2.1. Raman Spectra
Marker labeling of Raman peak is shown in
Table 1. Raman spectrum in
Figure 1 and
Figure 2 were corrected for baseline and normalized which allows for an effective comparison across a heterogeneous set of samples. Eventually, the baseline correction utilized the multiple point level method (Savitzgy-Golay) in which the baseline is leveled at a value that is the average of the baseline points. Normalization of Raman spectral was performed based on the common normalization method referring to min/max technique. The min/max (normalization) method is expressed by:
where
I is the intensity after baseline correction was performed,
Imin is the minimum intensity and
Imax is the maximum intensity on single spectral measured. Raman spectrum in
Figure 1 shows significant peak at <100 cm
−1 which indicate the strong presence of Pb and Te in the chemical bonding through vibrational mode due to addition of PbO and glass network. PbO stands out as unique because of its dual role [
8], one as modifier, if Pb–O bond is ionic and the other as glass former with PbO
4 structural units, if Pb–O bond is covalent. Occasionally, PbO concentration deteriorates glass forming ability [
9] of (TeO
2)
y[(PbO)
x(B
2O
3)
1−x]
1−y system. The addition of heavy metal oxide modifiers to pure TeO
2 leads to the progressive formation of distorted TeO
3+1 polyhedron followed by the creation of regular trigonal TeO
3 pyramids that contain non-bridging oxygen. In all compositions, the appearance of the low-frequency Boson peak (<200 cm
−1) affirms the presence of the glass structure. The increase broad shoulders at 410 cm
−1 indicate that new features to vibrations of one of the partially crystalline phase of Pb
3TeO
6. The existence of Pb
3TeO
6 is confirmed by X-ray analysis. Clearly, the shoulders at 410 cm
−1 were getting broader as the content of PbO increased, possibly due to the PbO unique ability. At low portions of PbO (up to 0.2% mol), it enters the glass network by breaking up the Te–O–Te and B–O–B bonds and introduces coordinate defects known as dangling bonds along with non-bridging oxygen ions (Te–O
−…Pb
2+…
−O–Te) which in turn neutralizes the negative charge of non-bridging oxygens (NBO) by forming TeO
3 and BO
4 units. Normally, the oxygen of PbO breaks the local symmetry while Pb
2+ ions occupy interstitial positions. As PbO increases (from 0.3% to 0.5% mol), a considerable portion may be acts as a double bridges between adjacent TeO
4 such as =Te–O–Pb–O–Te= which can formed besides the formation of PbO
4 and TeO
3 units. Therefore, for PbO ≥ 0.3% mol, Pb
2+ acts as glass forming agent and is incorporated in the glass network in the form of PbO
4 units. Decreasing on (ss) Te–O–Te and B–O–B bending shoulder at approximately 490 cm
−1 and 450 cm
−1 ascribe that the splitting of Te–O–Te and B–O–B bonds and hence, the bridging oxygen’s (BOs) are converted into NBOs. The pure B
2O
3 was known to consist of the boroxol rings by linking among trigonal-plane BO
3 units, but the network structure was altered through the addition of PbO. Some parts of the boroxol ring of BO
3 units were changed into BO
4 tetrahedral units [
10].
TeO
4 trigonal bipyramids is known to be the main structural unit of the network of all tellurite glasses [
11], as well as of the lattices of crystalline TeO
2 polymorphs. All stable tellurite glasses are multi-component and, what is important, cations on non-tellurite components have a coordination number other than four. The tellurite structural units of two types are always present in multi-component tellurite glass network, namely, fourfold coordinated Te atoms (TeO
4 trigonal bipyramids, where all O atoms from bridging bonds with the environment) and threefold coordinated Te atoms (O=TeO
2 trigonal pyramids, where one of O atoms is non-bridging, one forms O=Te double bond and one O atoms form bridging bonds with the environment). The spectral features from 710 cm
−1 to 730 cm
−1 and 790 cm
−1 in
Figure 1 correspond to the TeO
4 bi-pyramidal arrangement and the TeO
3+1 (or distorted TeO
4) and TeO
3 trigonal pyramids structures respectively. It can be clearly observed that the evolution of TeO
4 to TeO
3+1 and TeO
3 units which one of the Te sp3 hybrid orbital is occupied by a lone pair of electron. This transformation causes increases in the number of non-bridging oxygen (NBO) atoms. Legitimately, the increasing peak on the TeO
3 trigonal pyramids shows that modification of lattice and interstitial occurs in the system due to addition of PbO and B
2O
3.
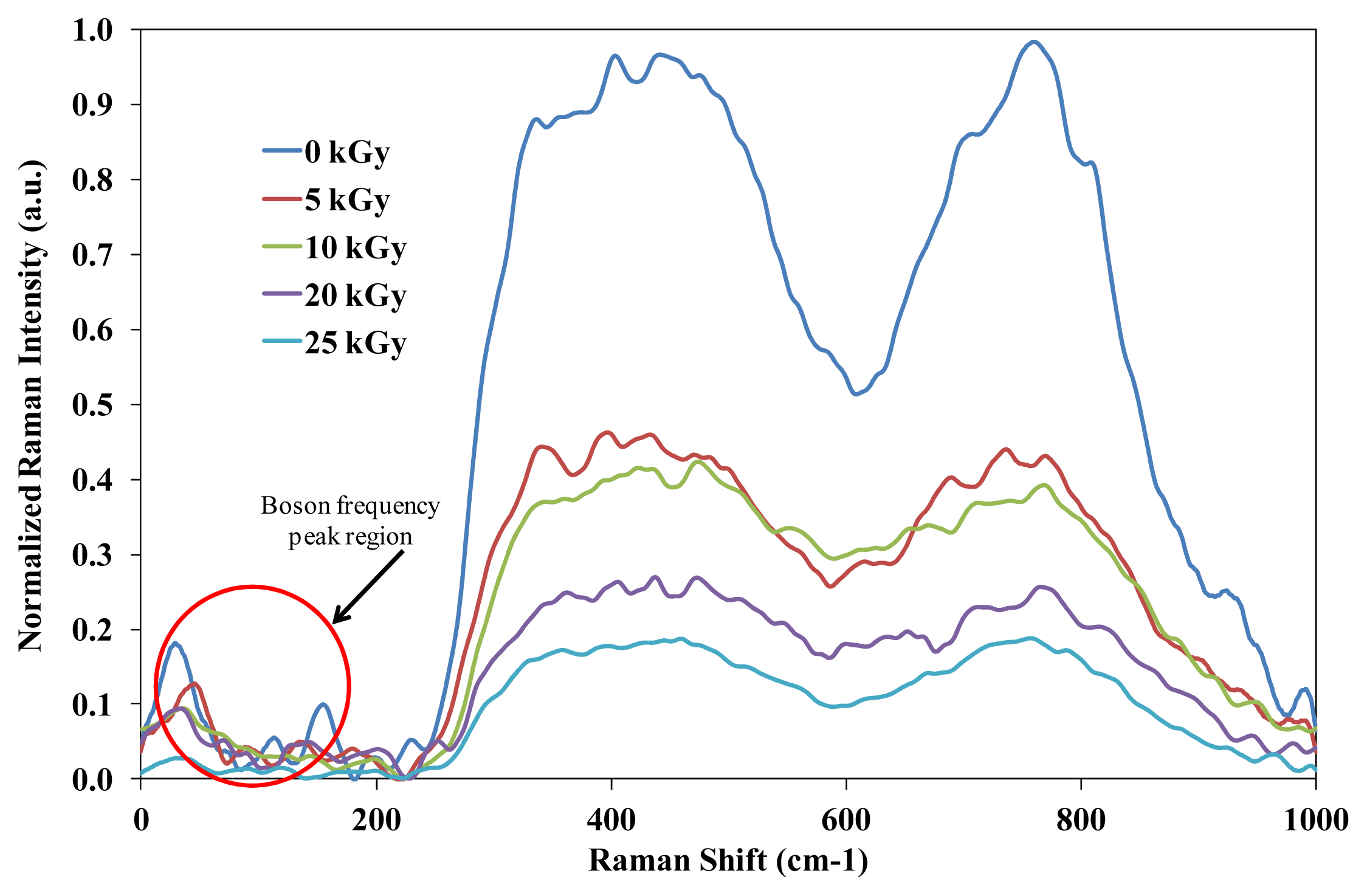
The presence peaks at <100 cm
−1 in
Figure 2 do not indicate any strong changes of intensity due to variation of gamma irradiation exposure. Eventually, the decrease in broad shoulders at 410 cm
−1 also indicates the existence of new features in the vibrations of the partially crystalline phase of Pb
3TeO
6. The existence of Pb
3TeO
6 is confirmed by X-ray analysis. Clearly, the shoulders at 410 cm
−1 were getting lower as the irradiation dose increased, possibly due to the network compaction. The spectral features from 710 cm
−1 to 730 cm
−1 and 790 cm
−1 correspond to the TeO
4 bi-pyramidal arrangement and the TeO
3+1 (or distorted TeO
4) and TeO
3 trigonal pyramids structures respectively. It can be clearly observed that the evolution of TeO
3+1 and TeO
3 units occurs as the TeO
2 concentration decreases. Legitimately, the decreasing peak on the TeO
4 bi-pyramidal arrangement and TeO
3+1 (or distorted TeO
4) is due to augmentation of irradiation dose which is attributed to an increase in degree of disorder of the amorphous phase. According to El-Alaily and Mohamed [
3], irradiation with gamma rays are assumed to create displacements, electronic defects and/or breaks in the network bonds, which allow the structure to relax and fill the relatively large interstices that exist in the interconnected network of boron and oxygen atoms causing expansion followed by compaction of the volume. Shelby [
13] also suggested that the boron-oxygen bond is more likely to be affected by irradiation.
2.3. Optical Absorption Spectra
The optical absorption spectra were taken in the ranges of 340 to 550 nm. The optical absorption is one of the most productive tools to understand the band gap of optical materials. The optical properties of a solid are governed by the interaction between the solid and the electric field of the electromagnetic wave. The optical absorption measurements coefficient α(ω) near the fundamental is calculated from absorbance
A, using the following Equation [
14]:
where
d is the thickness of the samples. The rapid change in α(ω) against ω is called “the fundamental absorption edge” and the corresponding energy is defined as “the optical energy gap (
Eopt). In the compound, a typical absorption edge can be broadly ascribed to any of the three processes: (i) residual below-gap absorption; (ii) Urbach tails; and (iii) interband absorption. In the second process, the absorption edge depends exponentially on the photon energy according to the Urbach relation. In crystalline materials the fundamental edge is directly related to the conduction band and valence band,
i.e., direct and indirect band gaps, while in the case of amorphous materials a different type of optical absorption edge is observed.
Figure 4 illustrates the variation of absorption coefficient, α with incident photon energy at different doses.
Urbach edge analysis is a useful way to parametrically characterize glass optical absorption edge and potentially distinguished intrinsic contributions to absorbance. Urbach’s absorption edge is formed in the region of photon energies below the forbidden gap. The interaction between lattice vibrations and localized states in tail of band gap from the glass samples has a significant effect on the optical properties. The plot of ln (α) against photon energy, ħω is linear for the absorption region near the fundamental absorption edge. Thus, it is evaluated that the absorption coefficient near the fundamental absorption edge is exponentially dependent on the photon energy and obeys the Ubach’s rule.
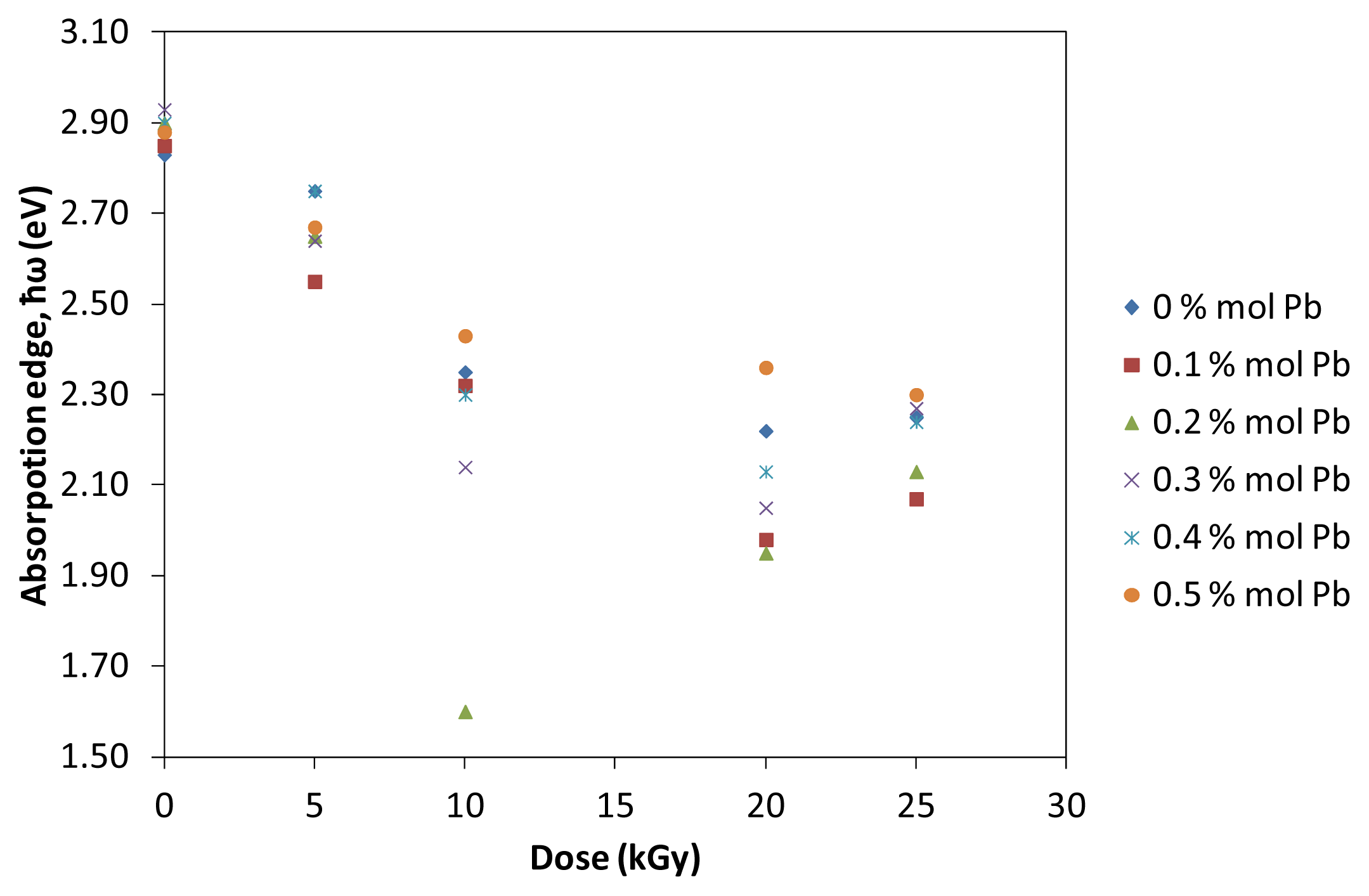
Figure 5 illustrates the dependence of Urbach’s absorption edge with different irradiation of prepared glasses. The absorption edge decreases with the increase of dose. Significantly, the decreasing values of absorption edge indicate that after irradiation processes, there is red shift effect on the (TeO
2)
y[(PbO)
x(B
2O
3)
1−x]
1−y glasses. The red shift effect is a process when the absorbance band shifts to longer wavelength and widens due to irradiation. A shift in the absorption edge can be attributed to an increase of the conjugation length. The number of Te atoms and Pb atoms per conjugation length is found to increase with increasing dose which create structure defect within the prepared (TeO
2)
y[(PbO)
x(B
2O
3)
1−x]
1−y glass.
The optical band gap energy is determined by using the following Equation [
15]
where α is the absorption coefficient, ħω is the incident photon energy,
A is a constant and
Eopt is the optical band gap. Values of
n are 2 and 1/2 for direct and indirect transitions, respectively.
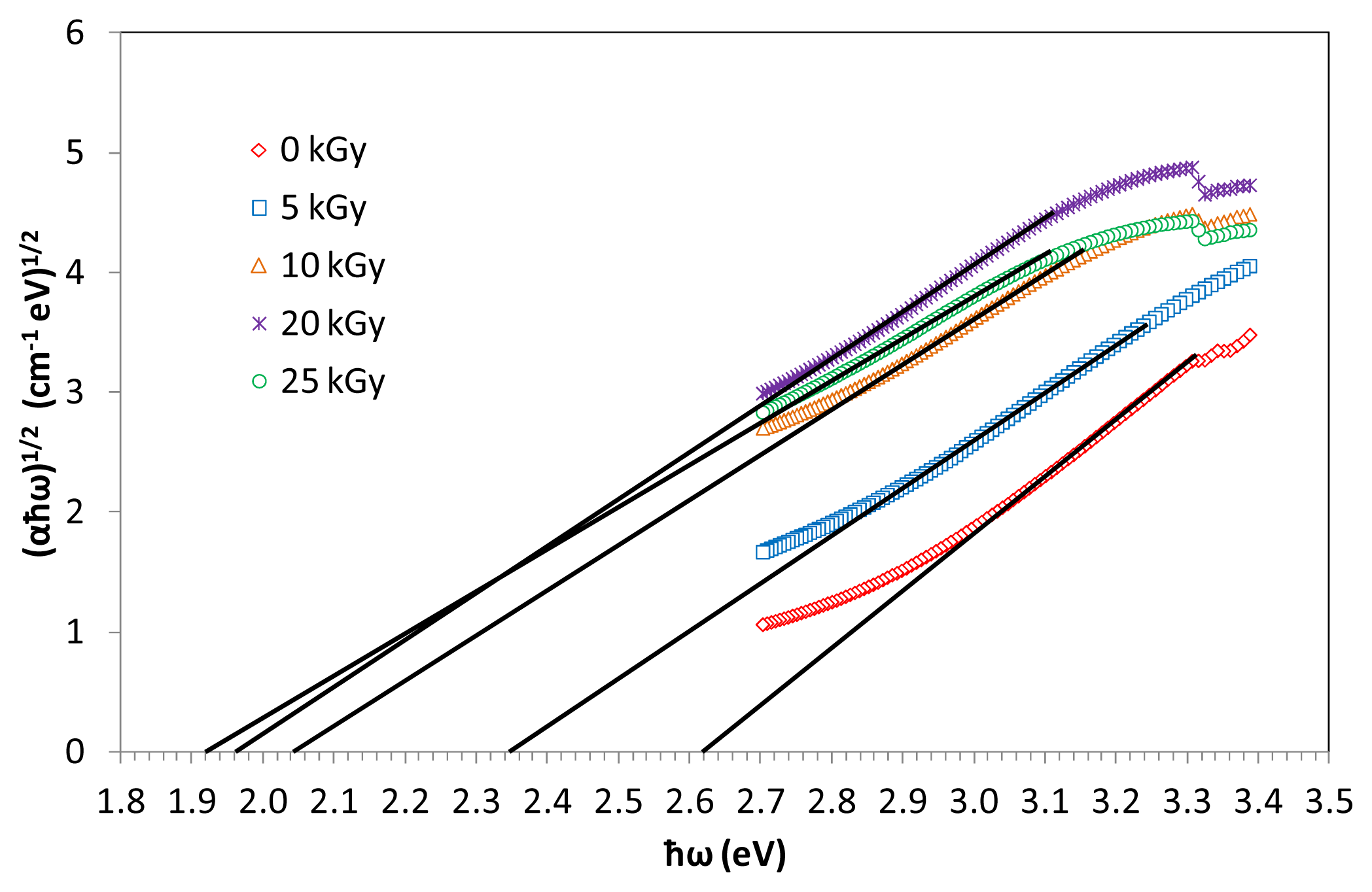
Figure 6 shows the information of indirect band gap (αħω)
1/2 against photon energy ħω of (TeO
2)
y[(PbO)
x(B
2O
3)
1−x]
1−y glasses with
x = 0.5 mol %;
y = 0.7 mol % at various irradiation exposure, plotted in the absorption region. Indirect energy gap is determined from the linear regions of the plots as shown in the figures and corresponding values presented in
Table 2. The
Eopt has been calculated approximately from the linear region of the arc extrapolating to meet the ħω axis at (αħω)
1/2=0.
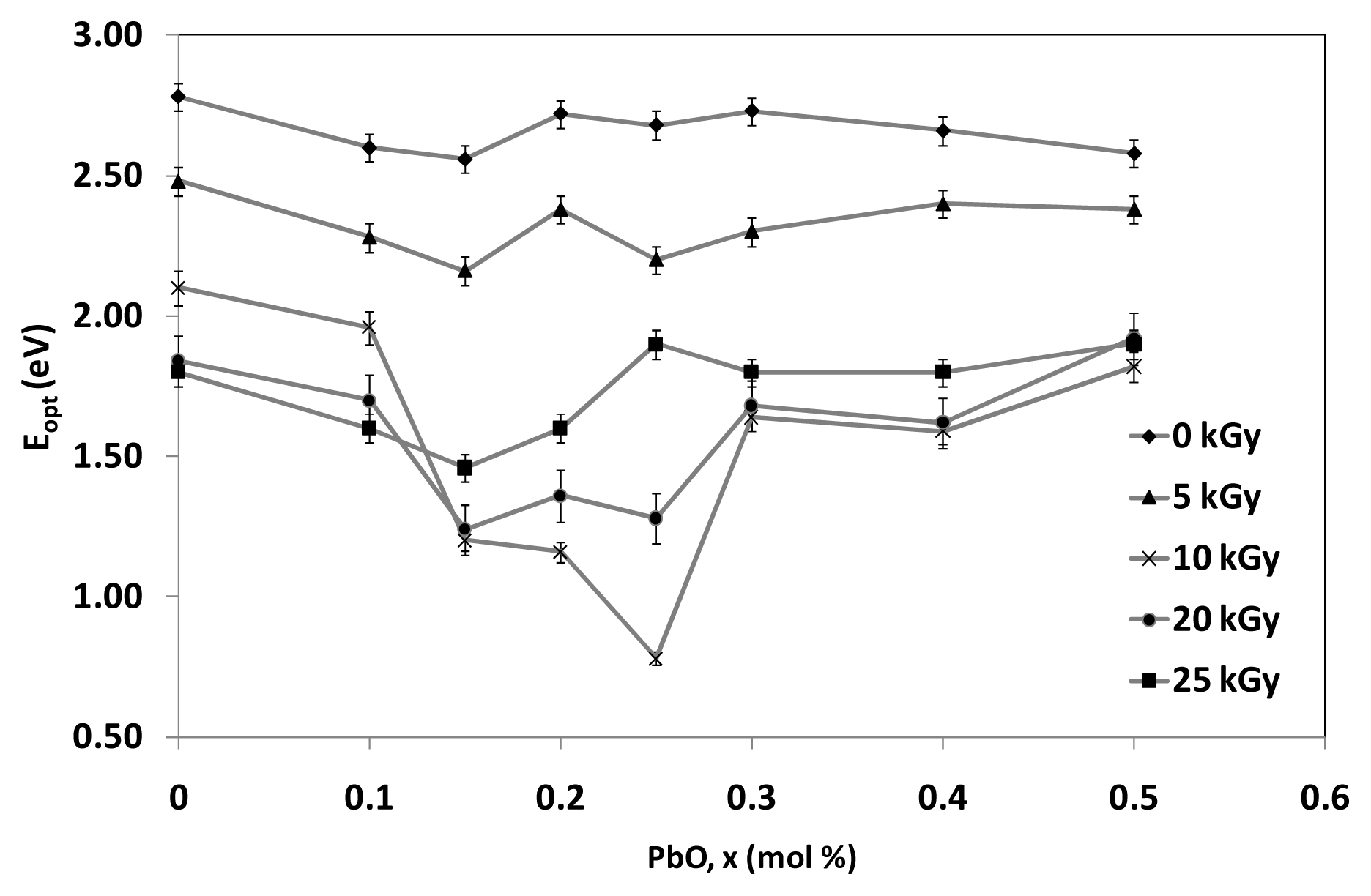
The variation of indirect optical band gap with mole fraction of PbO content before and after irradiation is shown in
Figure 7. The connected lines do not resemble any significant explanations rather than to show the decreasing and increasing pattern of the graph. The optical band gap values of the indirect process before irradiation decreases through the augment of PbO from 0% mol to 0.15% mol. This is due to the increase of the network disorder and consequently the extension of the localized states within the gap. More likely, PbO enters the glass network by breaking up the Te–O–Te and B–O–B bonds and introduces coordinate defects known as dangling bonds along with non-bridging oxygen. Normally, the oxygen of PbO breaks the local symmetry while Pb
2+ ions occupy interstitial positions. PbO content >0.2% mol shows a slight increase before tends to decline slowly for 0 kGy and increase for irradiated samples with 5 kGy up to 25 kGy (towards 0.5% mol). Consequently, as PbO increases (from 0.2% to 0.5% mol), modification of lattice and interstitial occurs in the system with the nearest neighboring atoms and arrangements, such as PbO
4 and/or BO
4. A considerable portion may act as double bridges between adjacent TeO
4 which can form in addition to the formation of PbO
4 and TeO
3 units. Therefore, for PbO ≥ 0.2% mol, Pb
2+ acts as glass forming agent and is incorporated in the glass network in the form of PbO
4 units. The optical band gap,
Eopt values for indirect transition decrease with increasing of irradiation dosage as the content of PbO ≤ 0.2% mol due to increase in degree of disorder of the amorphous phase. Obviously, the
Eopt values for indirect transition increase with increasing of irradiation dosage as the content of PbO > 0.2% mol. It is believed that the increasing of irradiation create displacements, electronic defects and/or breaks in the network bonds, which allow the structure to relax and fill the relative large interstices that exist in the interconnected network of boron and oxygen atoms causing expansion followed by compaction of the volume.
In many crystalline and non-crystalline semiconductors, the α(ω) depends exponentially on the ħω. This exponential dependence, known as the Urbach rule, can be written in the form [
16]:
where B is a constant and Δ
E is the width of the band tails of the localized states which also known as Urbach energy. The value of Urbach energy (Δ
E) is calculated by taking the reciprocals of the slopes of the linear portion of the ln α(ω) against ħω curves in the lower photon energy regions.
Figure 8 shows the reciprocal of the slopes of the linear portion from the ln α(ω) against ħω curves in the lower photon energy regions for (TeO
2)
y[(PbO)
x(B
2O
3)
1−x]
1−y glasses with
x = 0.5% mol;
y = 0.7% mol at various irradiation exposure. The reciprocal values will be used to calculate the value of Urbach energy (Δ
E) using the
Equation 3. The values of (Δ
E) of (TeO
2)
y[(PbO)
x(B
2O
3)
1−x]
1−y glasses’ various irradiation exposure with
x = 0.0%–0.5% mol;
y = 0.7% mol are visualized in
Figure 9 and tabulated in
Table 2. Generally, an increase and decrease in Urbach energy can be considered as being due to defects within the glass network.
Urbach’s energy is a characteristic energy which determines how rapidly the absorption coefficient decreases for below band gap energy. Urbach measured the absorption tail for different temperatures and showed that the Urbach’s energy is approximately
kT, the thermal energy. The temperature dependence of the urbach tail led to the conclusion that the below-bandgap transitions are assisted transition. The Urbach tail can be caused by mechanisms other than phonon-assisted absorption. Considering
Figure 9, before irradiation took place, the Ubach energy having a non-prominent increase pattern when the content PbO in the network increase which illustrating the saturation of defects at high content of PbO. Other than that, it is more likely due to the increase of the disorder and consequently the more extension of the localized states. For every irradiation dose of between 5 kGy and 25 kGy, Ubach energy increases as the content of PbO ≤ 0.2% mol suggesting the increasing of disorder and consequently the further extension of the localized states. Nevertheless, Ubach energy for every irradiation dose from 5 kGy up to 25 kGy decreases as the content of PbO > 0.2% mol advocating the possibility of long range order locally arising from the minimum in the number of defects.
With the increasing of irradiation dosage, the content of PbO ≤ 0.2% mol, the Ubach energy increases significantly, this suggests the increase in degree of disorder of the amorphous phase. As the content of PbO increases to be greater than 0.2% mol, the Ubach energy increases with 5 kGy and 10 kGy of irradiation dose and begins to decrease with irradiation dosage of 20 kGy and 25 kGy. It is believed that the increasing of irradiation creates displacements, electronic defects and/or breaks in the network bonds, which allow the structure to relax and fill the relative large interstices that exist in the interconnected network, causing expansion followed by compaction of the volume.