Antioxidant, Analgesic, Anti-Inflammatory, and Hepatoprotective Effects of the Ethanol Extract of Mahonia oiwakensis Stem
Abstract
:1. Introduction
2. Results and Discussion
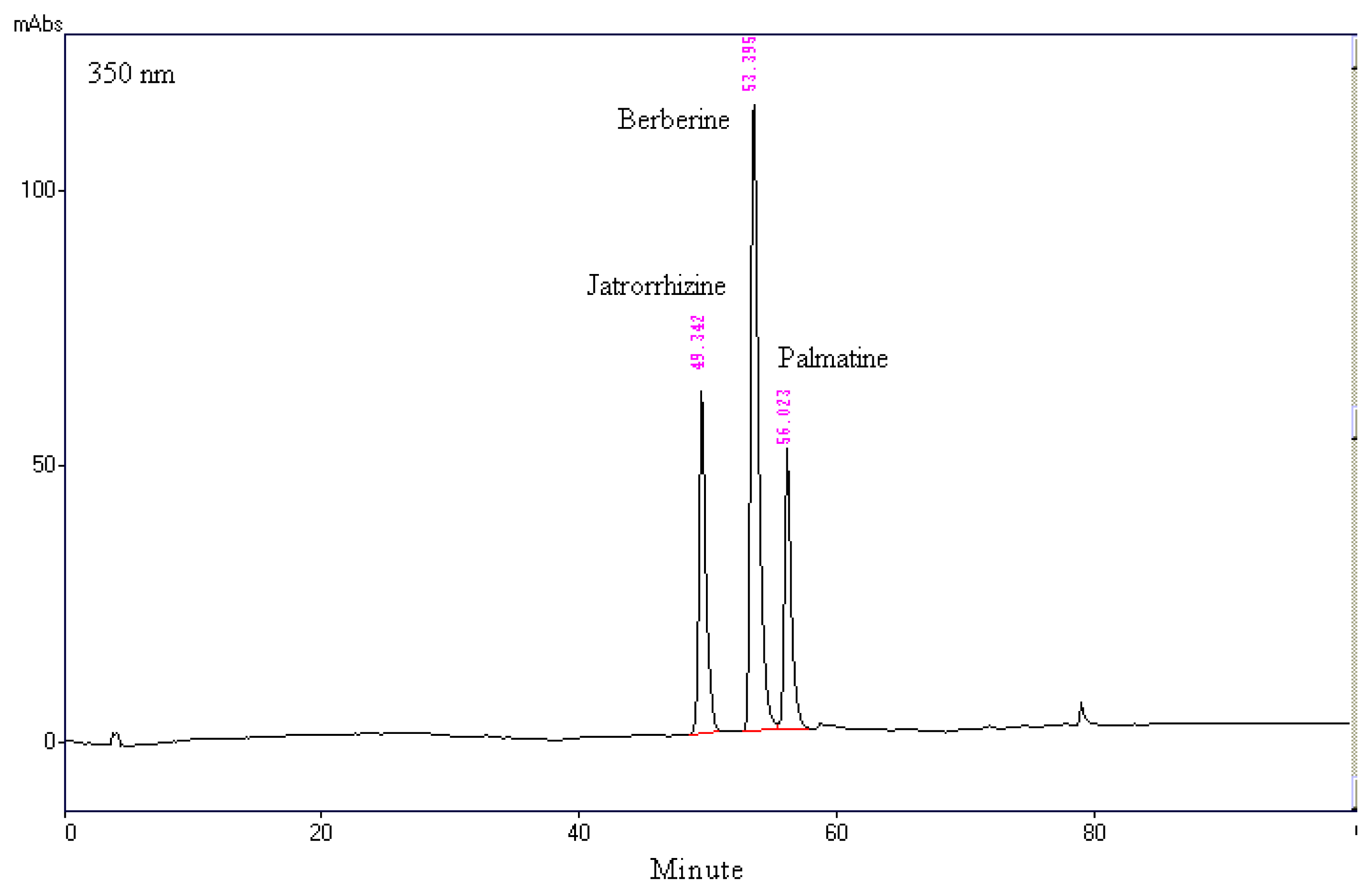
2.1. Chromatographic Analysis of MOSEtOH
2.2. The DPPH Radical Scavenging Activity of MOSEtOH
2.3. Toxicity Study
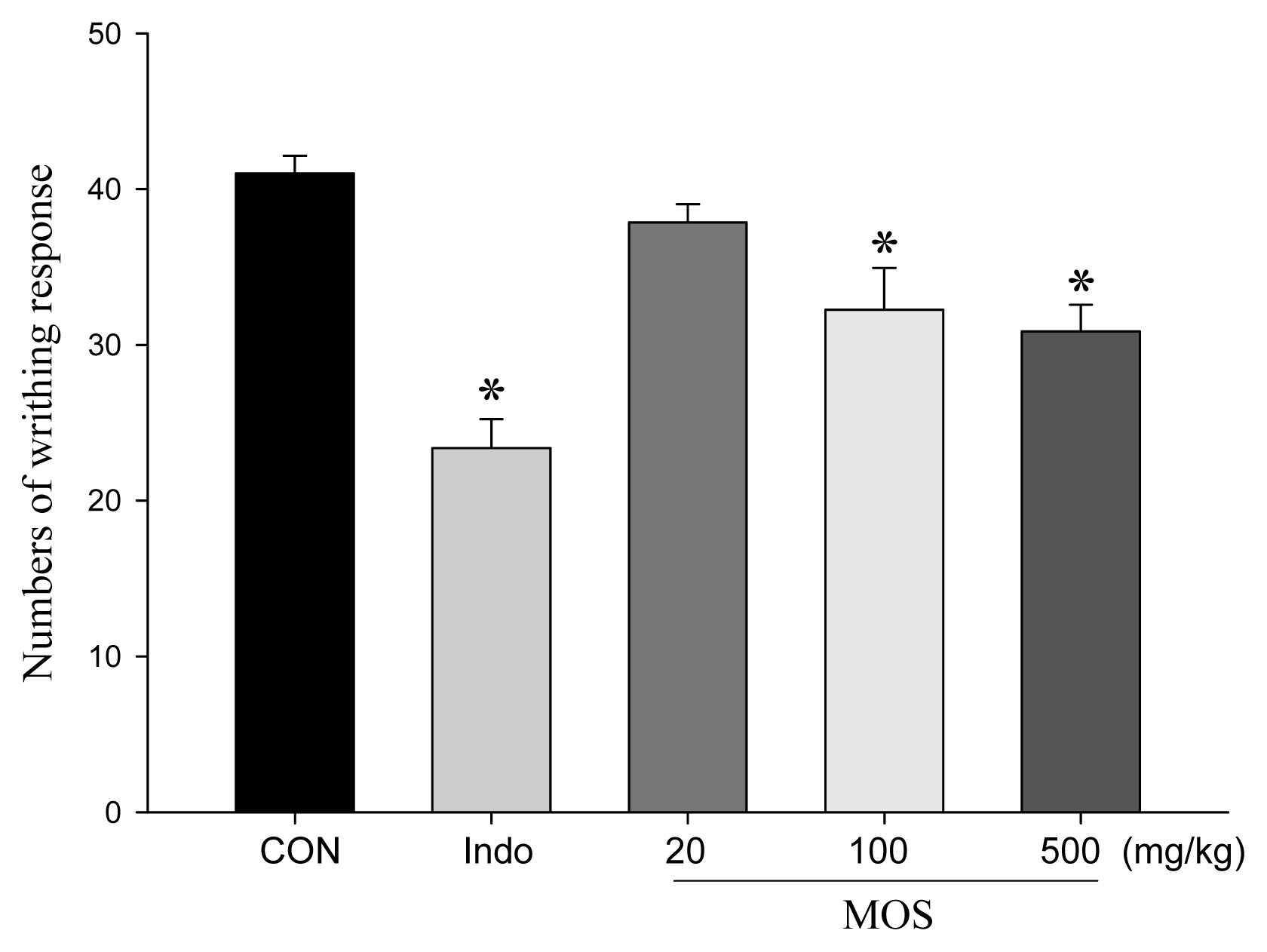
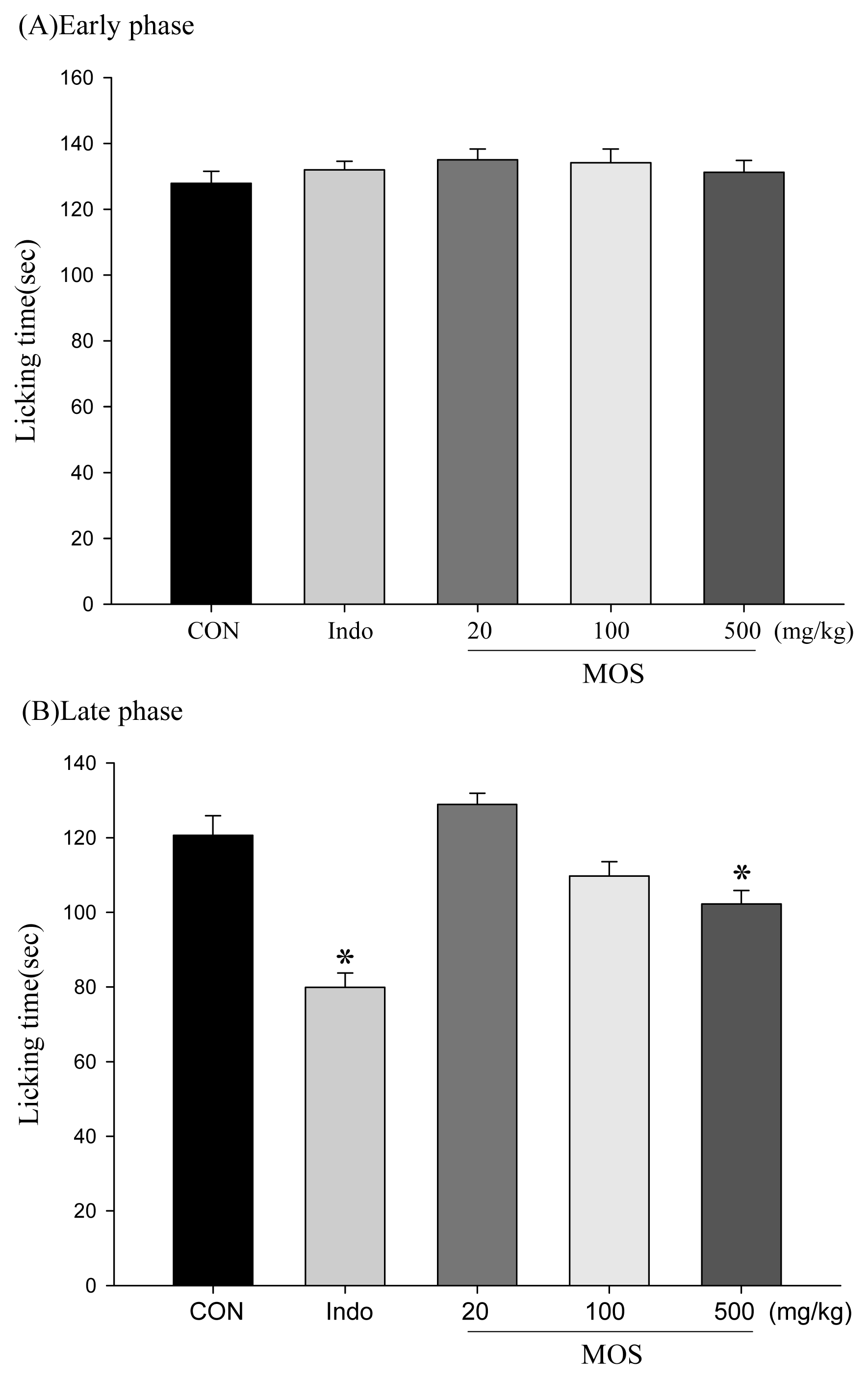
2.4. Analgesic and Anti-Inflammatory Activity of MOSEtOH
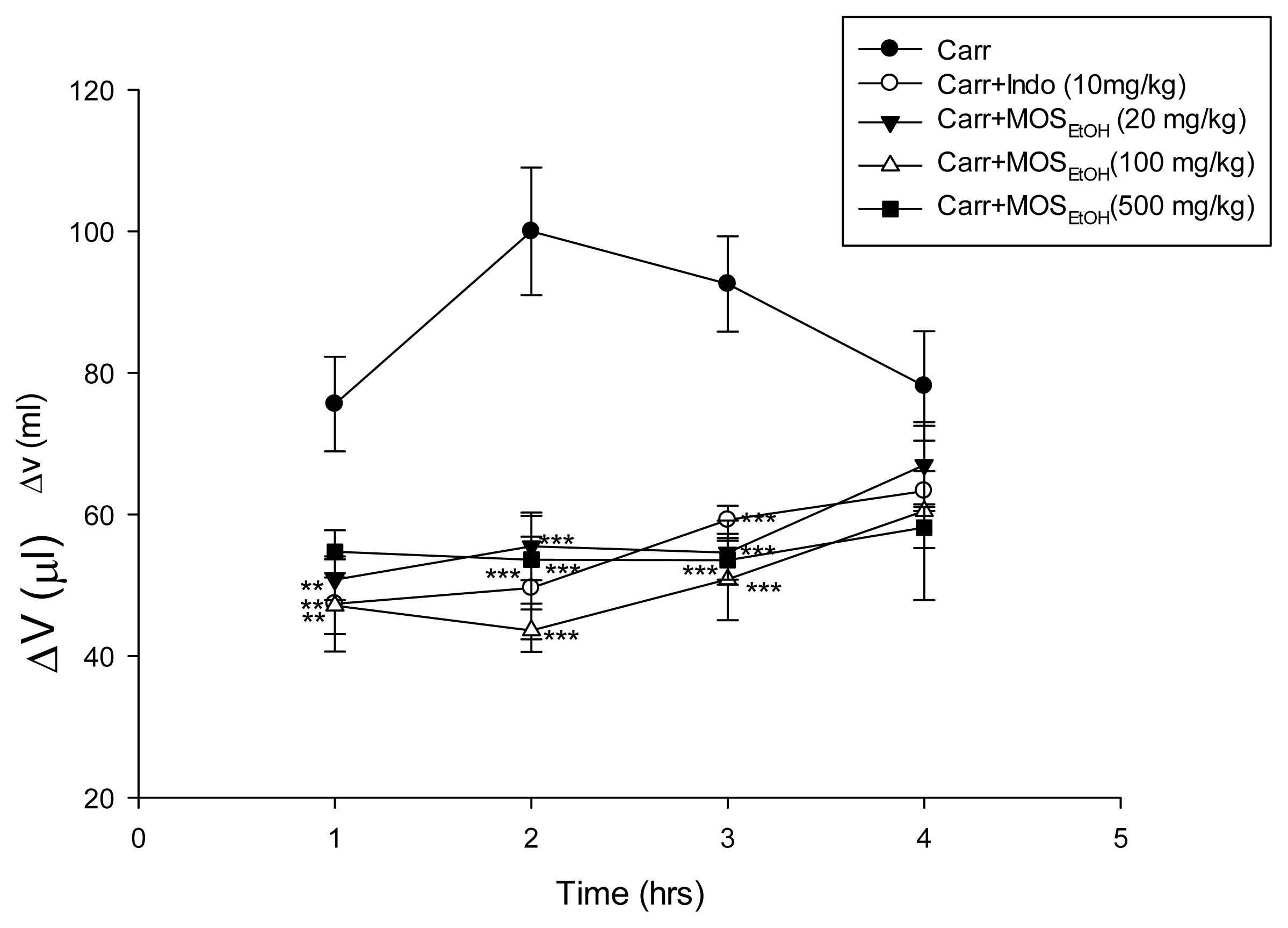
2.5. MOSEtOH-Inhibited Carrageenan-Induced Edema and Inflammation in Mice Paw Tissue
2.6. Hepatoprotective Effect of MOSEtOH
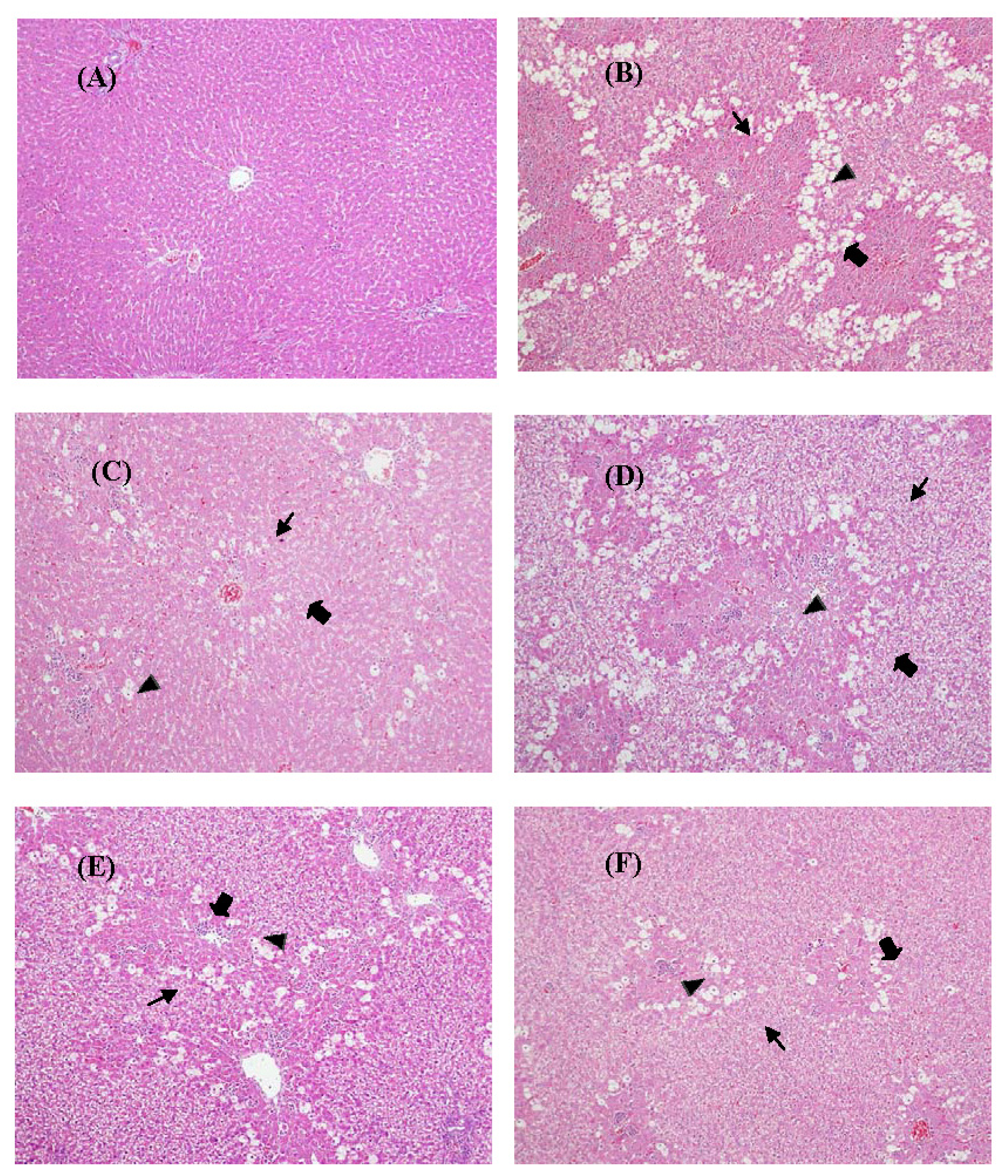
2.7. Histological Analysis
2.8. Hepatic Lipid Peroxidation
2.9. Antioxidative Enzyme Activity of Liver
2.10. Hepatic NO Level Changes
3. Discussion
4. Experimental Section
4.1. Chemicals
4.2. Plant Source and Preparation of Plant Extract
4.3. Chromatographic Identification of MOSEtOH
4.4. Determination of DPPH Radical Scavenging Ability
4.5. Animals
4.6. Acetic Acid-Induced Writhing Test
4.7. Formalin Test
4.8. Carrageenan-Induced Mice Paw Edema
4.9. Hepatoprotective Effect
4.10. Histopathological Evaluation
4.11. Antioxidative Enzyme Activity Measurements
4.12. Hepatic MDA and NO Assay
4.13. Statistical Analysis
5. Conclusions
Acknowledgements
References
- Lee, C.P.P.; Shih, H.; Hsu, C.L.; Yen, G.C. Hepatoprotection of tea seed oil (Camellia oleifera Abel.) against CCl4-induced oxidative damage in rats. Food Chem. Toxicol 2007, 6, 888–895. [Google Scholar]
- Recknagel, R.O.; Glende, E.A.; Dolak, J.A.; Waller, R.L. Mechanisms of carbon tetrachloride toxicity. Pharmacol. Ther 1989, 1, 139–154. [Google Scholar]
- Weber, L.W.; Boll, M.; Stampfl, A. Hepatotoxicity and mechanism of action of haloalkanes: Carbon tetrachloride as a toxicological model. Crit. Rev. Toxicol 2003, 2, 105–136. [Google Scholar]
- Forni, L.G.; Packer, J.E.; Slater, T.F.; Willson, R.L. Reaction of the trichloromethyl and halothane-derived peroxy radicals with unsaturated fatty acids: A pulse radiolysis study. Chem. Biol. Interact 1983, 2, 171–177. [Google Scholar]
- McCay, P.B.; Lai, E.K.; Poyer, J.L.; DuBose, C.M.; Janzen, E.G. Oxygen- and carbon-centered free radical formation during carbon tetrachloride metabolism. Observation of lipid radicals in vivo and in vitro. J. Biol. Chem 1984, 4, 2135–2143. [Google Scholar]
- Gancevici, G.G. Bioeffects of the plant Mahonia sempervirens. Arch. Roum. Pathol. Exp. Microbiol 1990, 2, 183–190. [Google Scholar]
- Rohrer, U.; Kunz, E.M.; Lenkeit, K.; Schaffner, W.; Meyer, J. Antimicrobial activity of Mahonia aquifolium and two of its alkaloids against oral bacteria. Schweiz. Monatsschr. Zahnmed 2007, 11, 1126–1131. [Google Scholar]
- Tseng, S.H.; Chien, T.Y.; Tzeng, C.F.; Lin, Y.H.; Wu, C.H.; Wang, C.C. Prevention of hepatic oxidative injury by Xiao-Chen-Chi-Tang in mice. J. Ethnopharmacol 2007, 2, 232–239. [Google Scholar]
- Kan, W.S. Pharmaceutical Botany; National Research Institute of Chinese Medicine: Taipei, Taiwan, 1993. [Google Scholar]
- Chao, J.; Lu, T.C.; Liao, J.W.; Huang, T.H.; Lee, M.S.; Cheng, H.Y.; Ho, L.K.; Kuo, C.L.; Peng, W.H. Analgesic and anti-inflammatory activities of ethanol root extract of Mahonia oiwakensis in mice. J. Ethnopharmacol 2009, 2, 297–303. [Google Scholar]
- Wong, B.S.; Hsiao, Y.C.; Lin, T.W.; Chen, K.S.; Chen, P.N.; Kuo, W.H.; Chu, S.C.; Hsieh, Y.S. The in vitro and in vivo apoptotic effects of Mahonia oiwakensis on human lung cancer cells. Chem. Biol. Interact 2009, 2, 165–174. [Google Scholar]
- Kan, W.S. Manual of Medicinal Plants in Taiwan (I); National Research Institute of Chinese Medicine: Taipei, Taiwan, 1980. [Google Scholar]
- Luo, Y.C.; Yeh, L.F.; Zhang, K.C.; Lu, F.Y. Ethnobotany in North Tsou tribe. Nat. Conserv. Q 2007, 58, 27–31. [Google Scholar]
- Rackova, L.; Majekova, M.; Kost’alova, D.; Stefek, M. Antiradical and antioxidant activities of alkaloids isolated from Mahonia aquifolium structural aspects. Bioorg. Med. Chem 2004, 12, 4709–4715. [Google Scholar]
- Moshage, H.; Kok, B.; Huizenga, J.R.; Jansen, P.L. Nitrite and nitrate determinations in plasma: A critical evaluation. Clin. Chem 1995, 41, 892–896. [Google Scholar]
- Knodell, R.G.; Ishak, K.G.; Black, W.C.; Chen, T.S.; Craig, R.; Kaplowitz, N.; Kiernan, T.W.; Wollman, J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1981, 1, 431–435. [Google Scholar]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem 1982, 126, 131–138. [Google Scholar]
- Basu, S. Carbon tetrachloride-induced lipid peroxidation: Eicosanoid formation and their regulation by antioxidant nutrients. Toxicology 2003, 189, 113–127. [Google Scholar]
- Seeff, L.B.; Lindsay, K.L.; Bacon, B.R.; Kresina, T.F.; Hoofnagle, J.H. Complementary and alternative medicine in chronic liver disease. Hepatology 2001, 34, 595–603. [Google Scholar]
- Strader, D.B.; Bacon, B.R.; Lindsay, K.L.; La Brecque, D.R.; Morgan, T.; Wright, E.C.; Allen, J.; Khokar, M.F.; Hoofnagle, J.H.; Seeff, L.B. Use of complementary and alternative medicine in patients with liver disease. Am. J. Gastroenterol 2002, 97, 2391–2397. [Google Scholar]
- Bissell, D.M.; Gores, G.J.; Laskina, D.L.; Hoofnagle, J.H. Drug-induced liver injury: Mechanisms and test systems. Hepatology 2001, 33, 1009–1013. [Google Scholar]
- Aeschke, H.; Gores, G.J.; Cederbaum, A.I.; Hinson, J.A.; Pessayre, D.; Lemasters, J.J. Mechanisms of hepatotoxicity. Toxicol. Sci 2002, 65, 166–176. [Google Scholar]
- Eller, D.A.; de Vera, M.E.; Russell, D.A.; Shapiro, R.A.; Nussler, A.K.; Simmons, R.L.; Billiar, T.R. A central role for IL-1β in the in vitro and in vivo regulation of hepatic inducible nitric oxide synthase. IL-1β induces hepatic nitric oxide synthesis. J. Immunol 1995, 155, 4890–4898. [Google Scholar]
- Rodenas, J.; Mitjavila, M.T.; Carbonell, T. Simultaneous generation of nitric oxide and superoxide by inflammatory cells in rats. Free Radic. Biol. Med 1995, 18, 869–875. [Google Scholar]
- Smith, J.A. Neutrophils, host defense, and inflammation: A double-edged sword. J. Leukoc. Biol 1994, 56, 672–686. [Google Scholar]
- Matsubara, N.; Fuchimoto, S.; Iwagaki, H.; Nonaka, Y.; Kimura, T.; Kashino, H.; Edamatsu, R.; Hiramatsu, M.; Orita, K. The possible involvement of free radical scavenging properties in the actions of cytokines. Res. Commun. Chem. Pathol. Pharmacol 1991, 71, 239–242. [Google Scholar]
- Ratty, A.K.; Sunamoto, J.; Das, N.P. Interaction of flavonoids with 1,1-diphenyl-2-picrylhydrazyl free radical, liposomal membranes and soybean lipoxygenase-1. Biochem. Pharmacol 1988, 37, 989–995. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem 1979, 95, 351–358. [Google Scholar]
- Rodrigues, A.S.; Perez-Gregorio, M.R.; Garcia-Falcon, M.S.; Simal-Gandara, J.; Almeida, D.P.F. Effect of meteorological conditions on antioxidant flavonoids in Portuguese cultivars of white and red onions. Food Chem 2011, 124, 303–308. [Google Scholar]
- Rodrigues, A.S.; Perez-Gregorio, M.R.; Garcia-Falcon, M.S.; Simal-Gandara, J. Effect of curing and cooking on flavonols and anthocyanins in traditional varieties of onion bulbs. Food Res. Int 2009, 42, 1331–1336. [Google Scholar]
- Perez-Gregorio, M.R.; Regueiro, J.; Gonzalez-Barreiro, C.; Rial-Otero, R.; Simal-Gandara, J. Changes in antioxidant flavonoids during freeze-drying of red onions and subsequent storage. Food Control 2011, 22, 1108–1113. [Google Scholar]
- Perez-Lammela, C.; Garcia-Falcon, M.S.; Simal-Gandara, J.; Orriols-Fernandez, I. Influence of grape variety, vine system and enological treatments on the colour stability of young red wines. Food Chem 2007, 101, 601–606. [Google Scholar]
- Alonso Garcia, A.; Cancho Grande, B.; Simal-Gandara, J. Development of a rapid method based on solid-phase extraction and liquid chromatography with ultraviolet detection for the determination of polyphenols in alcohol-free beers. J. Chromatogr. A 2004, 1054, 175–180. [Google Scholar]
- Yamaguchi, T.; Takamura, H.; Matoba, T.; Terao, J. HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1-diphenyl-2-picrylhydrazyl. Biosci. Biotechnol. Biochem 1998, 62, 1201–1204. [Google Scholar]
- Chiu, Y.J.; Huang, T.H.; Chiu, S.H.; Lu, T.C.; Chen, T.W.; Peng, W.H.; Chen, C.Y. Analgesic and anti-inflammatory activities of the extract from Plectranthus amboinicus (Lour.) spreng. both in vitro and in vivo animal models. Evid. Based Complement. Alternat. Med 2012, 2012, 1–11. [Google Scholar]
- Mitchell, J.R.; Jollow, D.J.; Potter, W.Z.; Gillette, J.R.; Brodie, B.B. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J. Pharmacol. Exp. Ther 1973, 187, 211–217. [Google Scholar]
- Hunskaar, S.O.; Berge, G.; Hole, K. Antinociceptive effects of orphenadrine citrate in mice. J. Ethnopharmacol 1985, 111, 221–226. [Google Scholar]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar]
- Lu, T.C.; Ko, Y.Z.; Huang, H.W.; Huang, Y.C.; Lin, Y.C.; Peng, W.H. Analgesic and anti-inflammatory activities of aqueous extract from Glycine tomentella root in mice. J. Ethnopharmacol 2007, 113, 142–148. [Google Scholar]
- Flohe, L.; Gunzler, W.A. Assays of glutathione peroxidase. Methods Enzymol 1984, 105, 114–121. [Google Scholar]
- Carlberg, I.; Mannervik, B. Glutathione reductase. Methods Enzymol 1985, 113, 484–490. [Google Scholar]

| Substance | DPPH radical scavenging activity (IC50, mg/mL) |
|---|---|
| MOSEtOH | 0.743 ± 0.023 |
| Vit. C | 0.134 ± 0.015 |
| BHT | 0.085 ± 0.002 |
| Histo-grade | Group | |||||
|---|---|---|---|---|---|---|
| Control | CCl4 | Silymarin 200 mg/kg | MOSEtOH (mg/kg) | |||
| 20 | 100 | 500 | ||||
| Vacuolization | 0 | 3.2 ± 0.2 ## | 1.7 ± 0.2 ** | 2.8 ± 0.3 | 2.5 ± 0.2 | 2.2 ± 0.2 ** |
| Inflammation | 0 | 2.7 ± 0.2 ## | 1.7 ± 0.2 * | 2.3 ± 0.2 | 2.0 ± 0.3 | 1.5 ± 0.2 * |
| Cellular necrosis | 0 | 3.5 ± 0.2 ## | 1.5 ± 0.2 ** | 3.0 ± 0.3 | 2.3 ± 0.2 ** | 1.7 ± 0.2 ** |
| Groups | MDA (nmoL/mg protein) | Activity (U/mg protein) | NO (μM/mg protein) | ||
|---|---|---|---|---|---|
| SOD | GPx | GRd | |||
| Control | 1.30 ± 0.02 | 19.26 ± 0.61 | 9.05 ± 0.43 | 0.193 ± 0.006 | 3.12 ± 0.19 |
| CCl4 | 2.11 ± 0.04 ### | 14.19 ± 0.28 ### | 4.31 ± 0.50 ### | 0.145 ± 0.001 ### | 7.71 ± 0.44 ### |
| Silymarin 200 mg/kg + CCl4 | 1.70 ± 0.04 *** | 18.99 ± 0.43 *** | 8.98 ± 0.65 *** | 0.169 ± 0.002 *** | 4.66 ± 0.33 *** |
| MOSEtOH 20 mg/kg + CCl4 | 1.99 ± 0.06 | 15.03 ± 0.46 | 5.60 ± 0.51 | 0.158 ± 0.002 | 6.49 ± 0.33 |
| MOSEtOH 100 mg/kg + CCl4 | 1.88 ± 0.06 * | 16.78 ± 0.42 ** | 6.89 ± 0.16 ** | 0.164 ± 0.003 ** | 5.53 ± 0.40 ** |
| MOSEtOH 500 mg/kg + CCl4 | 1.81 ± 0.05 ** | 18.23 ± 0.43 *** | 7.55 ± 0.15 *** | 0.171 ± 0.003 *** | 4.79 ± 0.32 *** |
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chao, J.; Liao, J.-W.; Peng, W.-H.; Lee, M.-S.; Pao, L.-H.; Cheng, H.-Y. Antioxidant, Analgesic, Anti-Inflammatory, and Hepatoprotective Effects of the Ethanol Extract of Mahonia oiwakensis Stem. Int. J. Mol. Sci. 2013, 14, 2928-2945. https://doi.org/10.3390/ijms14022928
Chao J, Liao J-W, Peng W-H, Lee M-S, Pao L-H, Cheng H-Y. Antioxidant, Analgesic, Anti-Inflammatory, and Hepatoprotective Effects of the Ethanol Extract of Mahonia oiwakensis Stem. International Journal of Molecular Sciences. 2013; 14(2):2928-2945. https://doi.org/10.3390/ijms14022928
Chicago/Turabian StyleChao, Jung, Jiunn-Wang Liao, Wen-Huang Peng, Meng-Shiou Lee, Li-Heng Pao, and Hao-Yuan Cheng. 2013. "Antioxidant, Analgesic, Anti-Inflammatory, and Hepatoprotective Effects of the Ethanol Extract of Mahonia oiwakensis Stem" International Journal of Molecular Sciences 14, no. 2: 2928-2945. https://doi.org/10.3390/ijms14022928
APA StyleChao, J., Liao, J.-W., Peng, W.-H., Lee, M.-S., Pao, L.-H., & Cheng, H.-Y. (2013). Antioxidant, Analgesic, Anti-Inflammatory, and Hepatoprotective Effects of the Ethanol Extract of Mahonia oiwakensis Stem. International Journal of Molecular Sciences, 14(2), 2928-2945. https://doi.org/10.3390/ijms14022928





