G. Shedding light on bioluminescence regulation in Vibrio fischeri. Mol. Microbiol 2012, 84, 795–806. [Google Scholar]
- Boylan, M.; Miyamoto, C.; Wall, L.; Graham, A.; Meighen, E. Lux C, D and E genes of the Vibrio fischeri luminescence operon code for the reductase, transferase, and synthetase enzymes involved in aldehyde biosynthesis. Photochem. Photobiol 1989, 49, 681–688. [Google Scholar]
- Nijvipakul, S.; Wongratana, J.; Suadee, C.; Entsch, B.; Ballou, D.P.; Chaiyen, P. LuxG is a functioning flavin reductase for bacterial luminescence. J. Bacteriol 2008, 190, 1531–1538. [Google Scholar]
- Schaefer, A.L.; Val, D.L.; Hanzelka, B.L.; Cronan, J.E., Jr; Greenberg, E.P. Generation of cell-to-cell signals in quorum sensing: Acyl homoserine lactone synthase activity of a purified Vibrio fischeri LuxI protein. Proc. Natl. Acad. Sci. USA 1996, 93, 9505–9509. [Google Scholar]
- Hanzelka, B.L.; Greenberg, E.P. Evidence that the N-terminal region of the Vibrio fischeri LuxR protein constitutes an autoinducer-binding domain. J. Bacteriol 1995, 177, 815–817. [Google Scholar]
- Stevens, A.M.; Dolan, K.M.; Greenberg, E.P. Synergistic binding of the Vibrio fischeri LuxR transcriptional activator domain and RNA polymerase to the lux promoter region. Proc. Natl. Acad. Sci. USA 1994, 91, 12619–12623. [Google Scholar]
- Perez, P.D.; Hagen, S.J. Heterogeneous response to a quorum-sensing signal in the luminescence of individual Vibrio fischeri. PLoS One 2010, 5, e15473. [Google Scholar]
- Lupp, C.; Urbanowski, M.; Greenberg, E.P.; Ruby, E.G. The Vibrio fischeri quorum-sensing systems ain and lux sequentially induce luminescence gene expression and are important for persistence in the squid host. Mol. Microbiol 2003, 50, 319–331. [Google Scholar]
- Neiditch, M.B.; Federle, M.J.; Pompeani, A. J.; Kelly, R.C.; Swem, D.L.; Jeffrey, P.D.; Bassler, B.L.; Hughson, F.M. Ligand-induced asymmetry in histidine sensor kinase complex regulates quorum sensing. Cell 2006, 126, 1095–1108. [Google Scholar]
- Miyashiro, T.; Wollenberg, M.S.; Cao, X.; Oehlert, D.; Ruby, E.G. A single qrr gene is necessary and sufficient for LuxO-mediated regulation in Vibrio fischeri. Mol. Microbiol 2010, 77, 1556–1567. [Google Scholar]
- Fidopiastis, P.M.; Miyamoto, C.M.; Jobling, M.G.; Meighen, E.A.; Ruby, E.G. LitR, a new transcriptional activator in Vibrio fischeri, regulates luminescence and symbiotic light organ colonization. Mol. Microbiol 2002, 45, 131–143. [Google Scholar]
- Milton, D.L. Quorum sensing in vibrios: Complexity for diversification. Int. J. Med. Microbiol 2006, 296, 61–71. [Google Scholar]
- Nelson, E.J.; Tunsjo, H.S.; Fidopiastis, P.M.; Sorum, H.; Ruby, E.G. A novel lux operon in the cryptically bioluminescent fish pathogen Vibrio salmonicida is associated with virulence. Appl. Environ. Microbiol 2007, 73, 1825–1833. [Google Scholar]
- Tu, K.C.; Bassler, B.L. Multiple small RNAs act additively to integrate sensory information and control quorum sensing in Vibrio harveyi. Genes Dev 2007, 21, 221–233. [Google Scholar]
- Lenz, D.H.; Mok, K.C.; Lilley, B.N.; Kulkarni, R.V.; Wingreen, N.S.; Bassler, B.L. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell 2004, 118, 69–82. [Google Scholar]
- Kuo, A.; Callahan, S.M.; Dunlap, P.V. Modulation of luminescence operon expression by N-octanoyl-L-homoserine lactone in ainS mutants of Vibrio fischeri. J. Bacteriol 1996, 178, 971–976. [Google Scholar]
- Schaefer, A.L.; Hanzelka, B.L.; Eberhard, A.; Greenberg, E.P. Quorum sensing in Vibrio fischeri: Probing autoinducer-LuxR interactions with autoinducer analogs. J. Bacteriol 1996, 178, 2897–2901. [Google Scholar]
- Perez, P.D.; Weiss, J.T.; Hagen, S.J. Noise and crosstalk in two quorum-sensing inputs of Vibrio fischeri. BMC Syst. Biol. 2011, 5. [Google Scholar] [CrossRef]
- Lupp, C.; Ruby, E.G. Vibrio fischeri LuxS and AinS: Comparative study of two signal synthases. J. Bacteriol 2004, 186, 3873–3881. [Google Scholar]
- Lupp, C.; Ruby, E.G. Vibrio fischeri uses two quorum-sensing systems for the regulation of early and late colonization factors. J. Bacteriol 2005, 187, 3620–3629. [Google Scholar]
- Studer, S.V.; Mandel, M.J.; Ruby, E.G. AinS quorum sensing regulates the Vibrio fischeri acetate switch. J. Bacteriol 2008, 190, 5915–5923. [Google Scholar]
- Ray, V.A.; Visick, K.L. LuxU connects quorum sensing to biofilm formation in Vibrio fischeri. Mol. Microbiol 2012, 86, 954–970. [Google Scholar]
- Sommer, F.; Backhed, F. The gut microbiota—Masters of host development and physiology. Nat. Rev. Microbiol 2013, 11, 227–238. [Google Scholar]
- Visick, K.L.; Ruby, E.G. Vibrio fischeri and its host: It takes two to tango. Curr. Opin. Microbiol 2006, 9, 632–638. [Google Scholar]
- Mandel, M.J.; Wollenberg, M.S.; Stabb, E.V.; Visick, K.L.; Ruby, E.G. A single regulatory gene is sufficient to alter bacterial host range. Nature 2009, 458, 215–218. [Google Scholar]
- Nyholm, S.V.; Deplancke, B.; Gaskins, H.R.; Apicella, M.A.; McFall-Ngai, M.J. Roles of Vibrio fischeri and nonsymbiotic bacteria in the dynamics of mucus secretion during symbiont colonization of the Euprymna scolopes light organ. Appl. Environ. Microbiol 2002, 68, 5113–5122. [Google Scholar]
- Nyholm, S.V.; McFall-Ngai, M.J. The winnowing: Establishing the squid-vibrio symbiosis. Nat. Rev. Microbiol 2004, 2, 632–642. [Google Scholar]
- Jones, B.W.; Nishiguchi, M.K. Counterillumination in the Hawaiian bobtail squid, Euprymna scolopes Berry (Mollusca: Cephalopoda). Mar. Biol 2004, 144, 1151–1155. [Google Scholar]
- Sycuro, L.K.; Ruby, E.G.; McFall-Ngai, M. Confocal microscopy of the light organ crypts in juvenile Euprymna scolopes reveals their morphological complexity and dynamic function in symbiosis. J. Morphol 2006, 267, 555–568. [Google Scholar]
- Lee, K.H.; Ruby, E.G. Detection of the light organ symbiont, Vibrio fischeri, in Hawaiian seawater by using lux gene probes. Appl. Environ. Microbiol 1992, 58, 942–947. [Google Scholar]
- Nyholm, S.V.; Stabb, E.V.; Ruby, E.G.; McFall-Ngai, M.J. Establishment of an animal-bacterial association: Recruiting symbiotic vibrios from the environment. Proc. Natl. Acad. Sci. USA 2000, 97, 10231–10235. [Google Scholar]
- Altura, M.A.; Heath-Heckman, E.A.; Gillette, A.; Kremer, N.; Krachler, A.M.; Brennan, C.; Ruby, E.G.; Orth, K.; McFall-Ngai, M.J. The first engagement of partners in the Euprymna scolopes-Vibrio fischeri symbiosis is a two-step process initiated by a few environmental symbiont cells. Environ. Microbiol. 2013. [Google Scholar] [CrossRef]
- Millikan, D.S.; Ruby, E.G. Alterations in Vibrio fischeri motility correlate with a delay in symbiosis initiation and are associated with additional symbiotic colonization defects. Appl. Environ. Microbiol 2002, 68, 2519–2528. [Google Scholar]
- Nyholm, S.V.; McFall-Ngai, M.J. Dominance of Vibrio fischeri in secreted mucus outside the light organ of Euprymna scolopes: The first site of symbiont specificity. Appl. Environ. Microbiol 2003, 69, 3932–3937. [Google Scholar]
- DeLoney-Marino, C.R.; Wolfe, A.J.; Visick, K.L. Chemoattraction of Vibrio fischeri to serine, nucleosides, and N-acetylneuraminic acid, a component of squid light-organ mucus. Appl. Environ. Microbiol 2003, 69, 7527–7530. [Google Scholar]
- Mandel, M.J.; Schaefer, A.L.; Brennan, C.A.; Heath-Heckman, E.A.; Deloney-Marino, C.R.; McFall-Ngai, M.J.; Ruby, E.G. Squid-derived chitin oligosaccharides are a chemotactic signal during colonization by Vibrio fischeri. Appl. Environ. Microbiol 2012, 78, 4620–4626. [Google Scholar]
- Ruby, E.G.; Asato, L.M. Growth and flagellation of Vibrio fischeri during initiation of the sepiolid squid light organ symbiosis. Arch. Microbiol 1993, 159, 160–167. [Google Scholar]
- Visick, K.L.; Foster, J.; Doino, J.; McFall-Ngai, M.; Ruby, E.G. Vibrio fischeri lux genes play an important role in colonization and development of the host light organ. J. Bacteriol 2000, 182, 4578–4586. [Google Scholar]
- Bose, J.L.; Rosenberg, C.S.; Stabb, E.V. Effects of luxCDABEG induction in Vibrio fischeri: Enhancement of symbiotic colonization and conditional attenuation of growth in culture. Arch. Microbiol 2008, 190, 169–183. [Google Scholar]
- McFall-Ngai, M.; Heath-Heckman, E.A.; Gillette, A.A.; Peyer, S.M.; Harvie, E.A. The secret languages of coevolved symbioses: Insights from the Euprymna scolopes-Vibrio fischeri symbiosis. Semin. Immunol 2012, 24, 3–8. [Google Scholar]
- Chun, C.K.; Troll, J.V.; Koroleva, I.; Brown, B.; Manzella, L.; Snir, E.; Almabrazi, H.; Scheetz, T.E.; de Bonaldo, M.F.; Casavant, T.L.; et al. Effects of colonization, luminescence, and autoinducer on host transcription during development of the squid-vibrio association. Proc. Natl. Acad. Sci. USA 2008, 105, 11323–11328. [Google Scholar]
- Heath-Heckman, E.A.; Peyer, S.M.; Whistler, C.A.; Apicella, M.A.; Goldman, W.E.; McFall-Ngai, M.J. Bacterial bioluminescence regulates expression of a host cryptochrome gene in the squid-vibrio symbiosis. MBio 2013, 4. [Google Scholar] [CrossRef]
- Koropatnick, T.A.; Engle, J.T.; Apicella, M.A.; Stabb, E.V.; Goldman, W.E.; McFall-Ngai, M.J. Microbial factor-mediated development in a host-bacterial mutualism. Science 2004, 306, 1186–1188. [Google Scholar]
- Koropatnick, T.A.; Kimbell, J.R.; McFall-Ngai, M.J. Responses of host hemocytes during the initiation of the squid-vibrio symbiosis. Biol. Bull 2007, 212, 29–39. [Google Scholar]
- Tong, D.; Rozas, N.S.; Oakley, T.H.; Mitchell, J.; Colley, N.J.; McFall-Ngai, M.J. Evidence for light perception in a bioluminescent organ. Proc. Natl. Acad. Sci. USA 2009, 106, 9836–9841. [Google Scholar]
- Callahan, S.M.; Dunlap, P.V. LuxR- and acyl-homoserine-lactone-controlled non-lux genes define a quorum-sensing regulon in Vibrio fischeri. J. Bacteriol 2000, 182, 2811–2822. [Google Scholar]
- Antunes, L.C.; Schaefer, A.L.; Ferreira, R.B.; Qin, N.; Stevens, A.M.; Ruby, E.G.; Greenberg, E.P. Transcriptome analysis of the Vibrio fischeri LuxR-LuxI regulon. J. Bacteriol 2007, 189, 8387–8391. [Google Scholar]
- Schleicher, T.R.; Nyholm, S.V. Characterizing the host and symbiont proteomes in the association between the Bobtail squid, Euprymna scolopes, and the bacterium, Vibrio fischeri. PLoS One 2011, 6, e25649. [Google Scholar]
- Egland, K.A.; Greenberg, E.P. Conversion of the Vibrio fischeri transcriptional activator, LuxR, to a repressor. J. Bacteriol 2000, 182, 805–811. [Google Scholar]
- Graf, J.; Dunlap, P.V.; Ruby, E.G. Effect of transposon-induced motility mutations on colonization of the host light organ by Vibrio fischeri. J. Bacteriol 1994, 176, 6986–6991. [Google Scholar]
- Millikan, D.S.; Ruby, E.G. Vibrio fischeri flagellin A is essential for normal motility and for symbiotic competence during initial squid light organ colonization. J. Bacteriol 2004, 186, 4315–4325. [Google Scholar]
- Millikan, D.S.; Ruby, E.G. FlrA, a sigma54-dependent transcriptional activator in Vibrio fischeri, is required for motility and symbiotic light-organ colonization. J. Bacteriol 2003, 185, 3547–3557. [Google Scholar]
- Cao, X.; Studer, S.V.; Wassarman, K.; Zhang, Y.; Ruby, E.G.; Miyashiro, T. The novel sigma factor-like regulator RpoQ controls luminescence, chitinase activity, and motility in Vibrio fischeri. MBio 2012, 3. [Google Scholar] [CrossRef]
- Boettcher, K.J.; Ruby, E.G. Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J. Bacteriol 1990, 172, 3701–3706. [Google Scholar]
- Stabb, E.V.; Schaefer, A.; Bose, J.L.; Ruby, E.G. Quorum Signaling and Symbiosis in the Marine Luminous Bacterium Vibrio fischeri. In Chemical Communication among Bacteria; Winans, S.C., Bassler, B.L., Eds.; ASM Press: Washington, DC, USA, 2008. [Google Scholar]
- Septer, A.N.; Lyell, N.L.; Stabb, E.V. The iron-dependent regulator fur controls pheromone signaling systems and luminescence in the squid symbiont Vibrio fischeri ES114. Appl. Environ. Microbiol 2013, 79, 1826–1834. [Google Scholar]
- Septer, A.N.; Wang, Y.; Ruby, E.G.; Stabb, E.V.; Dunn, A.K. The haem-uptake gene cluster in Vibrio fischeri is regulated by Fur and contributes to symbiotic colonization. Environ. Microbiol 2011, 13, 2855–2864. [Google Scholar]
- Lyell, N.L.; Dunn, A.K.; Bose, J.L.; Stabb, E.V. Bright mutants of Vibrio fischeri ES114 reveal conditions and regulators that control bioluminescence and expression of the lux operon. J. Bacteriol 2010, 192, 5103–5114. [Google Scholar]
- Makino, K.; Shinagawa, H.; Amemura, M.; Kawamoto, T.; Yamada, M.; Nakata, A. Signal transduction in the phosphate regulon of Escherichia coli involves phosphotransfer between PhoR and PhoB proteins. J. Mol. Biol 1989, 210, 551–559. [Google Scholar]
- Septer, A.N.; Stabb, E.V. Coordination of the arc regulatory system and pheromone-mediated positive feedback in controlling the Vibrio fischeri lux operon. PLoS One 2012, 7, e49590. [Google Scholar]
| Strain | Luminescence at different stages of symbiosis | References | |||
|---|---|---|---|---|---|
| 12 h | 24 h | 48 h | 72 h | ||
| ainS | 10%–20% | 10%–20% | 10%–40% | ND | [14,25] |
| luxO | 100% | 100% | 100% | ND | [14] |
| ainS, luxO | 100% | 100% | 100% | ND | [14] |
| luxS | 100% | 100% | ND | ND | [25] |
| luxS, ainS† | 50% | 50% | ND | ND | [25] |
| litR | 100% | 100% | 100% | ND | [17] |
| luxR | BD | BD | ND | ND | [44] |
| luxI | BD | BD | ND | ND | [44] |
| luxA | BD | BD | ND | ND | [44] |
| Strain | Colonization level at different stages of symbiosis | References | |||
|---|---|---|---|---|---|
| 12 h | 24 h | 48 h | 72 h | ||
| ainS | 45% | 75% | 40% | 20% | [14,26] |
| luxO | 37% | ND | ND | 100% | [14,26] |
| LuxO(D47E) | 52% | ND | ND | 97% | [26] |
| ainS, luxO | 36% | ND | ND | 100% | [14,26] |
| luxS | 95% | 75% | 90% | ND | [25,26] |
| luxS, ainS | 48% | 50% | 20% | ND | [25,26] |
| litR | 51% | 100% | 100% | ND | [17,26] |
| luxR | 119% | 100% | 25%–35% | ND | [26,44] |
| luxI | 115% | 100% | 25%–35% | ND | [26,44] |
| luxA | ND | 100% | 25%–35% | ND | [44] |
| luxCDABEG | ND | ND | 25%–35% | ND | [45] |
| ainS, luxI | 79% | 75% | 30% | 20% | [14,26] |
| Strains in mixed inoculums (1:1 ratio) | Dominant strain at different stages of symbiosis | References | |||
|---|---|---|---|---|---|
| Strain 1 | Strain 2 | 12 h | 24 h | 48 h | |
| ESR1 | luxA | ND | ESR1 | ESR1 | [44] |
| ESR1 | luxR | ND | - | ESR1 | [44] |
| ES114 | litR | ND | ND | litR | [16,17] |
| ES114 | qrr1 | ND | ND | ES114 | [16] |
| ES114 | luxO | ND | ND | ES114 | [16] |
| ES114 | luxO, litR | ND | ND | None | [16] |
| ES114 | qrr1, litR | ND | ND | None | [16] |
| ES114 | ainS | ND | ND | ES114 | [14] |
| ES114 | luxCDABEG | ND | ND | ES114 | [45] |
| Strain | Motility behavior on agar | Flagellation | Aggregation behavior | Colonization level at different stages of symbiosis | Reference | |||
|---|---|---|---|---|---|---|---|---|
| 0.3%–0.7% | 0.25% | 12 h | 24 h | 48 h | ||||
| ES114 | Motile | Motile | Normal flagella | Normal | 100% | 100% | 100% | [56] |
| N210 | Non-motile | ND | Normal flagella | ND | ND | BD | ND | [56] |
| NF201 | Non-motile | ND | No flagella | ND | ND | BD | ND | [56] |
| NM200 | Non-motile | ND | Abnormal flagella | ND | ND | BD | ND | [56] |
| DM66 | Hypermotile | ND | Hyper flagellation | Delayed | 50% | 60% | 100% | [39] |
| DM73 | Hypermotile | ND | Hyper flagellation | Delayed | ND | 40% | ND | [39] |
| DM61 | Hypermotile | ND | Hyper flagellation | Delayed | 0.1%–10% | 0.1%–10% | 0.1%–10% | [39] |
| flaA | ND | Less motile | Hypo flagellation | Normal | ND | 20%–25% | ND | [58] |
| flrA | Non-motile | ND | No flagella | Normal | ND | BD | ND | [58] |
| ainS | Motile | Hyper-motile | ND | Normal | [26] | |||
| litR | ND | Hyper-motile | ND | ND | [26] | |||
| luxO | ND | Non-motile | ND | ND | See Table 1 | [26] | ||
| luxR | ND | Motile | ND | ND | [26] | |||
| luxI | Motile | Motile | ND | ND | [26] | |||
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Verma, S.C.; Miyashiro, T. Quorum Sensing in the Squid-Vibrio Symbiosis. Int. J. Mol. Sci. 2013, 14, 16386-16401. https://doi.org/10.3390/ijms140816386
Verma SC, Miyashiro T. Quorum Sensing in the Squid-Vibrio Symbiosis. International Journal of Molecular Sciences. 2013; 14(8):16386-16401. https://doi.org/10.3390/ijms140816386
Chicago/Turabian StyleVerma, Subhash C., and Tim Miyashiro. 2013. "Quorum Sensing in the Squid-Vibrio Symbiosis" International Journal of Molecular Sciences 14, no. 8: 16386-16401. https://doi.org/10.3390/ijms140816386
APA StyleVerma, S. C., & Miyashiro, T. (2013). Quorum Sensing in the Squid-Vibrio Symbiosis. International Journal of Molecular Sciences, 14(8), 16386-16401. https://doi.org/10.3390/ijms140816386




