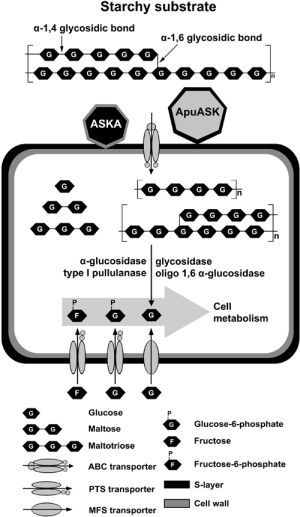
A High Molecular-Mass Anoxybacillus sp. SK3-4 Amylopullulanase: Characterization and Its Relationship in Carbohydrate Utilization
Abstract
:1. Introduction
2. Results
2.1. Genomic Sequencing of Strain SK3-4
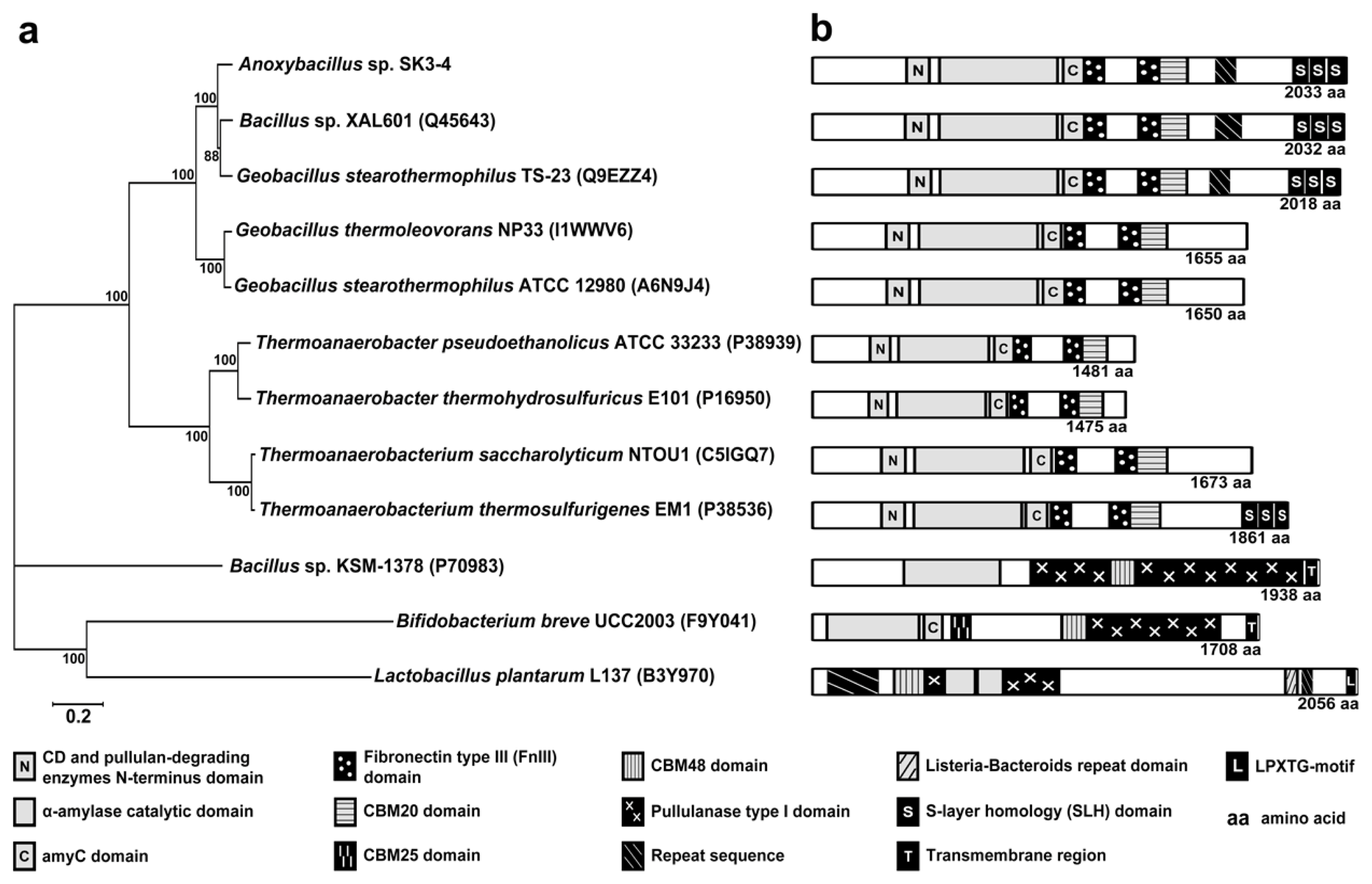
2.2. Analysis of the ApuASK Sequence
2.3. Purification of Apu
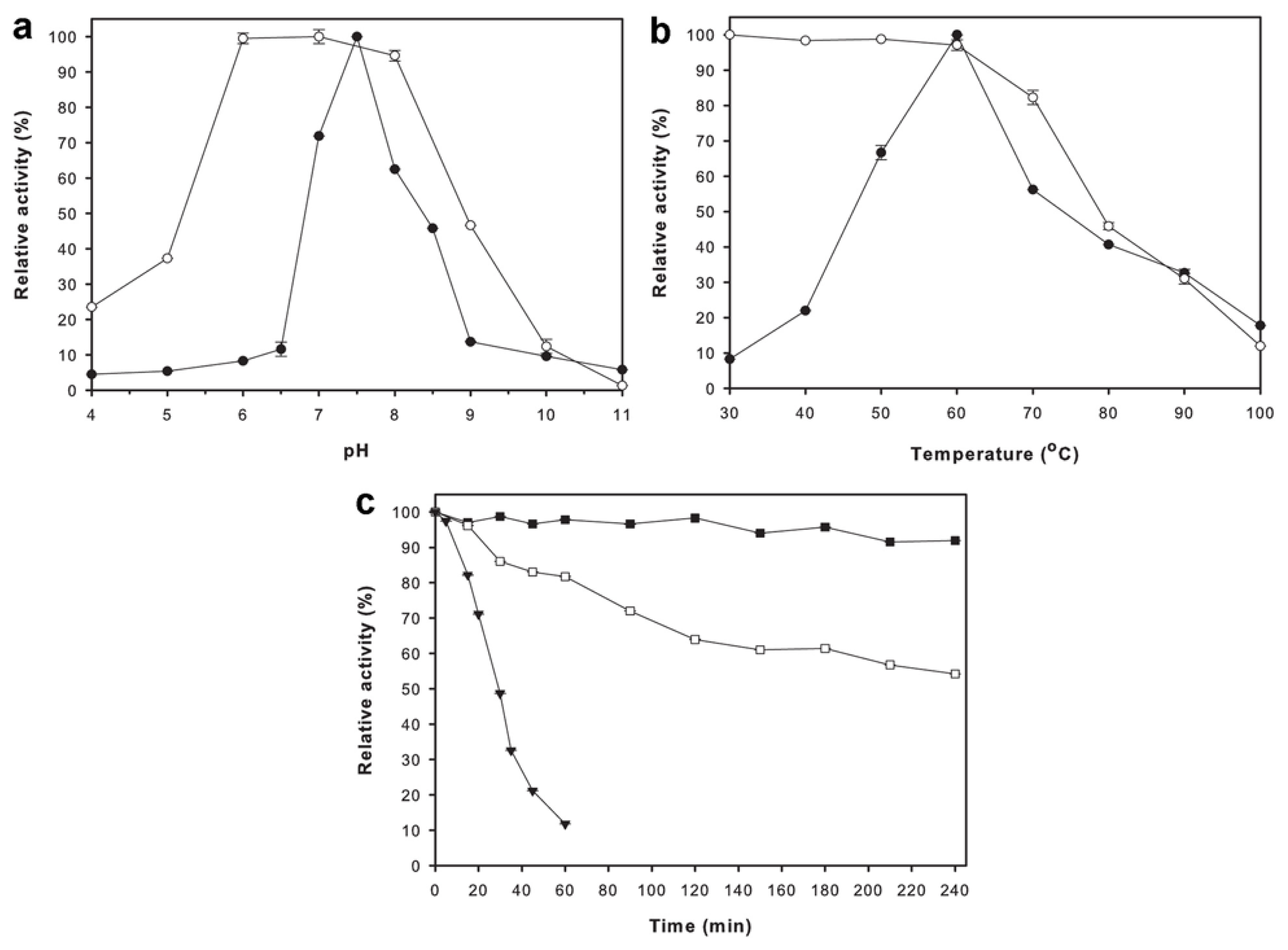
2.4. Effects of pH and Temperature on Enzyme Activity and Stability
2.5. Effects of Buffers, Metal Ions, and Chemical Reagents
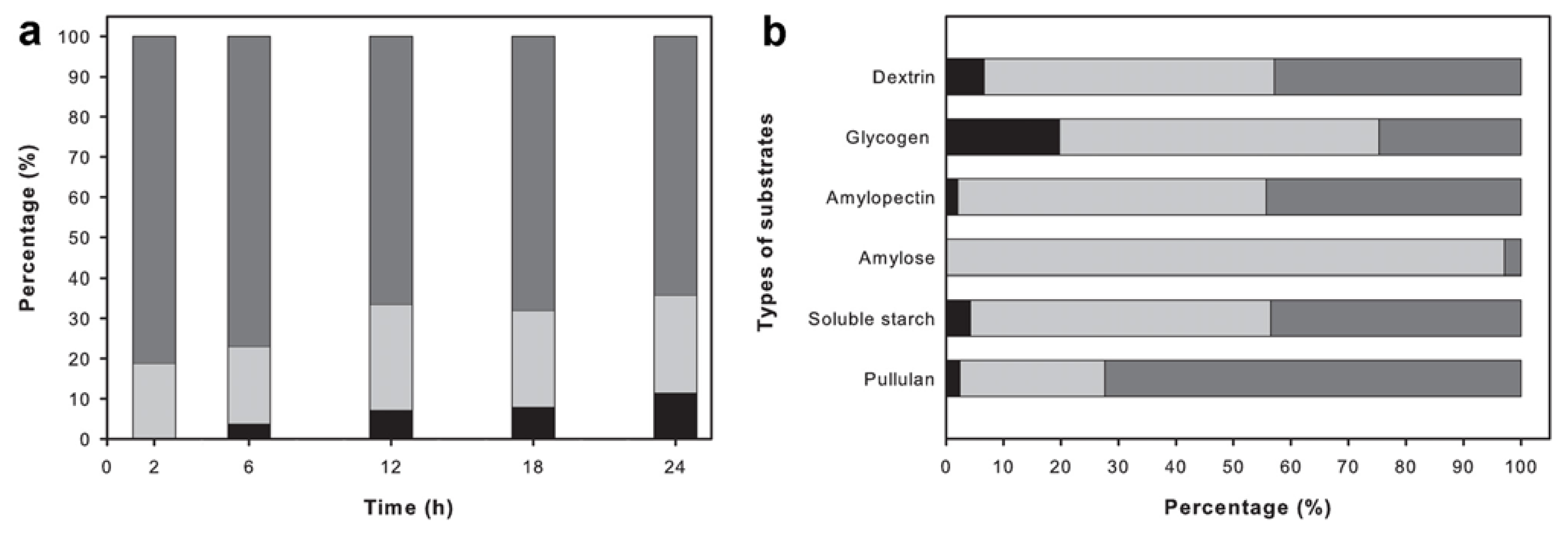
2.6. Analysis of the Reaction Products
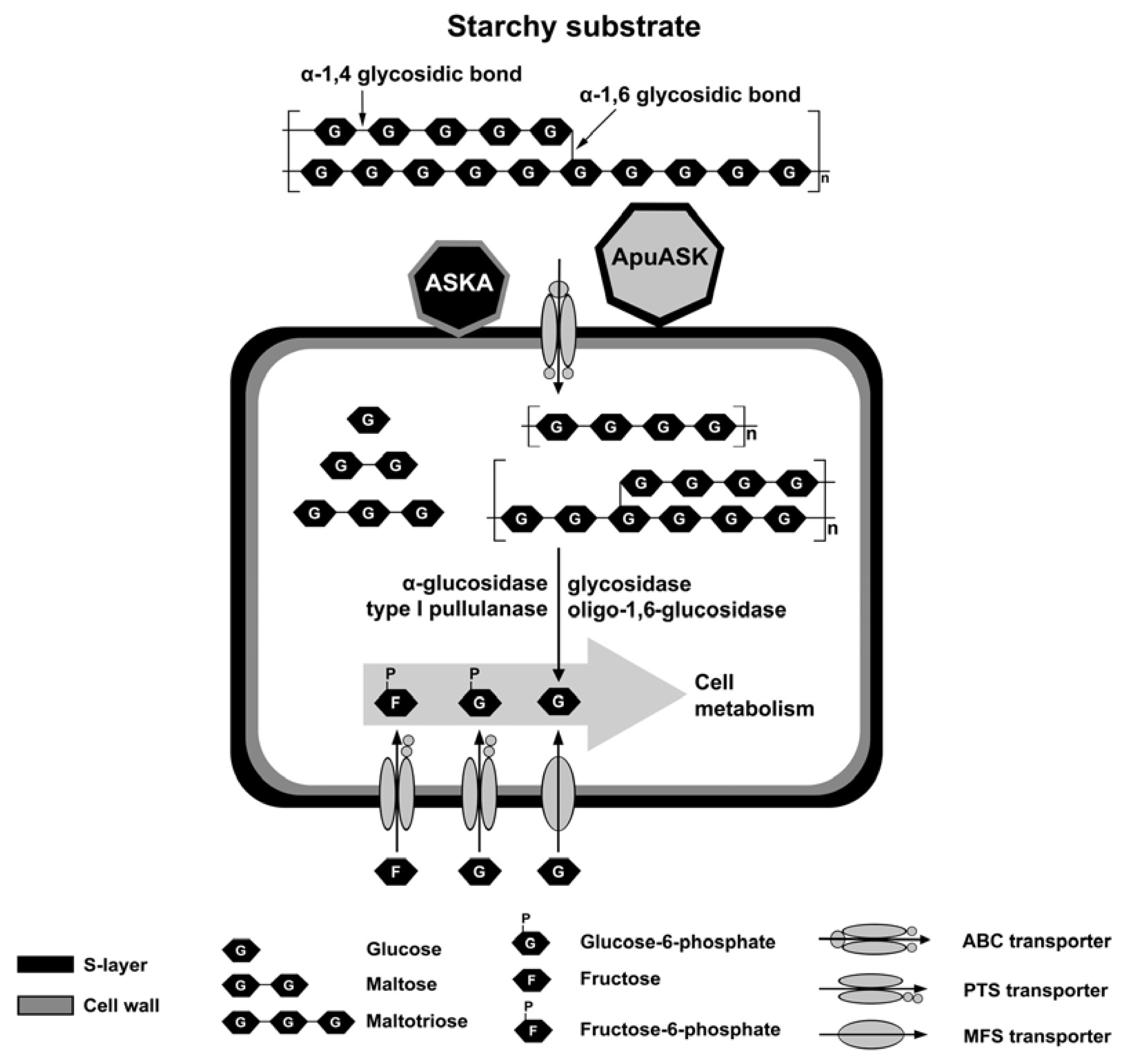
3. Discussion
4. Experimental Section
4.1. Chemicals
4.2. Bacterial Strain, Genome Sequencing, and Protein Sequence Analysis
4.3. Bacterial Culture Conditions
4.4. Determination of Enzyme Activity and Protein Concentration
4.5. Purification of Apu
4.6. Gel Electrophoresis and Zymography
4.7. Effects of pH and Temperature on Enzyme Activity and Stability
4.8. Effects of Buffers, Metal Ions, and Chemical Reagents
4.9. Analysis of the Reaction Products
4.10. Statistical Analysis
5. Conclusions
Supplementary Information
ijms-14-11302-s001.pdfAcknowledgments
Conflict of Interest
References
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The carbohydrate-active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res 2009, 37, D233–D238. [Google Scholar]
- Janeček, Š. How many conserved sequence regions are there in the α-amylase family? Biologia 2002, 57, 29–41. [Google Scholar]
- Christiansen, C.; Abou Hachem, M.; Janeček, Š.; Viks⊘-Nielsen, A.; Blennow, A.; Svensson, B. The carbohydrate-binding module family 20—diversity, structure, and function. FEBS J. 2009, 276, 5006–5029. [Google Scholar]
- Domań-Pytka, M.; Bardowski, J. Pullulan degrading enzymes of bacterial origin. Crit. Rev. Microbiol 2004, 30, 107–121. [Google Scholar]
- Cheng, K.-C.; Demirci, A.; Catchmark, J.M. Pullulan: Biosynthesis, production, and applications. Appl. Microbiol. Biotechnol 2011, 92, 29–44. [Google Scholar]
- Bertoldo, C.; Antranikian, G. Starch-hydrolyzing enzymes from thermophilic archaea and bacteria. Curr. Opin. Chem. Biol 2002, 6, 151–160. [Google Scholar]
- Ara, K.; Saeki, K.; Igarashi, K.; Takaiwa, M.; Uemura, T.; Hagihara, H.; Kawai, S.; Ito, S. Purification and characterization of an alkaline amylopullulanase with both α-1,4 and α-1,6 hydrolytic activity from alkalophilic Bacillus sp. KSM-1378. Biochim. Biophys. Acta 1995, 1243, 315–324. [Google Scholar]
- Goh, K.M.; Kahar, U.M.; Chai, Y.Y.; Chong, C.S.; Chai, K.P.; Ranjani, V.; Illias, R.M.; Chan, K.-G. Recent discoveries and applications of Anoxybacillus. Appl. Microbiol. Biotechnol 2013, 97, 1475–1488. [Google Scholar]
- Chai, Y.Y.; Kahar, U.M.; Salleh, M.M.; Illias, R.M.; Goh, K.M. Isolation and characterization of pullulan-degrading Anoxybacillus species isolated from Malaysian hot springs. Environ. Technol 2012, 33, 1231–1238. [Google Scholar]
- Lee, S.-P.; Morikawa, M.; Takagi, M.; Imanaka, T. Cloning of the aapT gene and characterization of its product, α-amylase-pullulanase (AapT), from thermophilic and alkaliphilic Bacillus sp. strain XAL601. Appl. Environ. Microbiol 1994, 60, 3764–3773. [Google Scholar]
- Chen, J.-T.; Chen, M.-C.; Chen, L.-L.; Chu, W.-S. Structure and expression of an amylopullulanase gene from Bacillus stearothermophilus TS-23. Biotechnol. Appl. Biochem 2001, 33, 189–199. [Google Scholar]
- Nisha, M.; Satyanarayana, T. Characterization of recombinant amylopullulanase (gt-apu) and truncated amylopullulanase (gt-apuT) of the extreme thermophile Geobacillus thermoleovorans NP33 and their action in starch saccharification. Appl. Microbiol. Biotechnol. 2012. [Google Scholar] [CrossRef]
- Ferner-Ortner-Bleckmann, J.; Huber-Gries, C.; Pavkov, T.; Keller, W.; Mader, C.; Ilk, N.; Sleytr, U.B.; Egelseer, E.M. The high-molecular-mass amylase (HMMA) of Geobacillus stearothermophilus ATCC 12980 interacts with the cell wall components by virtue of three specific binding regions. Mol. Microbiol 2009, 72, 1448–1461. [Google Scholar]
- Mathupala, S.P.; Lowe, S.E.; Podkovyrov, S.M.; Zeikus, J.G. Sequencing of the amylopullulanase (apu) gene of Thermoanaerobacter ethanolicus 39E, and identification of the active site by site-directed mutagenesis. J. Biol. Chem 1993, 268, 16332–16344. [Google Scholar]
- Melasniemi, H.; Paloheimo, M. Cloning and expression of the Clostridium thermohydrosulfuricum α-amylase-pullulanase gene in Escherichia coli. J. Gen. Microbiol 1989, 135, 1755–1762. [Google Scholar]
- Lin, F.-P.; Ma, H.-Y.; Lin, H.-J.; Liu, S.-M.; Tzou, W.-S. Biochemical characterization of two truncated forms of amylopullulanase from Thermoanaerobacterium saccharolyticum NTOU1 to identify its enzymatically active region. Appl. Biochem. Biotechnol 2011, 165, 1047–1056. [Google Scholar]
- Matuschek, M.; Burchhardt, G.; Sahm, K.; Bahl, H. Pullulanase of Thermoanaerobacterium thermosulfurigenes EM1 (Clostridium thermosulfurogenes): Molecular analysis of the gene, composite structure of the enzyme, and a common model for its attachment to the cell surface. J. Bacteriol 1994, 176, 3295–3302. [Google Scholar]
- Motherway, M.O.C.; Fitzgerald, G.F.; Neirynck, S.; Ryan, S.; Steidler, L.; van Sinderen, D. Characterization of ApuB, an extracellular type II amylopullulanase from Bifidobacterium breve UCC2003. Appl. Environ. Microbiol 2008, 74, 6271–6279. [Google Scholar]
- Kim, J.-H.; Sunako, M.; Ono, H.; Murooka, Y.; Fukusaki, E.; Yamashita, M. Characterization of gene encoding amylopullulanase from plant-originated lactic acid bacterium, Lactobacillus plantarum L137. J. Biosci. Bioeng 2008, 106, 449–459. [Google Scholar]
- Zareian, S.; Khajeh, K.; Ranjbar, B.; Dabirmanesh, B.; Ghollasi, M.; Mollania, N. Purification and characterization of a novel amylopullulanase that converts pullulan to glucose, maltose, and maltotriose and starch to glucose and maltose. Enzyme Microb. Technol 2010, 46, 57–63. [Google Scholar]
- Götz, S.; García-Gómez, J.M.; Terol, J.; Williams, T.D.; Nagaraj, S.H.; Nueda, M.J.; Robles, M.; Talón, M.; Dopazo, J.; Conesa, A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res 2008, 36, 3420–3435. [Google Scholar]
- Yin, Y.; Mao, X.; Yang, J.; Chen, X.; Mao, F.; Xu, Y. dbCAN: A web resource for automated carbohydrate-active enzyme annotation. Nucleic Acids Res 2012, 40, W445–W451. [Google Scholar]
- Hatada, Y.; Igarashi, K.; Ozaki, K.; Ara, K.; Hitomi, J.; Kobayashi, T.; Kawai, S.; Watabe, T.; Ito, S. Amino acid sequence and molecular structure of an alkaline amylopullulanase from Bacillus that hydrolyzes α-1,4 and α-1,6 linkages in polysaccharides at different active sites. J. Biol. Chem 1996, 271, 24075–24083. [Google Scholar]
- Kim, J.-H.; Sunako, M.; Ono, H.; Murooka, Y.; Fukusaki, E.; Yamashita, M. Characterization of the C-terminal truncated form of amylopullulanase from Lactobacillus plantarum L137. J. Biosci. Bioeng 2009, 107, 124–129. [Google Scholar]
- Rüdiger, A.; Jorgensen, P.L.; Antranikian, G. Isolation and characterization of a heat-stable pullulanase from the hyperthermophilic archaeon Pyrococcus woesei after cloning and expression of its gene in Escherichia coli. Appl. Environ. Microbiol 1995, 61, 567–575. [Google Scholar]
- Kamitori, S.; Kondo, S.; Okuyama, K.; Yokota, T.; Shimura, Y.; Tonozuka, T.; Sakano, Y. Crystal structure of Thermoactinomyces vulgaris R-47 α-amylase II (TVAII) hydrolyzing cyclodextrins and pullulan at 2.6 Å resolution. J. Mol. Biol 1999, 287, 907–921. [Google Scholar]
- Robert, X.; Haser, R.; Gottschalk, T.E.; Ratajczak, F.; Driguez, H.; Svensson, B.; Aghajari, N. The structure of barley α-amylase isozyme 1 reveals a novel role of domain C in substrate recognition and binding: A pair of sugar tongs. Structure 2003, 11, 973–984. [Google Scholar]
- Polekhina, G.; Gupta, A.; Michell, B.J.; van Denderen, B.; Murthy, S.; Feil, S.C.; Jennings, I.G.; Campbell, D.J.; Witters, L.A.; Parker, M.W.; et al. AMPK β subunit targets metabolic stress sensing to glycogen. Curr. Biol 2003, 13, 867–871. [Google Scholar]
- Boraston, A.B.; Healey, M.; Klassen, J.; Ficko-Blean, E.; van Bueren, A.L.; Law, V. A structural and functional analysis of α-glucan recognition by family 25 and 26 carbohydrate-binding modules reveals a conserved mode of starch recognition. J. Biol. Chem 2006, 281, 587–598. [Google Scholar]
- Kataeva, I.A.; Seidel, R.D., III; Shah, A.; West, L.T.; Li, X.-L.; Ljungdahl, L.G. The fibronectin type 3-like repeat from the Clostridium thermocellum cellobiohydrolase CbhA promotes hydrolysis of cellulose by modifying its surface. Appl. Environ. Microbiol. 2002, 68, 4292–4300. [Google Scholar]
- Takagi, M.; Lee, S.-P.; Imanaka, T. Diversity in size and alkaliphily of thermostable α-amylase-pullulanases (AapT) produced by recombinant Escherichia coli, Bacillus subtilis and the wild-type Bacillus sp. J. Ferment. Bioeng 1996, 81, 557–559. [Google Scholar]
- Lin, H.-Y.; Chuang, H.-H.; Lin, F.-P. Biochemical characterization of engineered amylopullulanase from Thermoanaerobacter ethanolicus 39E-implicating the non-necessity of its 100 C-terminal amino acid residues. Extremophiles 2008, 12, 641–650. [Google Scholar]
- Chai, Y.Y.; Rahman, R.N.Z.R.A.; Illias, R.M.; Goh, K.M. Cloning and characterization of two new thermostable and alkalitolerant α-amylases from the Anoxybacillus species that produce high levels of maltose. J. Ind. Microbiol. Biotechnol. 2012, 39, 731–741. [Google Scholar]
- Finn, R.D.; Mistry, J.; Tate, J.; Coggill, P.; Heger, A.; Pollington, J.E.; Gavin, O.L.; Gunasekaran, P.; Ceric, G.; Forslund, K.; et al. The Pfam protein families database. Nucleic Acids Res 2010, 38, D211–D222. [Google Scholar]
- Letunic, I.; Doerks, T.; Bork, P. SMART 7: Recent updates to the protein domain annotation resource. Nucleic Acids Res 2012, 40, D302–D305. [Google Scholar]
- Sigrist, C.J.A.; de Castro, E.; Cerutti, L.; Cuche, B.A.; Hulo, N.; Bridge, A.; Bougueleret, L.; Xenarios, I. New and continuing developments at PROSITE. Nucleic Acids Res 2013, 41, D344–D347. [Google Scholar]
- Yu, N.Y.; Wagner, J.R.; Laird, M.R.; Melli, G.; Rey, S.; Lo, R.; Dao, P.; Cenk Sahinalp, S.; Ester, M.; Foster, L.J.; et al. PSORTb 3.0: Improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 2010, 26, 1608–1615. [Google Scholar]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar]
- Crooks, G.E.; Hon, G.; Chandonia, J.-M.; Brenner, S.E. WebLogo: A sequence logo generator. Genome Res 2004, 14, 1188–1190. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol 2011, 28, 2731–2739. [Google Scholar]
- Kahar, U.M.; Salleh, M.M.; Goh, K.M. Medium optimisation for pullulanase production from Anoxybacillus species using experimental design. Indian J. Biotechnol. 2013, in press. [Google Scholar]
- Mahajan, P.M.; Desai, K.M.; Lele, S.S. Production of cell membrane-bound α- and β-glucosidase by Lactobacillus acidophilus. Food Bioprocess Technol 2012, 5, 706–718. [Google Scholar]
- Miller, G. L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem 1959, 31, 426–428. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem 1951, 193, 265–275. [Google Scholar]
- Furegon, L.; Curioni, A.; Peruffo, A.D.B. Direct detection of pullulanase activity in electrophoretic polyacrylamide gels. Anal. Biochem 1994, 221, 200–201. [Google Scholar]
- Yang, S.-J.; Lee, H.-S.; Park, C.-S.; Kim, Y.-R.; Moon, T.-W.; Park, K.-H. Enzymatic analysis of an amylolytic enzyme from the hyperthermophilic archaeon Pyrococcus furiosus reveals its novel catalytic properties as both an α-amylase and a cyclodextrin-hydrolyzing enzyme. Appl. Environ. Microbiol 2004, 70, 5988–5995. [Google Scholar]
| Source | TE | MW (kDa) | Opt. temp. (°C) | Opt. pH | Reaction product from pullulan | Ref. |
|---|---|---|---|---|---|---|
| Anoxybacillus sp. SK3-4 | N | 225 | 60 | 7.5 | Maltotriose, maltose and glucose | This study |
| Bacillus sp. XAL601 | R | 224 | 70 | 9.0 | Maltotriose | [10] |
| Geobacillus stearothermophilus TS-23 | R | 220 | ND | ND | ND | [11] |
| Geobacillus thermoleovorans NP33 | R | 182 | 60 | 7.0 | Maltotriose | [12] |
| Geobacillus stearothermophilus ATCC 12980 | R | 184 | ND | ND | ND | [13] |
| Thermoanaerobacter pseudoethanolicus ATCC 33233 | R | 160 | ND | ND | ND | [14] |
| Thermoanaerobacter thermohydrosulfuricus E101 | R | 165 | 80 | ND | Maltotriose | [15] |
| Thermoanaerobacterium saccharolyticum NTOU1 | R | 100 a | 70 | 5.0 | Maltotriose and maltose | [16] |
| Thermoanaerobacterium thermosulfurigenes EM1 | R | 205 | ND | ND | ND | [17] |
| Bacillus sp. KSM-1378 | N | 210 | 50 | 9.5 | Maltotriose, maltohexaose and maltononaose | [7] |
| Bifidobacterium breve UCC2003 | R | 182.3 a | ND | ND | Maltotriose and maltohexaose | [18] |
| Lactobacillus plantarum L137 | R | 211 | 40 | 4.0 | Maltotriose | [19] |
| Geobacillus sp. L14 | N | 100 a | 65 | 5.5 | Maltotriose, maltose and glucose | [20] |
| Buffers, metal ions, and chemical reagents | Relative activity (%) |
|---|---|
| Buffers (100 mM, pH 7.5) | |
| Sodium phosphate | 47 ± 0.04 |
| Potassium phosphate | 100 ± 0.08 |
| Tris-HCl | 45 ± 0.05 |
| MOPS | 94 ± 0.02 |
| HEPES-NaOH | 15 ± 0.02 |
| Metal ions (2 mM) | |
| None | 100 ± 0.02 |
| Na+ | 91 ± 0.01 |
| K+ | 128 ± 0.01 |
| Fe2+ | 108 ± 0.01 |
| Fe3+ | 196 ± 0.50 |
| Mg2+ | 114 ± 0.02 |
| Mn2+ | 182 ± 0.02 |
| Co2+ | 217 ± 0.03 |
| Cu2+ | 135 ± 0.03 |
| NH4+ | 83 ± 0.01 |
| Hg2+ | 16 ± 0.02 |
| Zn2+ | 70 ± 0.02 |
| Ni2+ | 154 ± 0.02 |
| Rb2+ | 14 ± 0.02 |
| Chemical reagents | |
| None | 100 ± 0.02 |
| 5 mM EDTA | 3 ± 0.04 |
| 1 mM SDS | 33 ± 0.06 |
| 10 mM DTT | 45 ± 0.08 |
| 10 mM β-mercaptoethanol | 27 ± 0.05 |
| 3 mM Urea | 25 ± 0.06 |
| 1% (v/v) Tween-20 | 20 ± 0.09 |
| 1% (v/v) Triton X-100 | 15 ± 0.02 |
| 0.1% (w/v) α-CD | 19 ± 0.02 |
| 0.1% (w/v) β-CD | 6 ± 0.01 |
| 0.1% (w/v) γ-CD | 2 ± 0.01 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kahar, U.M.; Chan, K.-G.; Salleh, M.M.; Hii, S.M.; Goh, K.M. A High Molecular-Mass Anoxybacillus sp. SK3-4 Amylopullulanase: Characterization and Its Relationship in Carbohydrate Utilization. Int. J. Mol. Sci. 2013, 14, 11302-11318. https://doi.org/10.3390/ijms140611302
Kahar UM, Chan K-G, Salleh MM, Hii SM, Goh KM. A High Molecular-Mass Anoxybacillus sp. SK3-4 Amylopullulanase: Characterization and Its Relationship in Carbohydrate Utilization. International Journal of Molecular Sciences. 2013; 14(6):11302-11318. https://doi.org/10.3390/ijms140611302
Chicago/Turabian StyleKahar, Ummirul Mukminin, Kok-Gan Chan, Madihah Md. Salleh, Siew Mee Hii, and Kian Mau Goh. 2013. "A High Molecular-Mass Anoxybacillus sp. SK3-4 Amylopullulanase: Characterization and Its Relationship in Carbohydrate Utilization" International Journal of Molecular Sciences 14, no. 6: 11302-11318. https://doi.org/10.3390/ijms140611302
APA StyleKahar, U. M., Chan, K.-G., Salleh, M. M., Hii, S. M., & Goh, K. M. (2013). A High Molecular-Mass Anoxybacillus sp. SK3-4 Amylopullulanase: Characterization and Its Relationship in Carbohydrate Utilization. International Journal of Molecular Sciences, 14(6), 11302-11318. https://doi.org/10.3390/ijms140611302