Xylitol Affects the Intestinal Microbiota and Metabolism of Daidzein in Adult Male Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. General Observations
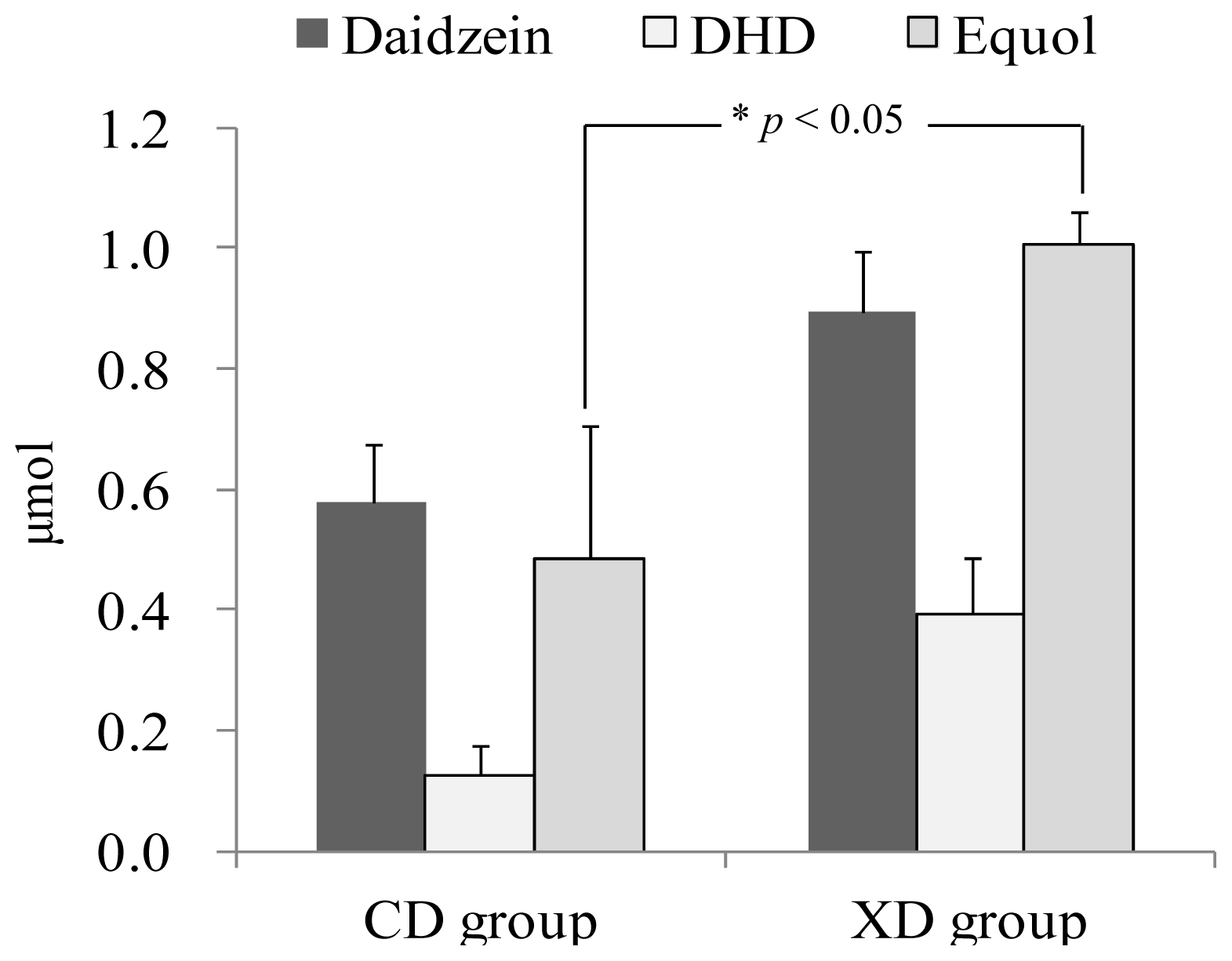
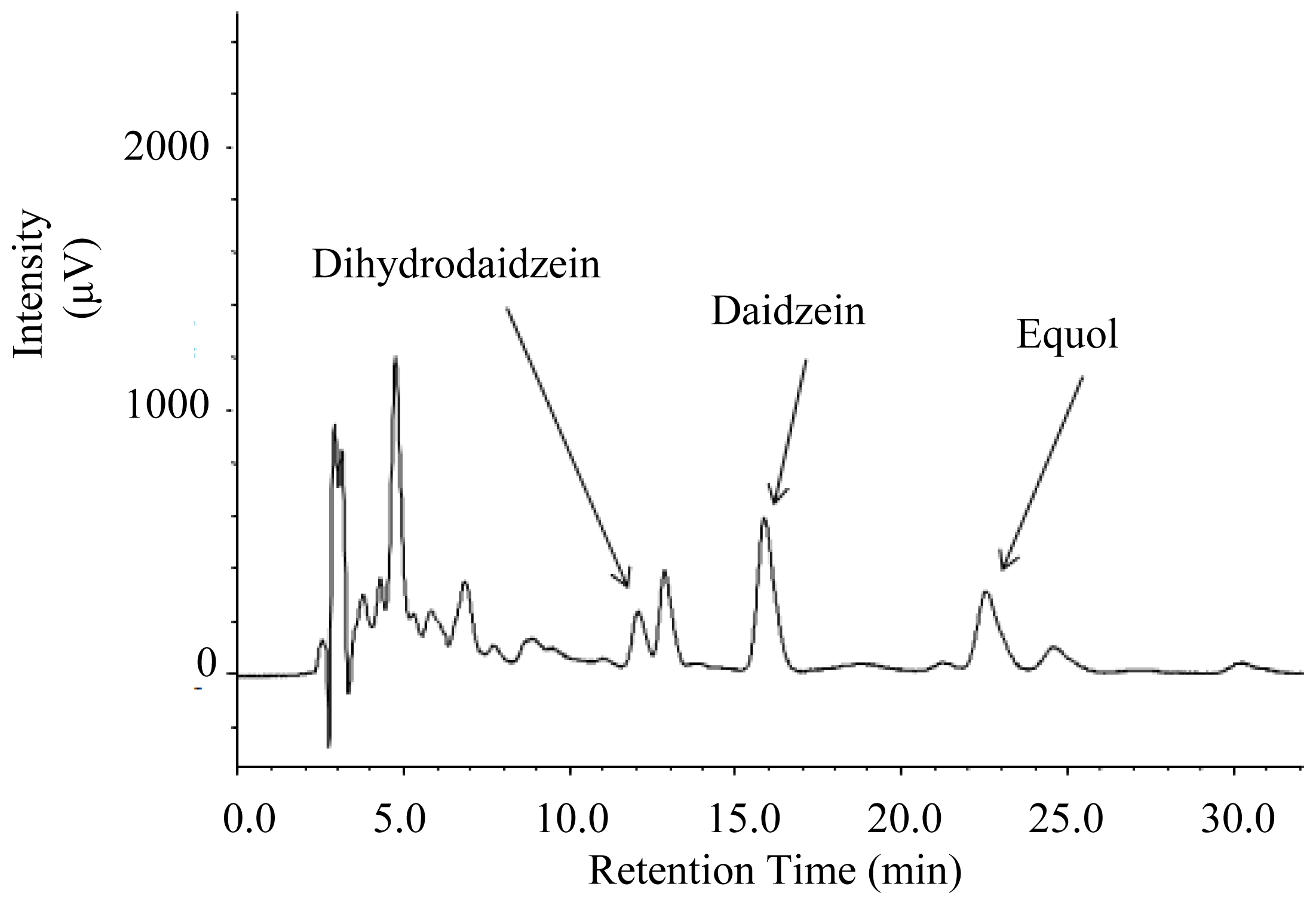
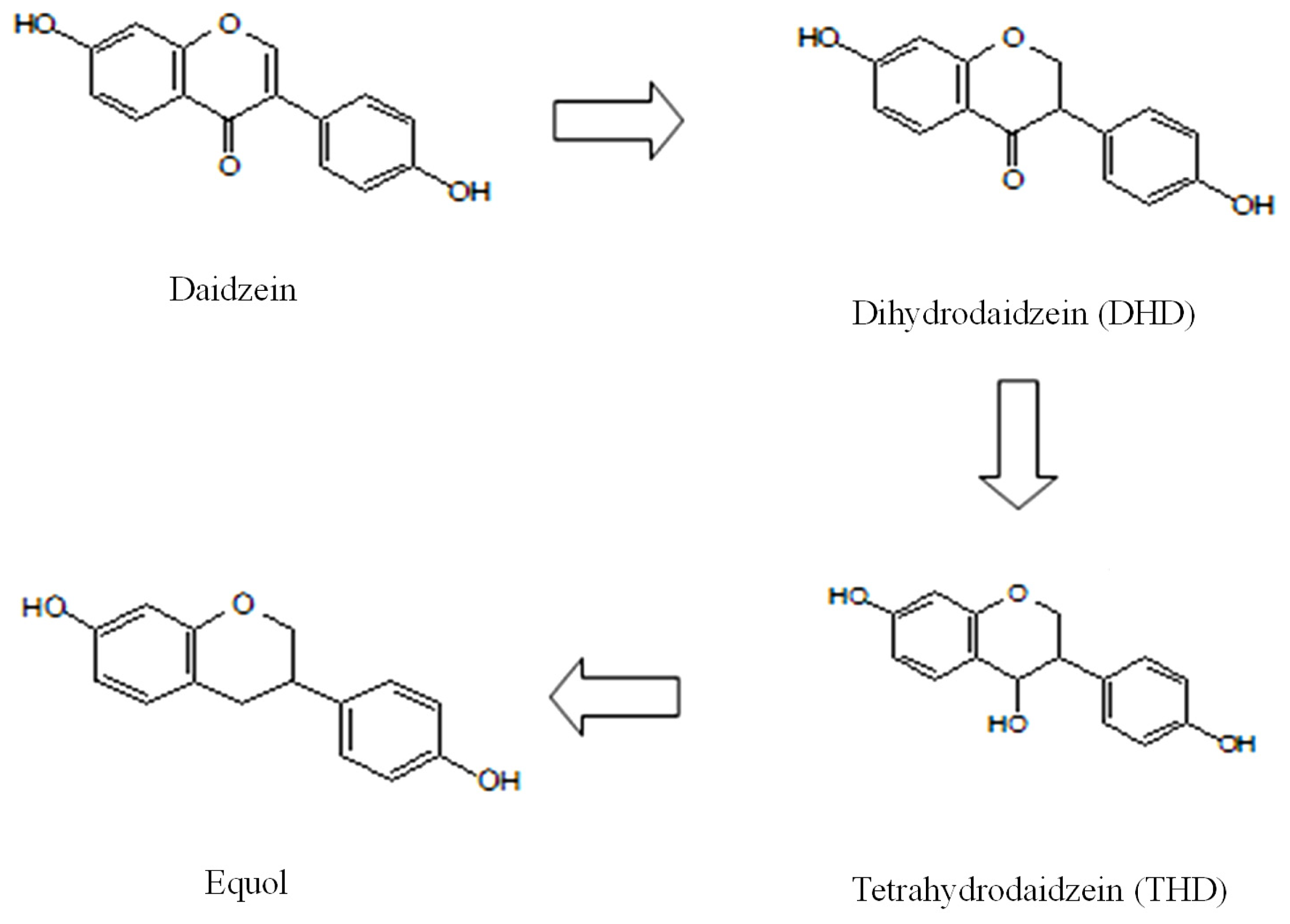
2.2. Urinary Isoflavonoids
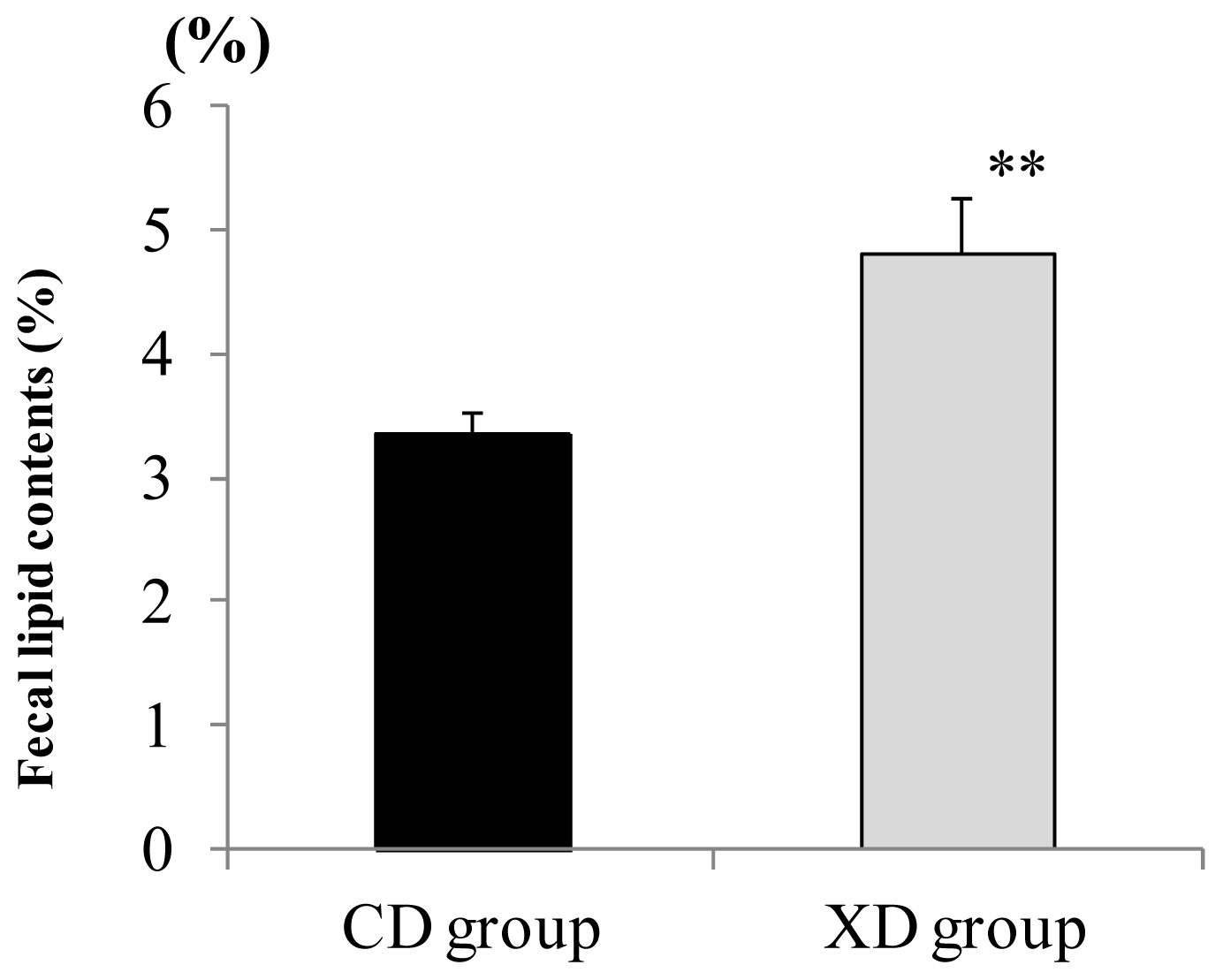
2.3. Amount of Fecal Lipid Contents
2.4. Plasma Total Cholesterol, Triglycerides, and Phospholipids
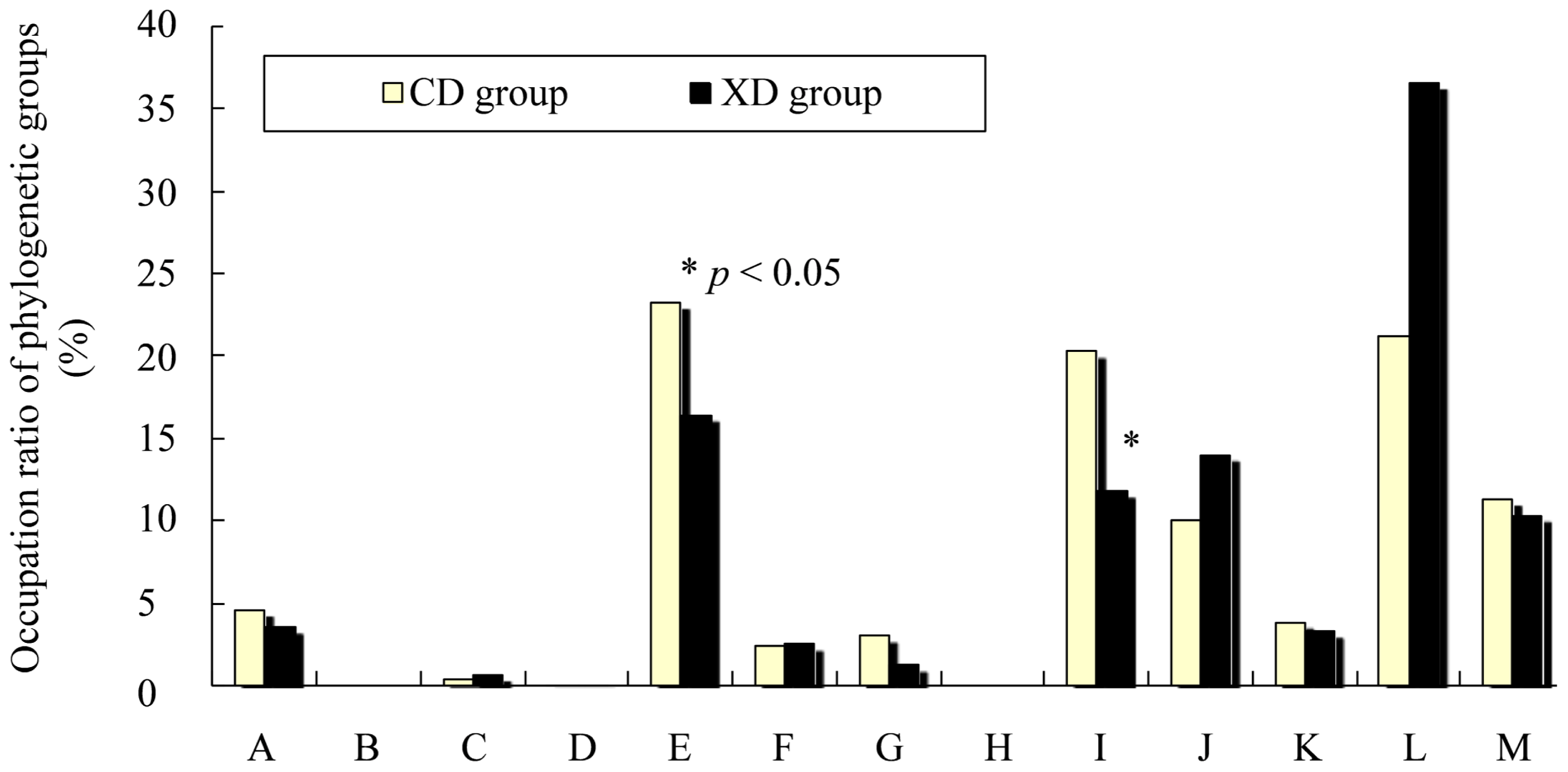
2.5. Effects of Diet on Cecal Microbiota of Mice
3. Experimental Section
3.1. Materials
3.2. Treatment of Animals
3.3. Measurement of Plasma Cholesterol, Triglycerides, and Phospholipids
3.4. Measurements of Fecal Weight and Fecal Lipid Extraction
3.5. Analysis of Urinary Isoflavonoids
3.7. PCR Conditions and Restriction Enzyme Digestion
3.8. T-RFLP (Terminal Restriction Fragment Length Polymorphism) Analysis
3.9. Statistics
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Goto, Y.; Anzai, M.; Chiba, M.; Ohneda, A.; Kawashima, S. Clinical effects of xylitol on carbohydrate and lipid metabolism in diabetes. Lancet 1965, 2, 918–921. [Google Scholar]
- Amo, K.; Arai, H.; Uebanso, T.; Fukaya, M.; Koganei, M.; Sasaki, H.; Yamamoto, H.; Taketani, Y.; Takeda, E. Effects of xylitol on metabolic parameters and visceral fat accumulation. J. Clin. Biochem. Nutr 2011, 49, 1–7. [Google Scholar]
- Ishii, H.; Takahashi, H.; Mamori, H.; Murai, S.; Kanno, T. Effects of xylitol on carbohydrate metabolism in rat liver treated with carbon tetrachloride or alloxan. Keio J. Med 1969, 18, 109–114. [Google Scholar]
- Mattila, P.T.; Svanberg, M.J.; Pökkä, P.; Knuuttila, M. Dietary xylitol protects against weakening of bone biomechanical properties in ovariectomized rats. J. Nutr 1998, 128, 1811–1814. [Google Scholar]
- Mattila, P.T.; Svanberg, M.J.; Knuuttila, M.L. Increased bone volume and bone mineral content in xylitol-fed aged rats. Gerontology 2001, 47, 300–305. [Google Scholar]
- Mattila, P.; Knuuttila, M.; Kovanen, V.; Svanberg, M. Improved bone biomechanical properties in rats after oral xylitol administration. Calcif. Tissue Int 1999, 64, 340–344. [Google Scholar]
- Svanberg, M.; Mattila, P.; Knuuttila, M. Dietary xylitol retards the ovariectomy-induced increase of bone turnover in rats. Calcif. Tissue Int 1997, 60, 462–466. [Google Scholar]
- Brommage, R.; Binacua, C.; Antille, S.; Carrie, A.L. Intestinal calcium absorption in rats is stimulated by dietary lactulose and other resistant sugars. J. Nutr 1993, 123, 2186–2194. [Google Scholar]
- Barnes, S. The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products. Lymphat. Res. Biol 2010, 8, 89–98. [Google Scholar]
- Uesugi, T.; Toda, T.; Tsuji, K.; Ishida, H. Comparative study on reduction of bone loss and lipid metabolism abnormality in ovariectomized rats by soy isoflavones, daidzin, genistin, and glycitin. Biol. Pharm. Bull 2001, 24, 368–372. [Google Scholar]
- Breinholt, V.; Larsen, J.C. Detection of weak estrogenic flavonoids using a recombinant yeast strain and a modified MCF7 cell proliferation assay. Chem. Res. Toxicol 1998, 11, 622–629. [Google Scholar]
- Chang, Y.C.; Nair, M.G. Metabolism of daidzein and genistein by intestinal bacteria. J. Nat. Prod 1995, 58, 1892–1896. [Google Scholar]
- Setchell, K.D.; Borriello, S.P.; Hulme, P.; Kirk, D.N.; Axelson, M. Nonsteroidal estrogens of dietary origin: Possible roles in hormone-dependent disease. Am. J. Clin. Nutr 1984, 40, 569–578. [Google Scholar]
- Bolca, S.; Possemiers, S.; Herregat, A.; Huybrechts, I.; Heyerick, A.; de Vriese, S.; Verbruggen, M.; Depypere, H.; de Keukeleire, D.; Bracke, M.; et al. Microbial and dietary factors are associated with the equol producer phenotype in healthy postmenopausal women. J. Nutr 2007, 137, 2242–2246. [Google Scholar]
- Setchell, K.D.; Clerici, C.; Lephart, E.D.; Cole, S.J.; Heenan, C.; Castellani, D.; Wolfe, B.E.; Nechemias-Zimmer, L.; Brown, N.M.; Lund, T.D.; et al. S-equol, a potent ligand for estrogen receptor β, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am. J. Clin. Nutr 2005, 81, 1072–1079. [Google Scholar]
- Atkinson, C.; Frankenfeld, C.L.; Lampe, J.W. Gut bacterial metabolism of the soy isoflavone daidzein: Exploring the relevance to human health. Exp. Biol. Med 2005, 230, 155–170. [Google Scholar]
- Bowey, E.; Adlercreutz, H.; Rowland, I. Metabolism of isoflavones and lignans by the gut microflora: A study in germ-free and human flora associated rats. Food Chem. Toxicol 2003, 41, 631–636. [Google Scholar]
- Akaza, H.; Miyanaga, N.; Takashima, N.; Naito, S.; Hirao, Y.; Tsukamoto, T.; Fujioka, T.; Mori, M.; Kim, W.J.; Song, J.M.; et al. Comparisons of percent equol producers between prostate cancer patients and controls: Case-controlled studies of isoflavones in Japanese, Korean and American residents. Jpn. J. Clin. Oncol 2004, 34, 86–89. [Google Scholar]
- Frankenfeld, C.L.; Atkinson, C.; Thomas, W.K.; Goode, E.L.; Gonzalez, A.; Jokela, T.; Wahala, K.; Schwartz, S.M.; Li, S.S.; Lampe, J.W. Familial correlations, segregation analysis, and nongenetic correlates of soy isoflavone-metabolizing phenotypes. Exp. Biol. Med 2004, 229, 902–913. [Google Scholar]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar]
- Salminen, S.; Salminen, E.; Koivistoinen, P.; Bridges, J.; Marks, V. Gut microflora interactions with xylitol in the mouse, rat and man. Food Chem. Toxicol 1985, 23, 985–990. [Google Scholar]
- Wang, X.L.; Hur, H.G.; Lee, J.H.; Kim, K.T.; Kim, S.I. Enantioselective synthesis of S-equol from dihydrodaidzein by a newly isolated anaerobic human intestinal bacterium. Appl. Environ. Microbiol 2005, 71, 214–219. [Google Scholar]
- Rafii, F.; Jackson, L.D.; Ross, I.; Heinze, T.M.; Lewis, S.M.; Aidoo, A.; Lyn-Cook, L.; Manjanatha, M. Metabolism of daidzein by fecal bacteria in rats. Comp. Med 2007, 57, 282–286. [Google Scholar]
- Mäkeläinen, H.S.; Mäkivuokko, H.A.; Salminen, S.J.; Rautonen, N.E.; Ouwehand, A.C. The effects of polydextrose and xylitol on microbial community and activity in a 4-stage colon simulator. J. Food Sci 2007, 72, M153–M159. [Google Scholar]
- Minamida, K.; Tanaka, M.; Abe, A.; Sone, T.; Tomita, F.; Hara, H.; Asano, K. Production of equol from daidzein by Gram-positive rod-shaped bacterium isolated from rat intestine. J. Biosci. Bioeng 2006, 102, 247–250. [Google Scholar]
- Salminen, E.K.; Salminen, S.J.; Porkka, L.; Kwasowski, P.; Marks, V.; Koivistoinen, P.E. Xylitol vs glucose: Effect on the rate of gastric emptying and motilin, insulin, and gastric inhibitory polypeptide release. Am. J. Clin. Nutr 1989, 49, 1228–1232. [Google Scholar]
- Mattila, P.T.; Svanberg, M.J.; Jämsä, T.; Knuuttila, M.L. Improved bone biomechanical properties in xylitol-fed aged rats. Metabolism 2002, 51, 92–96. [Google Scholar]
- Svanberg, M.; Knuuttila, M. Dietary xylitol prevents ovariectomy induced changes of bone inorganic fraction in rats. Bone Miner 1994, 26, 81–88. [Google Scholar]
- Fujioka, M.; Uehara, M.; Wu, J.; Adlercreutz, H.; Suzuki, K.; Kanazawa, K.; Takeda, K.; Yamada, K.; Ishimi, Y. Equol, a metabolite of daidzein, inhibits bone loss in ovariectomized mice. J. Nutr 2004, 134, 2623–2627. [Google Scholar]
- Ohta, A.; Uehara, M.; Sakai, K.; Takasaki, M.; Adlercreutz, H.; Morohashi, T.; Ishimi, Y. A combination of dietary fructooligosaccharides and isoflavone conjugates increases femoral bone mineral density and equol production in ovariectomized mice. J. Nutr 2002, 132, 2048–2054. [Google Scholar]
- Tousen, Y.; Ezaki, J.; Fujii, Y.; Ueno, T.; Nishimuta, M.; Ishimi, Y. Natural S-equol decreases bone resorption in postmenopausal, non-equol-producing Japanese women: A pilot randomized, placebo-controlled trial. Menopause 2011, 18, 563–574. [Google Scholar]
- Shafer, R.B.; Levine, A.S.; Marlette, J.M.; Morley, J.E. Effects of xylitol on gastric emptying and food intake. Am. J. Clin. Nutr 1987, 45, 744–747. [Google Scholar]
- Yamakoshi, J.; Piskula, M.K.; Izumi, T.; Tobe, K.; Saito, M.; Kataoka, S.; Obata, A.; Kikuchi, M. Isoflavone aglycone-rich extract without soy protein attenuates atherosclerosis development in cholesterol-fed rabbits. J. Nutr 2000, 130, 1887–1893. [Google Scholar]
- Mäkinen, K.K.; Hämäläinen, M.M. Metabolic effects in rats of high oral doses of galactitol, mannitol and xylitol. J. Nutr 1985, 115, 890–899. [Google Scholar]
- Islam, M.S. Effects of xylitol as a sugar substitute on diabetes-related parameters in nondiabetic rats. J. Med. Food 2011, 14, 505–511. [Google Scholar]
- Torok, V.A.; Ophel-Keller, K.; Loo, M.; Hughes, R.J. Application of methods for identifying broiler chicken gut bacterial species linked with increased energy metabolism. Appl. Environ. Microbiol 2008, 74, 783–791. [Google Scholar]
- Nagashima, K.; Hisada, T.; Sato, M.; Mochizuki, J. Application of new primer-enzyme combinations to terminal restriction fragment length polymorphism profiling of bacterial populations in human feces. Appl. Environ. Microbiol 2003, 69, 1251–1262. [Google Scholar]
- Nagashima, K.; Mochizuki, J.; Hisada, T.; Suzuki, S.; Shimomura, K. Phylogenetic analysis of 16S ribosomal RNA gene sequences from human fecal microbiota and improved utility of terminal restriction fragment length polymorphism profiling. Biosci. Microflora 2006, 25, 99–107. [Google Scholar]
- Walker, A.W.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.; Brown, D.; Stares, M.D.; Scott, P.; Bergerat, A.; et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J 2011, 5, 220–230. [Google Scholar]
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med 2009. [Google Scholar] [CrossRef]
- De La Serre, C.B.; Ellis, C.L.; Lee, J.; Hartman, A.L.; Rutledge, J.C.; Raybould, H.E. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am. J. Physiol. Gastrointest. Liver Physiol 2010, 299, G440–G448. [Google Scholar]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr 1993, 123, 1939–1951. [Google Scholar]
- Allain, C.C.; Poon, L.S.; Chan, C.S.; Richmond, W.; Fu, P.C. Enzymatic determination of total serum cholesterol. Clin. Chem 1974, 20, 470–475. [Google Scholar]
- Spayd, R.W.; Bruschi, B.; Burdick, B.A.; Dappen, G.M.; Eikenberry, J.N.; Esders, T.W.; Figueras, J.; Goodhue, C.T.; LaRossa, D.D.; Nelson, R.W.; et al. Multilayer film elements for clinical analysis: Applications to representative chemical determinations. Clin. Chem 1978, 24, 1343–1350. [Google Scholar]
- Takayama, M.; Itoh, S.; Nagasaki, T.; Tanimizu, I. A new enzymatic method for determination of serum choline-containing phospholipids. Clin. Chim. Acta 1977, 79, 93–98. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol 1959, 37, 911–917. [Google Scholar]
- Tamura, M.; Hori, S.; Nakagawa, H. Dihydrodaidzein-producing Clostridium-like intestinal bacterium, strain TM-40, affects in vitro metabolism of daidzein by fecal microbiota of human male equol producer and non-producers. Biosci. Microflora 2011, 30, 65–71. [Google Scholar]
- Matsuki, T. Procedure of DNA extraction from fecal sample for the analysis of intestinal microflora. J. Intest. Microbiol 2006, 20, 259–262. [Google Scholar]

| Ingredient (g/kg Diet) | AIN-93M | CD Diet | XD Diet |
|---|---|---|---|
| Corn starch | 465.692 | 455.692 | 405.692 |
| Casein | 140 | 140 | 140 |
| α-Corn starch | 155 | 154.5 | 154.5 |
| Sucrose | 100 | 100 | 100 |
| Lard | - | 50 | 50 |
| Soy bean oil | 40 | - | - |
| Cellulose | 50 | 50 | 50 |
| Mineral mix (AIN-93M-Mix) | 35 | 35 | 35 |
| Vitamin mix (AIN-93-Mix) | 10 | 10 | 10 |
| l-Cystine | 1.8 | 1.8 | 1.8 |
| Tert-butylhydroquinone | 0.008 | 0.008 | 0.008 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 |
| Xylitol | - | - | 50 |
| Daidzein | - | 0.5 | 0.5 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tamura, M.; Hoshi, C.; Hori, S. Xylitol Affects the Intestinal Microbiota and Metabolism of Daidzein in Adult Male Mice. Int. J. Mol. Sci. 2013, 14, 23993-24007. https://doi.org/10.3390/ijms141223993
Tamura M, Hoshi C, Hori S. Xylitol Affects the Intestinal Microbiota and Metabolism of Daidzein in Adult Male Mice. International Journal of Molecular Sciences. 2013; 14(12):23993-24007. https://doi.org/10.3390/ijms141223993
Chicago/Turabian StyleTamura, Motoi, Chigusa Hoshi, and Sachiko Hori. 2013. "Xylitol Affects the Intestinal Microbiota and Metabolism of Daidzein in Adult Male Mice" International Journal of Molecular Sciences 14, no. 12: 23993-24007. https://doi.org/10.3390/ijms141223993
APA StyleTamura, M., Hoshi, C., & Hori, S. (2013). Xylitol Affects the Intestinal Microbiota and Metabolism of Daidzein in Adult Male Mice. International Journal of Molecular Sciences, 14(12), 23993-24007. https://doi.org/10.3390/ijms141223993




