Where Do They Come from and Where Do They Go: Candidates for Regulating Extracellular Vesicle Formation in Fungi
Abstract
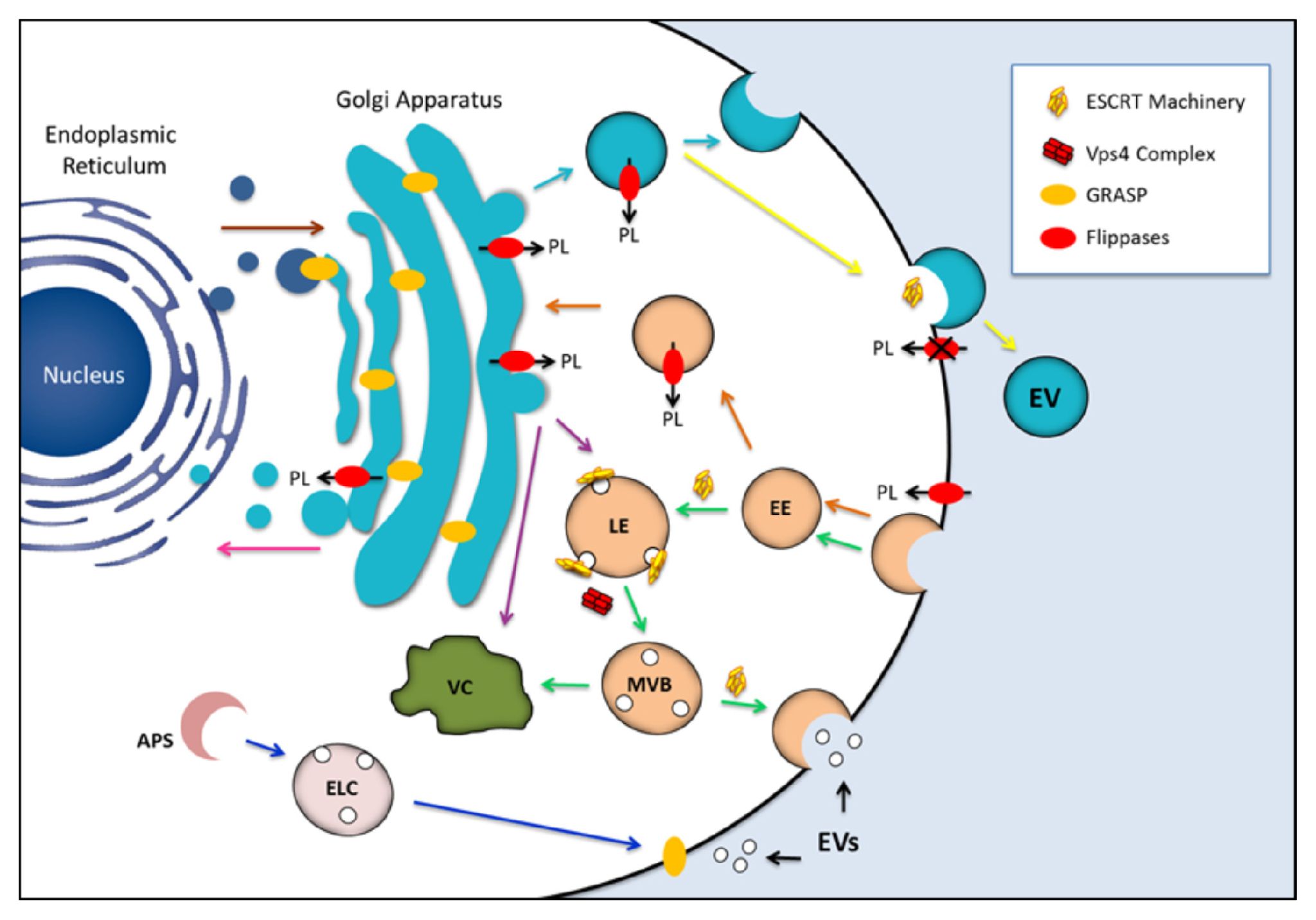
:1. Introduction
2. An Overview on Molecular Traffic and EV Release
3. Fungal EVs
4. Candidates for Regulating the Formation of Fungal EVs
4.1. Exosome Regulators
4.2. Flippases
4.3. Golgi Reassembly Stacking Protein (GRASP)
5. Concluding Remarks
Acknowledgments
References
- Latge, J.P.; Mouyna, I.; Tekaia, F.; Beauvais, A.; Debeaupuis, J.P.; Nierman, W. Specific molecular features in the organization and biosynthesis of the cell wall of Aspergillus fumigatus. Med. Mycol 2005, 43, S15–S22. [Google Scholar]
- Nimrichter, L.; Rodrigues, M.L.; Rodrigues, E.G.; Travassos, L.R. The multitude of targets for the immune system and drug therapy in the fungal cell wall. Microbes Infect 2005, 7, 789–798. [Google Scholar]
- Mitchell, A.P. Cryptococcal virulence: Beyond the usual suspects. J. Clin. Invest 2006, 116, 1481–1483. [Google Scholar]
- Nosanchuk, J.D.; Steenbergen, J.N.; Shi, L.; Deepe, G.S., Jr; Casadevall, A. Antibodies to a cell surface histone-like protein protect against Histoplasma capsulatum. J. Clin. Invest 2003, 112, 1164–1175. [Google Scholar]
- Barbosa, M.S.; Bao, S.N.; Andreotti, P.F.; de Faria, F.P.; Felipe, M.S.; dos Santos Feitosa, L.; Mendes-Giannini, M.J.; Soares, C.M. Glyceraldehyde-3-phosphate dehydrogenase of Paracoccidioides brasiliensis is a cell surface protein involved in fungal adhesion to extracellular matrix proteins and interaction with cells. Infect. Immun 2006, 74, 382–289. [Google Scholar]
- Rodrigues, M.L.; Nakayasu, E.S.; Oliveira, D.L.; Nimrichter, L.; Nosanchuk, J.D.; Almeida, I.C.; Casadevall, A. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot Cell 2008, 7, 58–67. [Google Scholar]
- Batista, W.L.; Matsuo, A.L.; Ganiko, L.; Barros, T.F.; Veiga, T.R.; Freymuller, E.; Puccia, R. The PbMDJ1 gene belongs to a conserved MDJ1/LON locus in thermodimorphic pathogenic fungi and encodes a heat shock protein that localizes to both the mitochondria and cell wall of Paracoccidioides brasiliensis. Eukaryot Cell 2006, 5, 379–390. [Google Scholar]
- Rodrigues, M.L.; Nimrichter, L.; Oliveira, D.B.L.; Frases, S.; Miranda, K.; Zaragoza, O.; Alvarez, M.; Nakouzi, A.; Feldmesser, M.; Casadevall, A. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Am. Soc. Microbiol 2007, 6, 48–59. [Google Scholar]
- Albuquerque, P.C.; Nakayasu, E.S.; Rodrigues, M.L.; Frases, S.; Casadevall, A.; Zancope-Oliveira, R.M.; Almeida, I.C.; Nosanchuk, J.D. Vesicular transport in Histoplasma capsulatum: An effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Cell Microbiol 2008, 10, 1695–1710. [Google Scholar]
- Vallejo, M.C.; Matsuo, A.L.; Ganiko, L.; Medeiros, L.C.; Miranda, K.; Silva, L.S.; Freymuller-Haapalainen, E.; Sinigaglia-Coimbra, R.; Almeida, I.C.; Puccia, R. The pathogenic fungus Paracoccidioides brasiliensis exports extracellular vesicles containing highly immunogenic alpha-Galactosyl epitopes. Eukaryot Cell 2011, 10, 343–351. [Google Scholar]
- Oliveira, D.L.; Nakayasu, E.S.; Joffe, L.S.; Guimaraes, A.J.; Sobreira, T.J.; Nosanchuk, J.D.; Cordero, R.J.; Frases, S.; Casadevall, A.; Almeida, I.C.; et al. Characterization of yeast extracellular vesicles: Evidence for the participation of different pathways of cellular traffic in vesicle biogenesis. PloS One 2010, 5, e11113. [Google Scholar]
- Barbosa, F.M.; Daffre, S.; Maldonado, R.A.; Miranda, A.; Nimrichter, L.; Rodrigues, M.L. Gomesin, a peptide produced by the spider Acanthoscurria gomesiana, is a potent anticryptococcal agent that acts in synergism with fluconazole. FEMS Microbiol. Lett 2007, 274, 279–286. [Google Scholar]
- Oliveira, D.B.L.; Nimrichter, L.; Miranda, K.; Frases, S.; Faull, K.F.; Casadevall, A.; Rodrigues, M.L. Cryptococcus neoformans cryoultramicrotomy and vesicle fractionation reveals an intimate association between membrane lipids and glucuronoxylomannan. Fungal Genet. Biol 2009, 46, 956–963. [Google Scholar]
- Gehrmann, U.; Qazi, K.R.; Johansson, C.; Hultenby, K.; Karlsson, M.; Lundeberg, L.; Gabrielsson, S.; Scheynius, A. Nanovesicles from Malassezia sympodialis and host exosomes induce cytokine responses—Novel mechanisms for host-microbe interactions in atopic eczema. PLoS One 2011, 6, e21480. [Google Scholar]
- Oliveira, D.L.; Freire-de-Lima, C.G.; Nosanchuk, J.D.; Casadevall, A.; Rodrigues, M.L.; Nimrichter, L. Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions. Infect. Immun 2010, 78, 1601–1609. [Google Scholar]
- Vallejo, M.C.; Nakayasu, E.S.; Longo, L.V.; Ganiko, L.; Lopes, F.G.; Matsuo, A.L.; Almeida, I.C.; Puccia, R. Lipidomic analysis of extracellular vesicles from the pathogenic phase of Paracoccidioides brasiliensis. PLoS One 2012, 7, e39463. [Google Scholar]
- Kuehn, M.J.; Kesty, N.C. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev 2005, 19, 2645–2655. [Google Scholar]
- Bomberger, J.M.; Maceachran, D.P.; Coutermarsh, B.A.; Ye, S.; O’Toole, G.A.; Stanton, B.A. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog 2009, 5, e1000382. [Google Scholar]
- Yonezawa, H.; Osaki, T.; Kurata, S.; Fukuda, M.; Kawakami, H.; Ochiai, K.; Hanawa, T.; Kamiya, S. Outer membrane vesicles of Helicobacter pylori TK1402 are involved in biofilm formation. BMC Microbiol 2009, 9, 197. [Google Scholar]
- Lee, E.Y.; Choi, D.Y.; Kim, D.K.; Kim, J.W.; Park, J.O.; Kim, S.; Kim, S.H.; Desiderio, D.M.; Kim, Y.K.; Kim, K.P.; et al. Gram-positive bacteria produce membrane vesicles: Proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics 2009, 9, 5425–5436. [Google Scholar]
- Rivera, J.; Cordero, R.J.; Nakouzi, A.S.; Frases, S.; Nicola, A.; Casadevall, A. Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proc. Natl Acad Sci USA 2010, 107, 19002–19007. [Google Scholar]
- Makarova, K.S.; Yutin, N.; Bell, S.D.; Koonin, E.V. Evolution of diverse cell division and vesicle formation systems in Archaea. Nat. Rev. Microbiol 2010, 8, 731–741. [Google Scholar]
- Ellen, A.F.; Albers, S.V.; Huibers, W.; Pitcher, A.; Hobel, C.F.; Schwarz, H.; Folea, M.; Schouten, S.; Boekema, E.J.; Poolman, B.; et al. Proteomic analysis of secreted membrane vesicles of archaeal Sulfolobus species reveals the presence of endosome sorting complex components. Extremophiles 2009, 13, 67–79. [Google Scholar]
- Soler, N.; Marguet, E.; Verbavatz, J.M.; Forterre, P. Virus-like vesicles and extracellular DNA produced by hyperthermophilic archaea of the order Thermococcales. Res. Microbiol 2008, 159, 390–399. [Google Scholar]
- Rabouille, C.; Malhotra, V.; Nickel, W. Diversity in unconventional protein secretion. J. Cell Sci 2012, 125, 5251–5255. [Google Scholar]
- Nickel, W. Pathways of unconventional protein secretion. Curr. Opin. Biotechnol 2010, 21, 621–626. [Google Scholar]
- Nickel, W.; Rabouille, C. Mechanisms of regulated unconventional protein secretion. Nat. Rev. Mol. Cell Biol 2009, 10, 148–155. [Google Scholar]
- Johnstone, R.M.; Adam, M.; Pan, B.T. The fate of the transferrin receptor during maturation of sheep reticulocytes in vitro. Can. J. Biochem. Cell Biol 1984, 62, 1246–1254. [Google Scholar]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med 1996, 183, 1161–1172. [Google Scholar]
- Zitvogel, L.; Regnault, A.; Lozier, A.; Wolfers, J.; Flament, C.; Tenza, D.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell-derived exosomes. Nat. Med 1998, 4, 594–600. [Google Scholar]
- Marzesco, A.M.; Janich, P.; Wilsch-Brauninger, M.; Dubreuil, V.; Langenfeld, K.; Corbeil, D.; Huttner, W.B. Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J. Cell Sci 2005, 118, 2849–2858. [Google Scholar]
- Gould, S.J.; Booth, A.M.; Hildreth, J.E. The Trojan exosome hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 10592–10597. [Google Scholar]
- Fang, Y.; Wu, N.; Gan, X.; Yan, W.; Morrell, J.C.; Gould, S.J. Higher-order oligomerization targets plasma membrane proteins and HIV gag to exosomes. PLoS Biol 2007, 5, e158. [Google Scholar]
- Bhatnagar, S.; Shinagawa, K.; Castellino, F.J.; Schorey, J.S. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood 2007, 110, 3234–3244. [Google Scholar]
- Beatty, W.L.; Russell, D.G. Identification of mycobacterial surface proteins released into subcellular compartments of infected macrophages. Infect. Immun 2000, 68, 6997–7002. [Google Scholar]
- Park, J.E.; Tan, H.S.; Datta, A.; Lai, R.C.; Zhang, H.; Meng, W.; Lim, S.K.; Sze, S.K. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol. Cell. Proteomics 2010, 9, 1085–1099. [Google Scholar]
- Marcilla, A.; Trelis, M.; Cortes, A.; Sotillo, J.; Cantalapiedra, F.; Minguez, M.T.; Valero, M.L.; Sanchez del Pino, M.M.; Munoz-Antoli, C.; Toledo, R.; et al. Extracellular vesicles from parasitic helminths contain specific excretory/secretory proteins and are internalized in intestinal host cells. PLoS One 2012, 7, e45974. [Google Scholar]
- Silverman, J.M.; Clos, J.; de’Oliveira, C.C.; Shirvani, O.; Fang, Y.; Wang, C.; Foster, L.J.; Reiner, N.E. An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J. Cell Sci 2010, 123, 842–852. [Google Scholar]
- Clayton, C.; Estevez, A. The exosomes of trypanosomes and other protists. Adv. Exp. Med. Biol 2011, 702, 39–49. [Google Scholar]
- Regente, M.; Corti-Monzon, G.; Maldonado, A.M.; Pinedo, M.; Jorrin, J.; de la Canal, L. Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Lett 2009, 583, 3363–3366. [Google Scholar]
- Takeo, K.; Uesaka, I.; Uehira, K.; Nishiura, M. Fine structure of Cryptococcus neoformans grown in vitro as observed by freeze-etching. J. Bacteriol 1973, 113, 1442–1448. [Google Scholar]
- Rodrigues, M.L.; Travassos, L.R.; Miranda, K.R.; Franzen, A.J.; Rozental, S.; de Souza, W.; Alviano, C.S.; Barreto-Bergter, E. Human antibodies against a purified glucosylceramide from Cryptococcus neoformans inhibit cell budding and fungal growth. Infect. Immun 2000, 68, 7049–7060. [Google Scholar]
- Anderson, J.; Mihalik, R.; Soll, D.R. Ultrastructure and antigenicity of the unique cell wall pimple of the Candida opaque phenotype. J. Bacteriol 1990, 172, 224–235. [Google Scholar]
- Bogo, M.R.; Queiroz, M.V.; Silva, D.M.; Giménez, M.P.; Azevedo, J.L.; Schrank, A. Double-stranded RNA and isometric virus-like particles in the entomopathogenic fungus Metarhizium anisopliae. Mycol. Res 1996, 100, 1468–1472. [Google Scholar]
- Chang, Y.C.; Penoyer, L.A.; Kwon-Chung, K.J. The second capsule gene of Cryptococcus neoformans, CAP64, is essential for virulence. Infect. Immun 1996, 64, 1977–1983. [Google Scholar]
- Chang, Y.C.; Kwon-Chung, K.J. Isolation of the third capsule-associated gene, CAP60, required for virulence in Cryptococcus neoformans. Infect. Immun 1998, 66, 2230–2236. [Google Scholar]
- Chang, Y.C.; Kwon-Chung, K.J. Isolation, characterization, and localization of a capsule-associated gene, CAP10, of Cryptococcus neoformans. J. Bacteriol 1999, 181, 5636–5643. [Google Scholar]
- Feldmesser, M.; Kress, Y.; Novikoff, P.; Casadevall, A. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect. Immun 2000, 68, 4225–37. [Google Scholar]
- Yoneda, A.; Doering, T.L. A eukaryotic capsular polysaccharide is synthesized intracellularly and secreted via exocytosis. Mol. Biol. Cell 2006, 17, 5131–5140. [Google Scholar]
- Bonifacino, J.S.; Glick, B.S. The mechanisms of vesicle budding and fusion. Cell 2004, 116, 153–166. [Google Scholar]
- Feldmesser, M.; Kress, Y.; Casadevall, A. Dynamic changes in the morphology of Cryptococcus neoformans during murine pulmonary infection. Microbiology 2001, 147, 2355–2365. [Google Scholar]
- Vallejo, M.C.; Nakayasu, E.S.; Matsuo, A.L.; Sobreira, T.J.; Longo, L.V.; Ganiko, L.; Almeida, I.C.; Puccia, R. Vesicle and vesicle-free extracellular proteome of Paracoccidioides brasiliensis: Comparative analysis with other pathogenic fungi. J. Proteome Res 2012, 11, 1676–85. [Google Scholar]
- Jeffery, C.J. Moonlighting proteins. Trends Biochem. Sci 1999, 24, 8–11. [Google Scholar]
- Eisenman, H.C.; Frases, S.; Nicola, A.M.; Rodrigues, M.L.; Casadevall, A. Vesicle-associated melanization in Cryptococcus neoformans. Microbiology 2009, 155, 3860–3867. [Google Scholar]
- Schorey, J.S.; Bhatnagar, S. Exosome function: From tumor immunology to pathogen biology. Traffic 2008, 9, 871–881. [Google Scholar]
- Huang, S.H.; Wu, C.H.; Chang, Y.C.; Kwon-Chung, K.J.; Brown, R.J.; Jong, A. Cryptococcus neoformans-derived microvesicles enhance the pathogenesis of fungal brain infection. PloS One 2012, 7, e48570. [Google Scholar]
- Panepinto, J.; Komperda, K.; Frases, S.; Park, Y.D.; Djordjevic, J.T.; Casadevall, A.; Williamson, P.R. Sec6-dependent sorting of fungal extracellular exosomes and laccase of Cryptococcus neoformans. Mol. Microbiol 2009, 71, 1165–1176. [Google Scholar]
- Kmetzsch, L.; Joffe, L.S.; Staats, C.C.; de Oliveira, D.L.; Fonseca, F.L.; Cordero, R.J.; Casadevall, A.; Nimrichter, L.; Schrank, A.; Vainstein, M.H.; et al. Role for Golgi reassembly and stacking protein (GRASP) in polysaccharide secretion and fungal virulence. Mol. Microbiol 2011, 81, 206–218. [Google Scholar]
- Trams, E.G.; Lauter, C.J.; Salem, N., Jr; Heine, U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta 1981, 645, 63–70. [Google Scholar]
- Pan, B.T.; Teng, K.; Wu, C.; Adam, M.; Johnstone, R.M. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J. Cell Biol 1985, 101, 942–948. [Google Scholar]
- Harding, C.; Heuser, J.; Stahl, P. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: Demonstration of a pathway for receptor shedding. Eur. J. Cell Biol 1984, 35, 256–263. [Google Scholar]
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol 2013, 200, 373–383. [Google Scholar]
- Soo, C.Y.; Song, Y.; Zheng, Y.; Campbell, E.C.; Riches, A.C.; Gunn-Moore, F.; Powis, S.J. Nanoparticle tracking analysis monitors microvesicle and exosome secretion from immune cells. Immunology 2012, 136, 192–197. [Google Scholar]
- Hoen, E.N.; van der Vlist, E.J.; Aalberts, M.; Mertens, H.C.; Bosch, B.J.; Bartelink, W.; Mastrobattista, E.; van Gaal, E.V.; Stoorvogel, W.; Arkesteijn, G.J.; et al. Quantitative and qualitative flow cytometric analysis of nanosized cell-derived membrane vesicles. Nanomedicine 2012, 8, 712–720. [Google Scholar]
- Van der Vlist, E.J.; Nolte-’t Hoen, E.N.; Stoorvogel, W.; Arkesteijn, G.J.; Wauben, M.H. Fluorescent labeling of nano-sized vesicles released by cells and subsequent quantitative and qualitative analysis by high-resolution flow cytometry. Nat. Protoc 2012, 7, 1311–1326. [Google Scholar]
- Escola, J.M.; Kleijmeer, M.J.; Stoorvogel, W.; Griffith, J.M.; Yoshie, O.; Geuze, H.J. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J. Biol. Chem 1998, 273, 20121–20127. [Google Scholar]
- Thery, C.; Regnault, A.; Garin, J.; Wolfers, J.; Zitvogel, L.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J. Cell Biol 1999, 147, 599–610. [Google Scholar]
- Wubbolts, R.; Leckie, R.S.; Veenhuizen, P.T.; Schwarzmann, G.; Mobius, W.; Hoernschemeyer, J.; Slot, J.W.; Geuze, H.J.; Stoorvogel, W. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J. Biol. Chem 2003, 278, 10963–10972. [Google Scholar]
- Laulagnier, K.; Motta, C.; Hamdi, S.; Roy, S.; Fauvelle, F.; Pageaux, J.F.; Kobayashi, T.; Salles, J.P.; Perret, B.; Bonnerot, C.; et al. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem. J 2004, 380, 161–171. [Google Scholar]
- Subra, C.; Laulagnier, K.; Perret, B.; Record, M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie 2007, 89, 205–212. [Google Scholar]
- Brouwers, J.F.; Aalberts, M.; Jansen, J.W.A.; van Niel, G.; Wauben, M.H.; Stout, T.A.E.; Helms, J.B.; Stoorvogel, W. Distinct lipid compositions of two types of human prostasomes. Proteomics 2013. [Google Scholar] [CrossRef]
- Palade, G.E. Studies on the endoplasmic reticulum. II. Simple dispositions in cells in situ. J. Biophys. Biochem. Cytol 1955, 1, 567–582. [Google Scholar]
- Sotelo, J.R.; Porter, K.R. An electron microscope study of the rat ovum. J. Biophys. Biochem. Cytol 1959, 5, 327–342. [Google Scholar]
- Katzmann, D.J.; Babst, M.; Emr, S.D. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 2001, 106, 145–155. [Google Scholar]
- Babst, M.; Katzmann, D.J.; Snyder, W.B.; Wendland, B.; Emr, S.D. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell 2002, 3, 283–289. [Google Scholar]
- Babst, M.; Katzmann, D.J.; Estepa-Sabal, E.J.; Meerloo, T.; Emr, S.D. Escrt-III: An endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell 2002, 3, 271–282. [Google Scholar]
- Asao, H.; Sasaki, Y.; Arita, T.; Tanaka, N.; Endo, K.; Kasai, H.; Takeshita, T.; Endo, Y.; Fujita, T.; Sugamura, K. Hrs is associated with STAM, a signal-transducing adaptor molecule. Its suppressive effect on cytokine-induced cell growth. J. Biol. Chem 1997, 272, 32785–32791. [Google Scholar]
- Babst, M.; Sato, T.K.; Banta, L.M.; Emr, S.D. Endosomal transport function in yeast requires a novel AAA-type ATPase, Vps4p. EMBO J 1997, 16, 1820–1831. [Google Scholar]
- Shih, S.C.; Katzmann, D.J.; Schnell, J.D.; Sutanto, M.; Emr, S.D.; Hicke, L. Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nat. Cell Biol 2002, 4, 389–393. [Google Scholar]
- Henne, W.M.; Buchkovich, N.J.; Emr, S.D. The ESCRT pathway. Dev. Cell 2011, 21, 77–91. [Google Scholar]
- Schmidt, O.; Teis, D. The ESCRT machinery. Curr. Biol 2012, 22, R116–R120. [Google Scholar]
- Raiborg, C.; Bremnes, B.; Mehlum, A.; Gillooly, D.J.; D'Arrigo, A.; Stang, E.; Stenmark, H. FYVE and coiled-coil domains determine the specific localisation of Hrs to early endosomes. J. Cell Sci 2001, 114, 2255–2263. [Google Scholar]
- Katzmann, D.J.; Stefan, C.J.; Babst, M.; Emr, S.D. Vps27 recruits ESCRT machinery to endosomes during MVB sorting. J. Cell Biol 2003, 162, 413–423. [Google Scholar]
- Kostelansky, M.S.; Sun, J.; Lee, S.; Kim, J.; Ghirlando, R.; Hierro, A.; Emr, S.D.; Hurley, J.H. Structural and functional organization of the ESCRT-I trafficking complex. Cell 2006, 125, 113–26. [Google Scholar]
- Bache, K.G.; Raiborg, C.; Mehlum, A.; Stenmark, H. STAM and Hrs are subunits of a multivalent ubiquitin-binding complex on early endosomes. J. Biol. Chem 2003, 278, 12513–12521. [Google Scholar]
- Lu, Q.; Hope, L.W.; Brasch, M.; Reinhard, C.; Cohen, S.N. TSG101 interaction with HRS mediates endosomal trafficking and receptor down-regulation. Proc. Natl Acad Sci USA 2003, 100, 7626–7631. [Google Scholar]
- Langelier, C.; von Schwedler, U.K.; Fisher, R.D.; De Domenico, I.; White, P.L.; Hill, C.P.; Kaplan, J.; Ward, D.; Sundquist, W.I. Human ESCRT-II complex and its role in human immunodeficiency virus type 1 release. J. Virol 2006, 80, 9465–9480. [Google Scholar]
- Gill, D.J.; Teo, H.; Sun, J.; Perisic, O.; Veprintsev, D.B.; Emr, S.D.; Williams, R.L. Structural insight into the ESCRT-I/-II link and its role in MVB trafficking. EMBO J 2007, 26, 600–612. [Google Scholar]
- Teo, H.; Gill, D.J.; Sun, J.; Perisic, O.; Veprintsev, D.B.; Vallis, Y.; Emr, S.D.; Williams, R.L. ESCRT-I core and ESCRT-II GLUE domain structures reveal role for GLUE in linking to ESCRT-I and membranes. Cell 2006, 125, 99–111. [Google Scholar]
- Hierro, A.; Sun, J.; Rusnak, A.S.; Kim, J.; Prag, G.; Emr, S.D.; Hurley, J.H. Structure of the ESCRT-II endosomal trafficking complex. Nature 2004, 431, 221–225. [Google Scholar]
- Teo, H.; Perisic, O.; Gonzalez, B.; Williams, R.L. ESCRT-II, an endosome-associated complex required for protein sorting: crystal structure and interactions with ESCRT-III and membranes. Dev. Cell 2004, 7, 559–569. [Google Scholar]
- Saksena, S.; Emr, S.D. ESCRTs and human disease. Biochem. Soc. Trans 2009, 37, 167–172. [Google Scholar]
- Teis, D.; Saksena, S.; Emr, S.D. Ordered assembly of the ESCRT-III complex on endosomes is required to sequester cargo during MVB formation. Dev. Cell 2008, 15, 578–589. [Google Scholar]
- Luhtala, N.; Odorizzi, G. Bro1 coordinates deubiquitination in the multivesicular body pathway by recruiting Doa4 to endosomes. J. Cell Biol 2004, 166, 717–729. [Google Scholar]
- Odorizzi, G.; Katzmann, D.J.; Babst, M.; Audhya, A.; Emr, S.D. Bro1 is an endosome-associated protein that functions in the MVB pathway in Saccharomyces cerevisiae. J. Cell Sci 2003, 116, 1893–1903. [Google Scholar]
- Finken-Eigen, M.; Rohricht, R.A.; Kohrer, K. The VPS4 gene is involved in protein transport out of a yeast pre-vacuolar endosome-like compartment. Curr. Genet 1997, 31, 469–480. [Google Scholar]
- Babst, M.; Wendland, B.; Estepa, E.J.; Emr, S.D. The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO J 1998, 17, 2982–2993. [Google Scholar]
- Scott, A.; Gaspar, J.; Stuchell-Brereton, M.D.; Alam, S.L.; Skalicky, J.J.; Sundquist, W.I. Structure and ESCRT-III protein interactions of the MIT domain of human VPS4A. Proc. Natl. Acad. Sci. USA 2005, 102, 13813–13818. [Google Scholar]
- Rue, S.M.; Mattei, S.; Saksena, S.; Emr, S.D. Novel Ist1-Did2 complex functions at a late step in multivesicular body sorting. Mol. Biol. Cell 2008, 19, 475–484. [Google Scholar]
- Shestakova, A.; Hanono, A.; Drosner, S.; Curtiss, M.; Davies, B.A.; Katzmann, D.J.; Babst, M. Assembly of the AAA ATPase Vps4 on ESCRT-III. Mol. Biol. Cell 2010, 21, 1059–1071. [Google Scholar]
- Henne, W.M.; Buchkovich, N.J.; Zhao, Y.; Emr, S.D. The endosomal sorting complex ESCRT-II mediates the assembly and architecture of ESCRT-III helices. Cell 2012, 151, 356–371. [Google Scholar]
- Teis, D.; Saksena, S.; Judson, B.L.; Emr, S.D. ESCRT-II coordinates the assembly of ESCRT-III filaments for cargo sorting and multivesicular body vesicle formation. EMBO J 2010, 29, 871–83. [Google Scholar]
- Lata, S.; Schoehn, G.; Jain, A.; Pires, R.; Piehler, J.; Gottlinger, H.G.; Weissenhorn, W. Helical structures of ESCRT-III are disassembled by VPS4. Science 2008, 321, 1354–1357. [Google Scholar]
- Fabrikant, G.; Lata, S.; Riches, J.D.; Briggs, J.A.; Weissenhorn, W.; Kozlov, M.M. Computational model of membrane fission catalyzed by ESCRT-III. PLoS Comput. Biol 2009, 5, e1000575. [Google Scholar]
- Theos, A.C.; Truschel, S.T.; Tenza, D.; Hurbain, I.; Harper, D.C.; Berson, J.F.; Thomas, P.C.; Raposo, G.; Marks, M.S. A lumenal domain-dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev. Cell 2006, 10, 343–354. [Google Scholar]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brugger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar]
- Matsuo, H.; Chevallier, J.; Mayran, N.; Le Blanc, I.; Ferguson, C.; Faure, J.; Blanc, N.S.; Matile, S.; Dubochet, J.; Sadoul, R.; et al. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science 2004, 303, 531–534. [Google Scholar]
- Takeo, K.; Uesaka, I.; Uehira, K.; Nishiura, M. Fine structure of Cryptococcus neoformans grown in vivo as observed by freeze-etching. J. Bacteriol 1973, 113, 1449–1454. [Google Scholar]
- Farge, E.; Devaux, P.F. Shape changes of giant liposomes induced by an asymmetric transmembrane distribution of phospholipids. Biophys. J 1992, 61, 347–357. [Google Scholar]
- Farge, E.; Ojcius, D.M.; Subtil, A.; Dautry-Varsat, A. Enhancement of endocytosis due to aminophospholipid transport across the plasma membrane of living cells. Am. J. Physiol 1999, 276, C725–C733. [Google Scholar]
- Graham, T.R.; Kozlov, M.M. Interplay of proteins and lipids in generating membrane curvature. Curr. Opin. Cell Biol 2010, 22, 430–436. [Google Scholar]
- Catty, P.; de Kerchove d’Exaerde, A.; Goffeau, A. The complete inventory of the yeast Saccharomyces cerevisiae P-type transport ATPases. FEBS Lett 1997, 409, 325–332. [Google Scholar]
- Axelsen, K.B.; Palmgren, M.G. Evolution of substrate specificities in the P-type ATPase superfamily. J. Mol. Evol 1998, 46, 84–101. [Google Scholar]
- Auland, M.E.; Roufogalis, B.D.; Devaux, P.F.; Zachowski, A. Reconstitution of ATP-dependent aminophospholipid translocation in proteoliposomes. Proc. Natl. Acad. Sci. USA 1994, 91, 10938–10942. [Google Scholar]
- Paulusma, C.C.; Oude Elferink, R.P. The type 4 subfamily of P-type ATPases, putative aminophospholipid translocases with a role in human disease. Biochim. Biophys. Acta 2005, 1741, 11–24. [Google Scholar]
- Pomorski, T.; Holthuis, J.C.; Herrmann, A.; van Meer, G. Tracking down lipid flippases and their biological functions. J. Cell Sci 2004, 117, 805–813. [Google Scholar]
- Tang, X.; Halleck, M.S.; Schlegel, R.A.; Williamson, P. A subfamily of P-type ATPases with aminophospholipid transporting activity. Science 1996, 272, 1495–1497. [Google Scholar]
- Alder-Baerens, N.; Lisman, Q.; Luong, L.; Pomorski, T.; Holthuis, J.C. Loss of P4 ATPases Drs2p and Dnf3p disrupts aminophospholipid transport and asymmetry in yeast post-Golgi secretory vesicles. Mol. Biol. Cell 2006, 17, 1632–1642. [Google Scholar]
- Coleman, J.A.; Kwok, M.C.; Molday, R.S. Localization, purification, and functional reconstitution of the P4-ATPase Atp8a2, a phosphatidylserine flippase in photoreceptor disc membranes. J. Biol. Chem 2009, 284, 32670–32679. [Google Scholar]
- Seigneuret, M.; Devaux, P.F. ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: Relation to shape changes. Proc. Natl. Acad. Sci. USA 1984, 81, 3751–3755. [Google Scholar]
- Lenoir, G.; Williamson, P.; Puts, C.F.; Holthuis, J.C. Cdc50p plays a vital role in the ATPase reaction cycle of the putative aminophospholipid transporter Drs2p. J. Biol. Chem 2009, 284, 17956–17967. [Google Scholar]
- Poulsen, L.R.; Lopez-Marques, R.L.; McDowell, S.C.; Okkeri, J.; Licht, D.; Schulz, A.; Pomorski, T.; Harper, J.F.; Palmgren, M.G. The Arabidopsis P4-ATPase ALA3 localizes to the golgi and requires a beta-subunit to function in lipid translocation and secretory vesicle formation. Plant Cell 2008, 20, 658–676. [Google Scholar]
- Saito, K.; Fujimura-Kamada, K.; Furuta, N.; Kato, U.; Umeda, M.; Tanaka, K. Cdc50p, a protein required for polarized growth, associates with the Drs2p P-type ATPase implicated in phospholipid translocation in Saccharomyces cerevisiae. Mol. Biol. Cell 2004, 15, 3418–3432. [Google Scholar]
- Weingartner, A.; Drobot, B.; Herrmann, A.; Sanchez-Canete, M.P.; Gamarro, F.; Castanys, S.; Gunther Pomorski, T. Disruption of the lipid-transporting LdMT-LdRos3 complex in Leishmania donovani affects membrane lipid asymmetry but not host cell invasion. PLoS One 2010, 5, e12443. [Google Scholar]
- Van der Velden, L.M.; van de Graaf, S.F.; Klomp, L.W. Biochemical and cellular functions of P4 ATPases. Biochem. J 2010, 431, 1–11. [Google Scholar]
- Sebastian, T.T.; Baldridge, R.D.; Xu, P.; Graham, T.R. Phospholipid flippases: Building asymmetric membranes and transport vesicles. Biochim. Biophys. Acta 2012, 1821, 1068–1077. [Google Scholar]
- Chen, C.Y.; Ingram, M.F.; Rosal, P.H.; Graham, T.R. Role for Drs2p, a P-type ATPase and potential aminophospholipid translocase, in yeast late Golgi function. J. Cell Biol 1999, 147, 1223–1236. [Google Scholar]
- Hua, Z.; Fatheddin, P.; Graham, T.R. An essential subfamily of Drs2p-related P-type ATPases is required for protein trafficking between Golgi complex and endosomal/vacuolar system. Mol. Biol. Cell 2002, 13, 3162–3177. [Google Scholar]
- Pomorski, T.; Lombardi, R.; Riezman, H.; Devaux, P.F.; van Meer, G.; Holthuis, J.C. Drs2p-related P-type ATPases Dnf1p and Dnf2p are required for phospholipid translocation across the yeast plasma membrane and serve a role in endocytosis. Mol. Biol. Cell 2003, 14, 1240–1254. [Google Scholar]
- Hua, Z.; Graham, T.R. Requirement for neo1p in retrograde transport from the Golgi complex to the endoplasmic reticulum. Mol. Biol. Cell 2003, 14, 4971–4983. [Google Scholar]
- Wicky, S.; Schwarz, H.; Singer-Kruger, B. Molecular interactions of yeast Neo1p, an essential member of the Drs2 family of aminophospholipid translocases, and its role in membrane trafficking within the endomembrane system. Mol. Cell. Biol 2004, 24, 7402–7418. [Google Scholar]
- Furuta, N.; Fujimura-Kamada, K.; Saito, K.; Yamamoto, T.; Tanaka, K. Endocytic recycling in yeast is regulated by putative phospholipid translocases and the Ypt31p/32p-Rcy1p pathway. Mol. Biol. Cell 2007, 18, 295–312. [Google Scholar]
- Kishimoto, T.; Yamamoto, T.; Tanaka, K. Defects in structural integrity of ergosterol and the Cdc50p-Drs2p putative phospholipid translocase cause accumulation of endocytic membranes, onto which actin patches are assembled in yeast. Mol. Biol. Cell 2005, 16, 5592–5609. [Google Scholar]
- Liu, K.; Hua, Z.; Nepute, J.A.; Graham, T.R. Yeast P4-ATPases Drs2p and Dnf1p are essential cargos of the NPFXD/Sla1p endocytic pathway. Mol. Biol. Cell 2007, 18, 487–500. [Google Scholar]
- Liu, K.; Surendhran, K.; Nothwehr, S.F.; Graham, T.R. P4-ATPase requirement for AP-1/clathrin function in protein transport from the trans-Golgi network and early endosomes. Mol. Biol. Cell 2008, 19, 3526–3535. [Google Scholar]
- Ruaud, A.F.; Nilsson, L.; Richard, F.; Larsen, M.K.; Bessereau, J.L.; Tuck, S. The C. elegans P4-ATPase TAT-1 regulates lysosome biogenesis and endocytosis. Traffic 2009, 10, 88–100. [Google Scholar]
- Wehman, A.M.; Poggioli, C.; Schweinsberg, P.; Grant, B.D.; Nance, J. The P4-ATPase TAT-5 inhibits the budding of extracellular vesicles in C. elegans embryos. Curr. Biol. CB 2011, 21, 1951–1959. [Google Scholar]
- Birchmeier, W.; Lanz, J.H.; Winterhalter, K.H.; Conrad, M.J. ATP-induced endocytosis in human erythrocyte ghosts. Characterization of the process and isolation of the endocytosed vesicles. J. Biol. Chem 1979, 254, 9298–9304. [Google Scholar]
- Muller, P.; Pomorski, T.; Herrmann, A. Incorporation of phospholipid analogues into the plasma membrane affects ATP-induced vesiculation of human erythrocyte ghosts. Biochem. Biophys. Res. Commun 1994, 199, 881–887. [Google Scholar]
- Devaux, P.F. Is lipid translocation involved during endo- and exocytosis? Biochimie 2000, 82, 497–509. [Google Scholar]
- Sheetz, M.P.; Singer, S.J. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc. Natl. Acad. Sci. USA 1974, 71, 4457–4461. [Google Scholar]
- Cox, R.; Mason-Gamer, R.J.; Jackson, C.L.; Segev, N. Phylogenetic analysis of Sec7-domain-containing Arf nucleotide exchangers. Mol. Biol. Cell 2004, 15, 1487–1505. [Google Scholar]
- Gall, W.E.; Geething, N.C.; Hua, Z.; Ingram, M.F.; Liu, K.; Chen, S.I.; Graham, T.R. Drs2p-dependent formation of exocytic clathrin-coated vesicles in vivo. Curr. Biol. CB 2002, 12, 1623–7. [Google Scholar]
- Valdivia, R.H.; Baggott, D.; Chuang, J.S.; Schekman, R.W. The yeast clathrin adaptor protein complex 1 is required for the efficient retention of a subset of late Golgi membrane proteins. Dev. Cell 2002, 2, 283–294. [Google Scholar]
- Gurunathan, S.; David, D.; Gerst, J.E. Dynamin and clathrin are required for the biogenesis of a distinct class of secretory vesicles in yeast. EMBO J 2002, 21, 602–614. [Google Scholar]
- Harsay, E.; Schekman, R. A subset of yeast vacuolar protein sorting mutants is blocked in one branch of the exocytic pathway. J. Cell Biol 2002, 156, 271–285. [Google Scholar]
- Balhadere, P.V.; Talbot, N.J. PDE1 encodes a P-type ATPase involved in appressorium-mediated plant infection by the rice blast fungus Magnaporthe grisea. Plant Cell 2001, 13, 1987–2004. [Google Scholar]
- Gilbert, M.J.; Thornton, C.R.; Wakley, G.E.; Talbot, N.J. A P-type ATPase required for rice blast disease and induction of host resistance. Nature 2006, 440, 535–539. [Google Scholar]
- Hu, G.; Kronstad, J.W. A putative P-type ATPase, Apt1, is involved in stress tolerance and virulence in Cryptococcus neoformans. Eukaryotic Cell 2010, 9, 74–83. [Google Scholar]
- Glick, B.S.; Malhotra, V. The curious status of the Golgi apparatus. Cell 1998, 95, 883–889. [Google Scholar]
- Ramirez, I.B.; Lowe, M. Golgins and GRASPs: Holding the Golgi together. Semin. Cell Dev. Biol 2009, 20, 770–779. [Google Scholar]
- Shorter, J.; Watson, R.; Giannakou, M.-E.; Clarke, M.; Warren, G.; Barr, F.A. GRASP55, a second mammalian GRASP protein involved in the stacking of Golgi cisternae in a cell-free system. EMBO J 1999, 18, 4949–4960. [Google Scholar]
- Levi, S.K.; Glick, B.S. GRASPing unconventional secretion. Cell 2007, 130, 407–409. [Google Scholar]
- Barr, F.A.; Puype, M.; Vandekerckhove, J.L.; Warren, G. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell 1997, 91, 253–262. [Google Scholar]
- Behnia, R.; Barr, F.A.; Flanagan, J.J.; Barlowe, C.; Munro, S. The yeast orthologue of GRASP65 forms a complex with a coiled-coil protein that contributes to ER to Golgi traffic. J. Cell Biol 2007, 176, 255–261. [Google Scholar]
- Short, B.; Preisinger, C.; Körner, R.; Kopajtich, R.; Byron, O.; Barr, F.A. A GRASP55-rab2 effector complex linking Golgi structure to membrane traffic. J. Cell Biol 2001, 155, 877–884. [Google Scholar]
- Sutterlin, C.; Polishchuk, R.; Pecot, M.; Malhotra, V. The Golgi-associated protein GRASP65 regulates spindle dynamics and is essential for cell division. Mol. Biol. Cell 2005, 16, 3211–3222. [Google Scholar]
- Puthenveedu, M.A.; Bachert, C.; Puri, S.; Lanni, F.; Linstedt, A.D. GM130 and GRASP65-dependent lateral cisternal fusion allows uniform Golgi-enzyme distribution. Nat. Cell Biol 2006, 8, 238–248. [Google Scholar]
- Kondylis, V.; Spoorendonk, K.M.; Rabouille, C. dGRASP localization and function in the early exocytic pathway in Drosophila S2 cells. Am. Soc. Cell Biol 2005, 16, 4061–4072. [Google Scholar]
- Kinseth, M.A.; Anjard, C.; Fuller, D.; Guizzunti, G.; Loomis, W.F.; Malhotra, V. The Golgi-associated protein GRASP is required for unconventional protein secretion during development. Cell 2007, 130, 524–534. [Google Scholar]
- Cabral, M.; Anjard, C.; Malhotra, V.; Loomis, W.F.; Kuspa, A. Unconventional secretion of AcbA in Dictyostelium discoideum through a vesicular intermediate. Eukaryot Cell 2010, 9, 1009–1017. [Google Scholar]
- Duran, J.M.; Anjard, C.; Stefan, C.; Loomis, W.F.; Malhotra, V. Unconventional secretion of Acb1 is mediated by autophagosomes. J. Cell Biol 2010, 188, 527–536. [Google Scholar]
- Manjithaya, R.; Anjard, C.; Loomis, W.F.; Subramani, S. Unconventional secretion of Pichia pastoris Acb1 is dependent on GRASP protein, peroxisomal functions, and autophagosome formation. J. Cell Biol 2010, 188, 537–546. [Google Scholar]
- Abrahamsen, H.; Stenmark, H. Protein secretion: Unconventional exit by exophagy. Curr. Biol. CB 2010, 20, R415–R418. [Google Scholar]
- Schotman, H.; Karhinen, L.; Rabouille, C. dGRASP-mediated noncanonical integrin secretion is required for Drosophila epithelial remodeling. Dev. Cell 2008, 14, 171–182. [Google Scholar]
- Rodrigues, M.L.; Nosanchuk, J.D.; Schrank, A.; Vainstein, M.H.; Casadevall, A.; Nimrichter, L. Vesicular transport systems in fungi. Future Microbiol 2011, 6, 1371–1381. [Google Scholar]
- Casadevall, A.; Rosas, A.L.; Nosanchuk, J.D. Melanin and virulence in Cryptococcus neoformans. Curr. Opin. Microbiol 2000, 3, 354–358. [Google Scholar]
- Rodrigues, M.L.; Alviano, C.S.; Travassos, L.R. Pathogenicity of Cryptococcus neoformans: Virulence factors and immunological mechanisms. Microbes Infect 1999, 1, 293–301. [Google Scholar]
- Levi, S.K.; Bhattacharyya, D.; Strack, R.L.; Austin, J.R., II; Glick, B.S. The yeast GRASP Grh1 colocalizes with COPII and is dispensable for organizing the secretory pathway. Traffic 2010, 11, 1168–1179. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Oliveira, D.L.; Rizzo, J.; Joffe, L.S.; Godinho, R.M.C.; Rodrigues, M.L. Where Do They Come from and Where Do They Go: Candidates for Regulating Extracellular Vesicle Formation in Fungi. Int. J. Mol. Sci. 2013, 14, 9581-9603. https://doi.org/10.3390/ijms14059581
Oliveira DL, Rizzo J, Joffe LS, Godinho RMC, Rodrigues ML. Where Do They Come from and Where Do They Go: Candidates for Regulating Extracellular Vesicle Formation in Fungi. International Journal of Molecular Sciences. 2013; 14(5):9581-9603. https://doi.org/10.3390/ijms14059581
Chicago/Turabian StyleOliveira, Débora L., Juliana Rizzo, Luna S. Joffe, Rodrigo M. C. Godinho, and Marcio L. Rodrigues. 2013. "Where Do They Come from and Where Do They Go: Candidates for Regulating Extracellular Vesicle Formation in Fungi" International Journal of Molecular Sciences 14, no. 5: 9581-9603. https://doi.org/10.3390/ijms14059581
APA StyleOliveira, D. L., Rizzo, J., Joffe, L. S., Godinho, R. M. C., & Rodrigues, M. L. (2013). Where Do They Come from and Where Do They Go: Candidates for Regulating Extracellular Vesicle Formation in Fungi. International Journal of Molecular Sciences, 14(5), 9581-9603. https://doi.org/10.3390/ijms14059581




