Superior Mechanical Properties of Double-Network Hydrogels Reinforced by Carbon Nanotubes without Organic Modification
Abstract
:1. Introduction
2. Results and Discussion
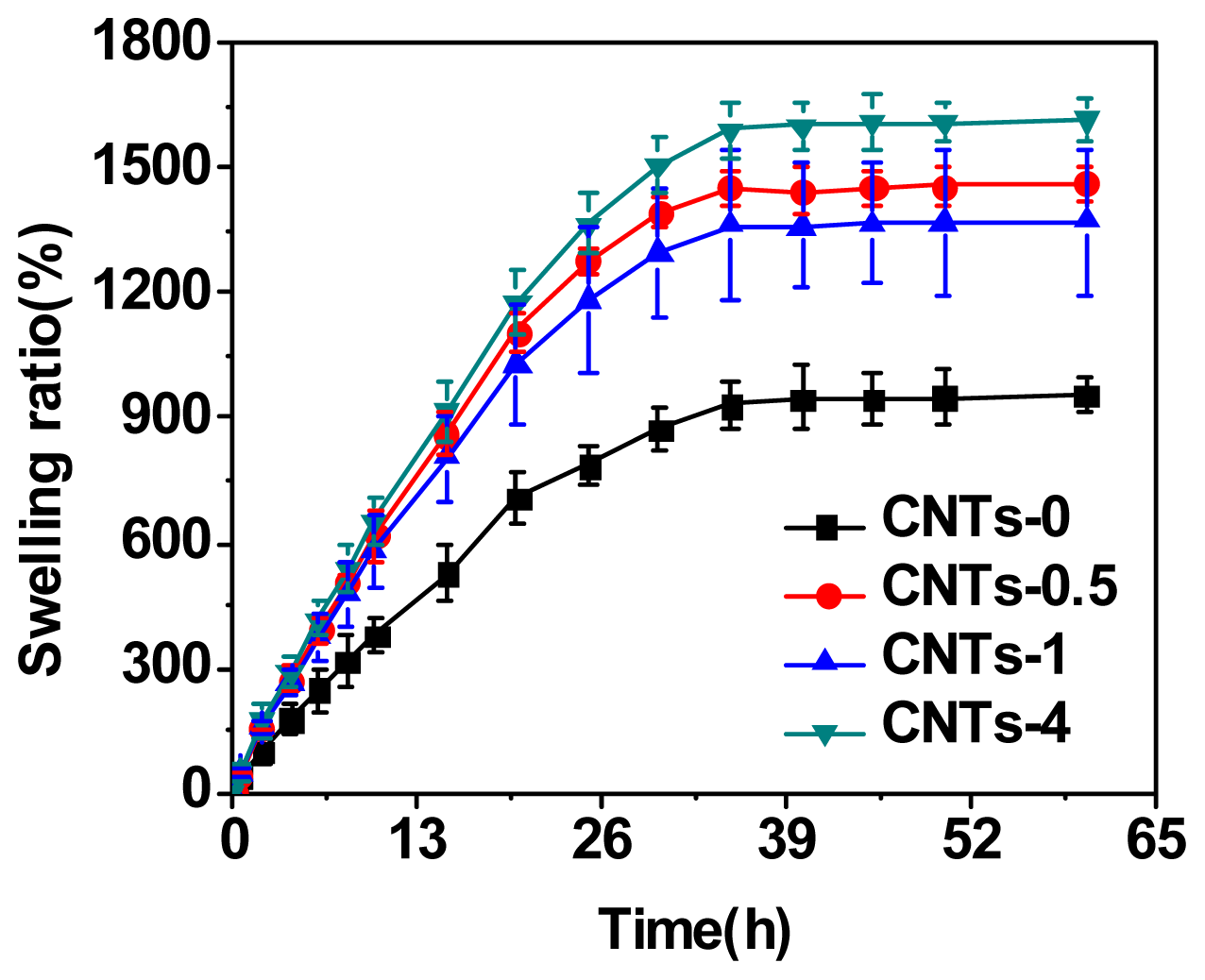
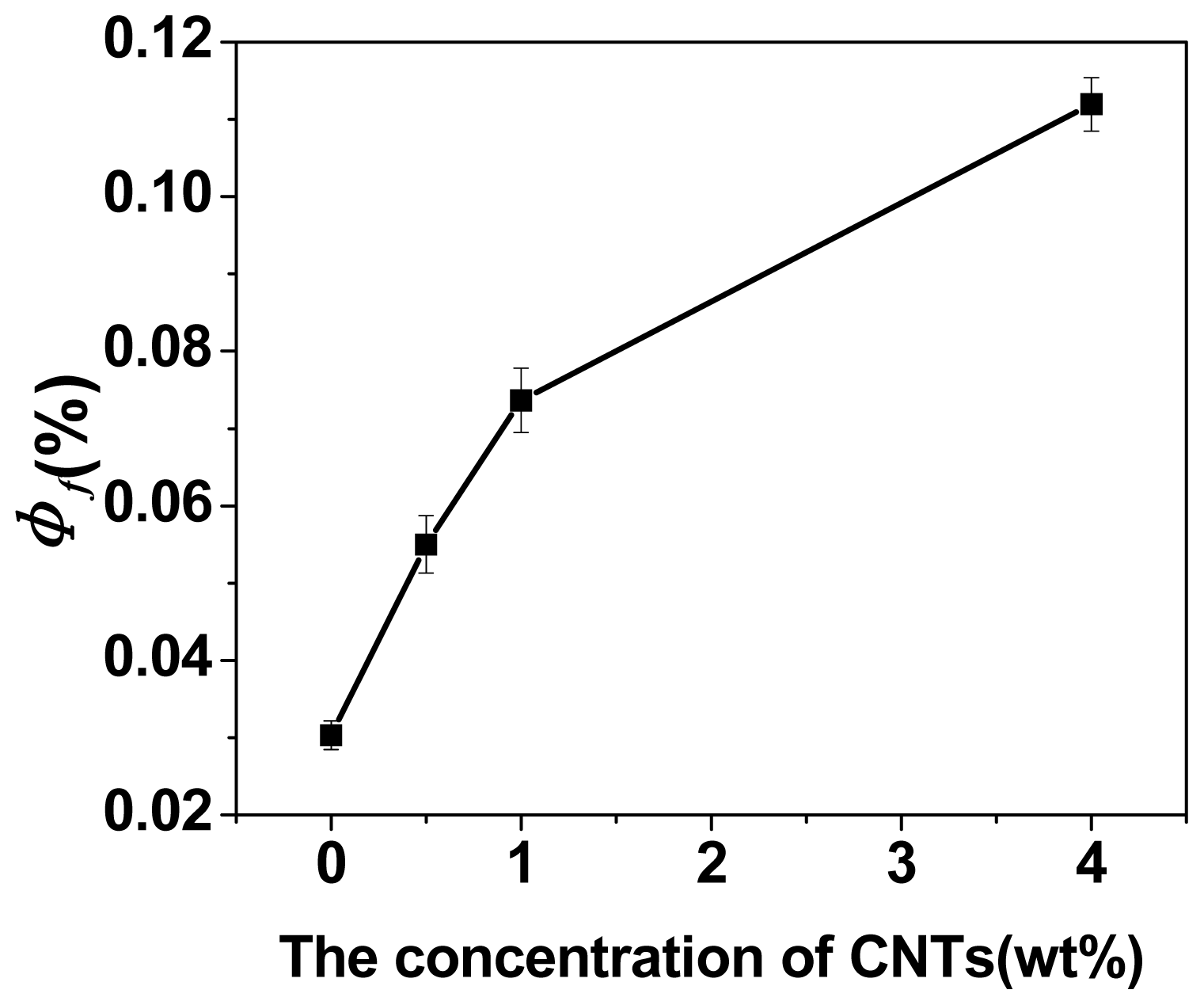
2.1. Swelling Behavior
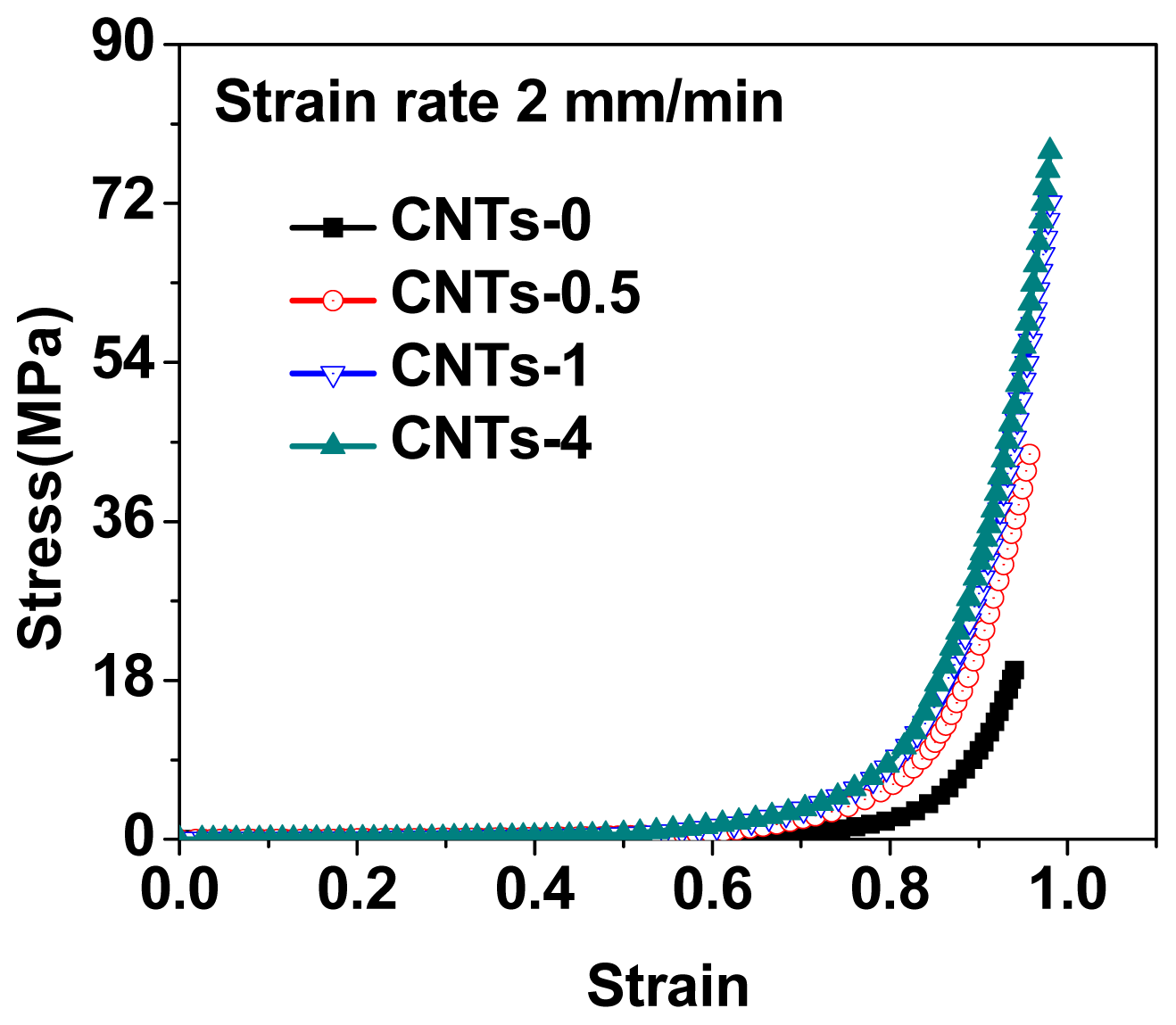
2.2. Mechanical Properties
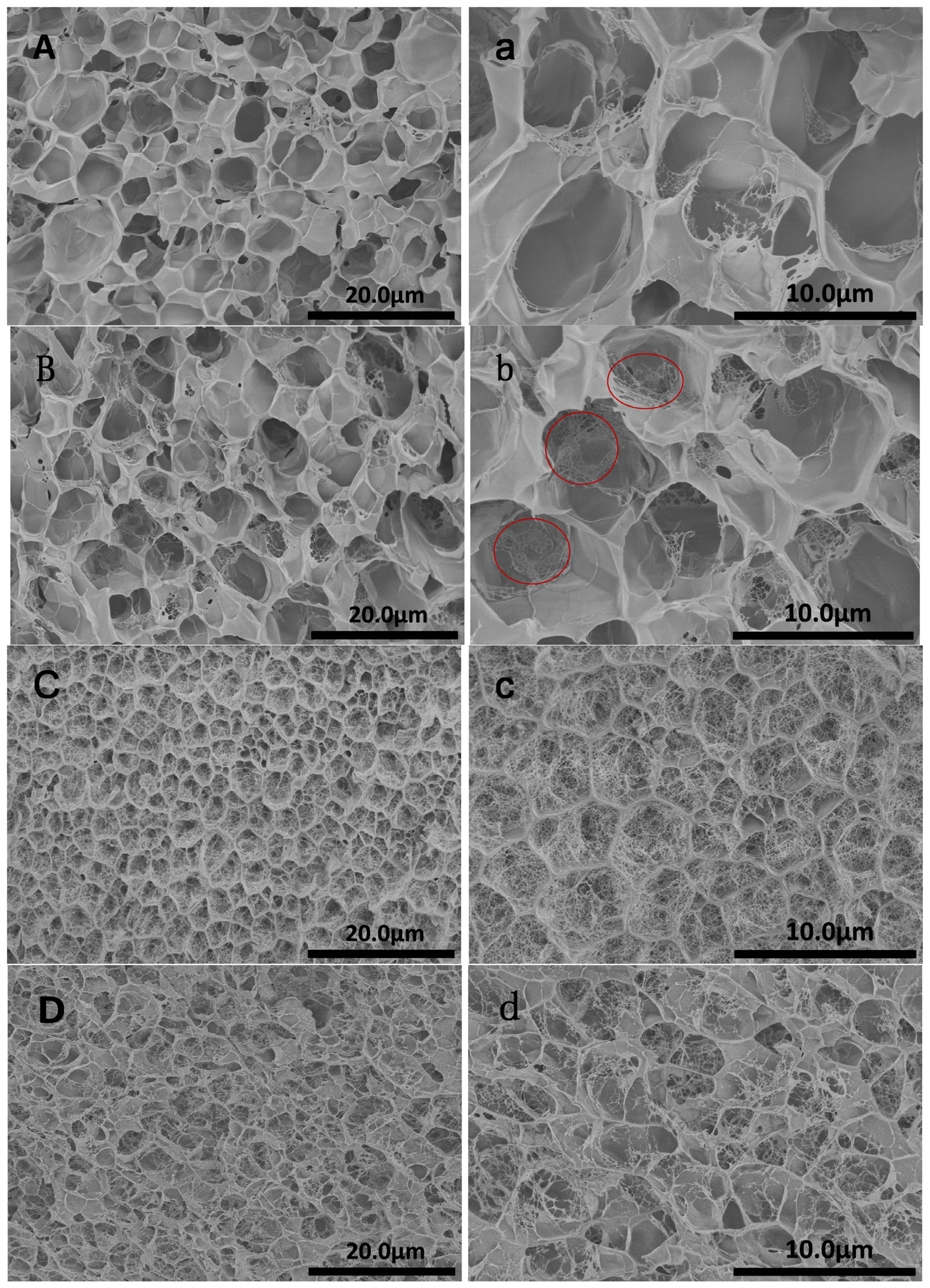
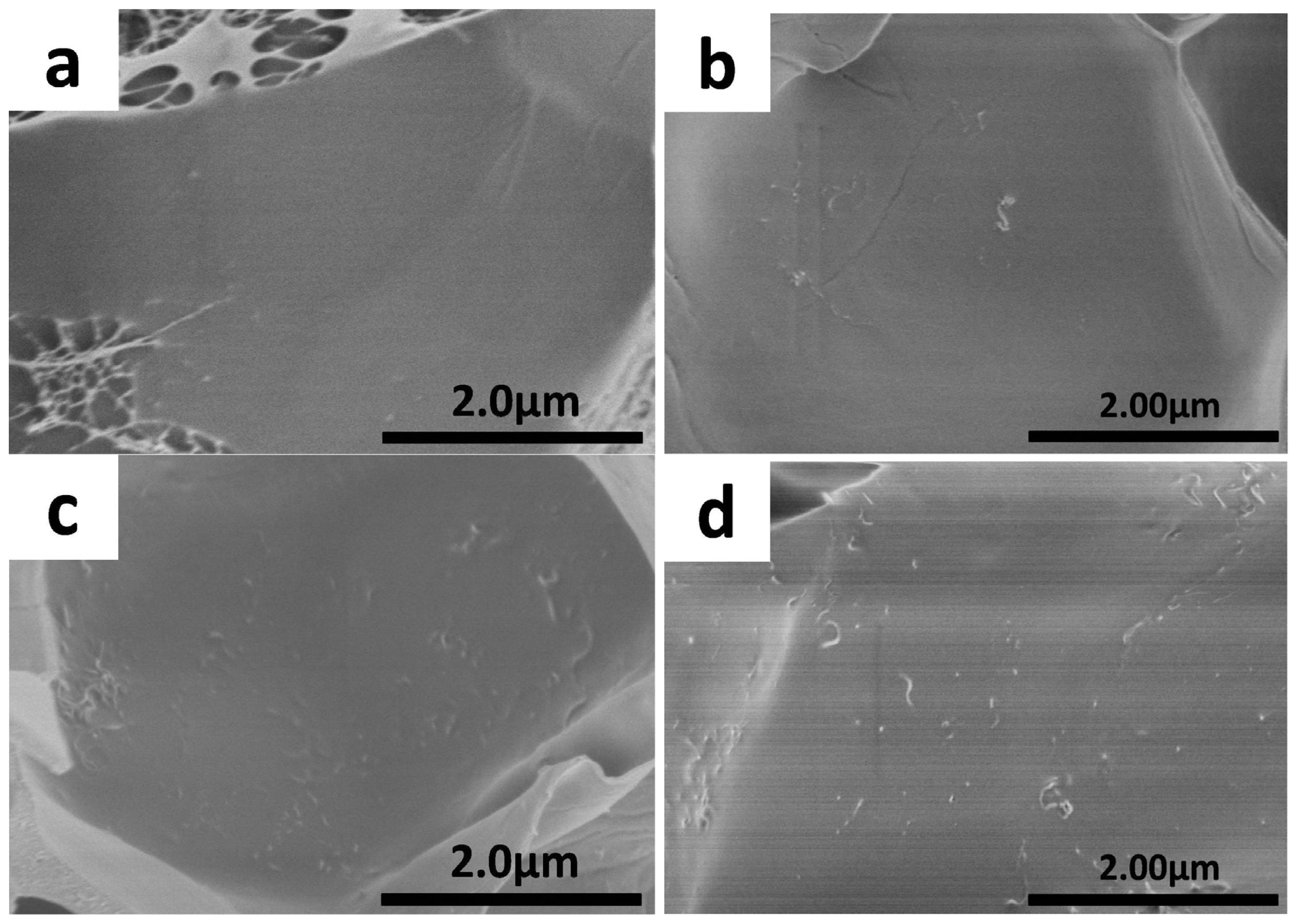
2.3. Morphology
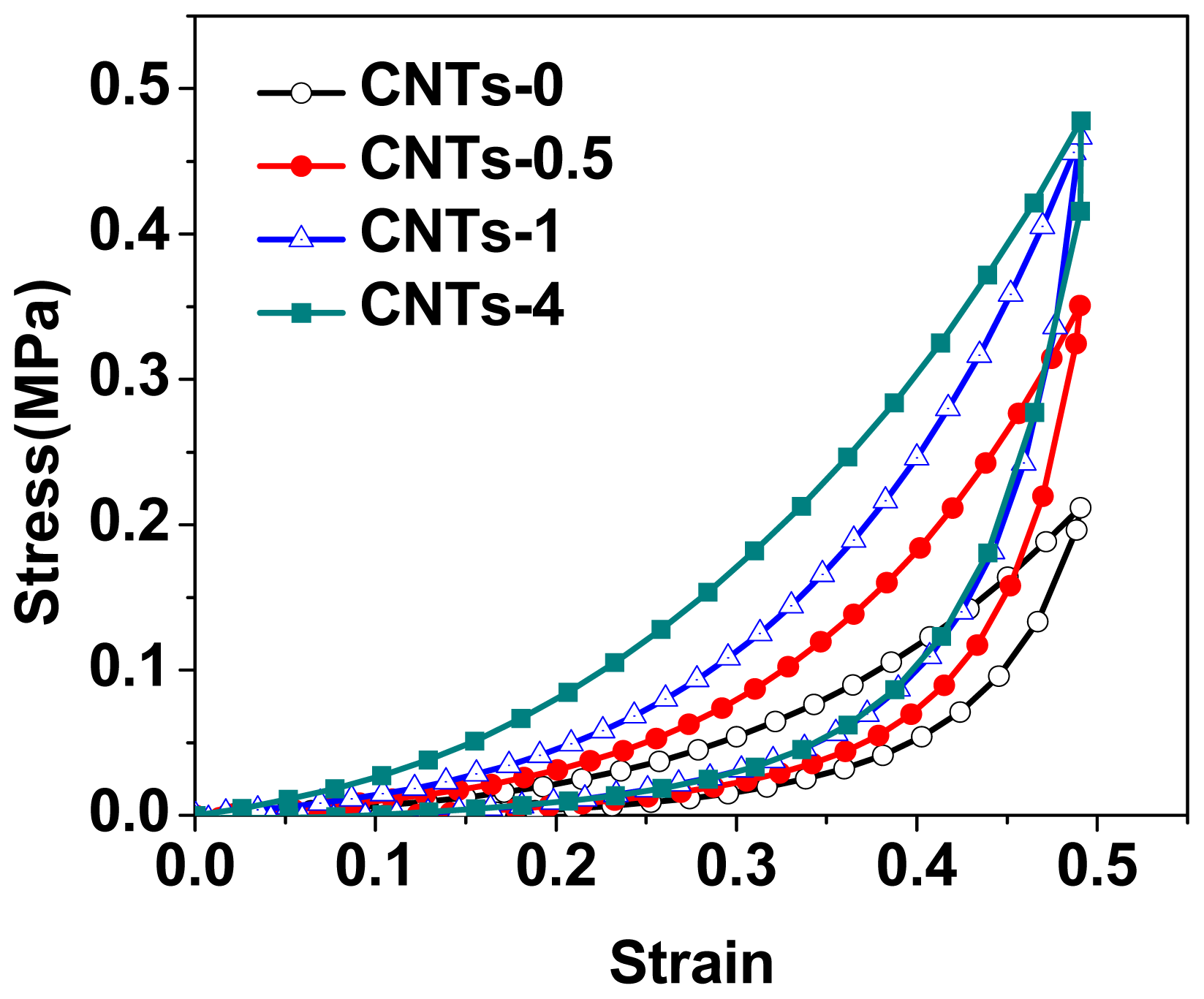
2.4. Compressive Loading-Unloading Behaviors and Analysis
3. Experimental Section
3.1. Materials
3.2. Experimental Section
3.3. Measurements and Characterization
3.3.1. Scanning Electron Microscopy (SEM)
3.3.2. Swelling Experiments
3.3.3. Tensile Measurements
3.3.4. Compressive Measurements
3.3.5. Statistical Analysis
4. Conclusions



| Samples | Compressive properties | |||
|---|---|---|---|---|
| Compressive modulus (kPa) | σc,0.9 (MPa) | Fracture stress | Fracture strain | |
| CNTs-0 | 60 ± 2 | 10.09 ± 1.1 | 19.17 ± 3.1 | 0.94 ± 0.01 |
| CNTs-0.5 | 93 ± 5 | 22.02 ± 2.9 | 43.61 ± 3.9 | 0.95 ± 0.01 |
| CNTs-1 | 144 ± 5 | 27.83 ± 3.7 | 72.05 ± 6.7 | 0.98 (no fracture) |
| CNTs-4 | 280 ± 14 | 31.63 ± 1.8 | 77.91 ± 6.8 | 0.98 (no fracture) |
| Samples | Tensile properties | |||
|---|---|---|---|---|
| Young’s modulus (kPa) | Yield stress (kPa) | Ultimate stress (kPa) | Ultimate strain | |
| CNTs-0 | 20.8 ± 0.5 | 241 ± 17 | 681 ± 43 | 13.39 ± 2.08 |
| CNTs-0.5 | 23.0 ± 0.3 | 366 ± 17 | 632 ± 35 | 11.46 ± 2.10 |
| CNTs-1 | 25.6 ± 0.5 | 432 ± 10 | 630 ± 23 | 8.36 ± 1.77 |
| CNTs-4 | 35.6 ± 0.9 | 533 ± 18 | 547 ± 28 | 3.45 ± 0.52 |
| Samples | 1st | 2nd | 3rd | 4th | 5th | |
|---|---|---|---|---|---|---|
| CNTs-0 | Ea/kPa | 33 ± 2 | 34 ± 2 | 34 ± 1 | 37 ± 3 | 34 ± 2 |
| σb/kPa | 210 ± 11 | 199 ± 9 | 191 ± 1 | 184 ± 4 | 180 ± 2 | |
| Ed/kJ m−3 | 13.1 ± 0.8 | 2.3 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.1 | |
| CNTs-0.5 | Ea/kPa | 77 ± 5 | 53 ± 7 | 51 ± 7 | 54 ± 3 | 57 ± 4 |
| σb/kPa | 348 ± 13 | 330 ± 15 | 317 ± 18 | 306 ± 15 | 298 ± 19 | |
| Ed/kJ m−3 | 23.8 ± 1.6 | 3.4 ± 0.2 | 2.9 ± 0.1 | 2.6 ± 0.1 | 2.5 ± 0.1 | |
| CNTs-1 | Ea/kPa | 103 ± 7 | 60 ± 4 | 66 ± 8 | 65 ± 5 | 61 ± 7 |
| σb/kPa | 466 ± 23 | 451 ± 17 | 432 ± 14 | 419 ± 14 | 410 ± 17 | |
| Ed/kJ m−3 | 31.8 ± 1.8 | 4.5 ± 0.4 | 3.6 ± 0.2 | 3.3 ± 0.3 | 3.0 ± 0.2 | |
| CNTs-4 | Ea/kPa | 165 ± 8 | 84 ± 7 | 85 ± 9 | 88 ± 7 | 86 ± 8 |
| σb/kPa | 478 ± 12 | 456 ± 16 | 437 ± 12 | 423 ± 19 | 415 ± 18 | |
| Ed/kJ m−3 | 48.4 ± 1.5 | 5.3 ± 0.4 | 3.9 ± 0.3 | 3.3 ± 0.2 | 3.2 ± 0.2 | |
| Nomenclature | Composition at hydrogel preparation conditions | ||||||
|---|---|---|---|---|---|---|---|
| First network | Second network | ||||||
| CCNTa (wt%) | CAMPS (mol/L) | CMBAA (mol/L) | CKPS (mol/L) | CAAm (mol/L) | CMBAA (mol/L) | CKPS (mol/L) | |
| CNTs-0 | 0 | 1.0 | 0.04 | 0.005 | 2.0 | 0.001 | 0.002 |
| CNTs-0.5 | 0.5 | 1.0 | 0.04 | 0.005 | 2.0 | 0.001 | 0.002 |
| CNTs-1 | 1 | 1.0 | 0.04 | 0.005 | 2.0 | 0.001 | 0.002 |
| CNTs-4 | 4 | 1.0 | 0.04 | 0.005 | 2.0 | 0.001 | 0.002 |
Acknowledgments
Conflicts of Interest
References
- Cushing, M.C.; Anseth, K.S. Hydrogel cell cultures. Science 2007, 316, 1133–1134. [Google Scholar]
- Hirst, A.R.; Escuder, B.; Miravet, J.F.; Smith, D.K. High-tech applications of self-assembling supramolecular nanostructured gel-phase materials: From regenerative medicine to electronic devices. Angew. Chem. Int. Ed. Engl 2008, 47, 8002–8018. [Google Scholar]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev 2002, 54, 3–12. [Google Scholar]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater 2006, 18, 1345–1360. [Google Scholar]
- Nicolson, P.C.; Vogt, J. Soft contact lens polymers: An evolution. Biomaterials 2001, 22, 3273–3283. [Google Scholar]
- Callwaert, M.; Rouxhet, P.G.; Boulange-Petermann, L. Modifying stainless steel surfaces with responsive polymers: Effect of PS-PAA and PNIPAAM on cell adhesion and oil removal. J. Adhes. Sci. Technol 2005, 19, 765–781. [Google Scholar]
- Chen, J.; Park, H.; Park, K. Synthesis of superporous hydrogels: Hydrogels with fast swelling and superabsorbent properties. J. Biomed. Mater. Res 1999, 44, 53–62. [Google Scholar]
- Gong, J.P. Why are double network hydrogels so tough. Soft Matter 2010, 6, 2583–2590. [Google Scholar]
- Calvert, P. Hydrogels for soft machines. Adv. Mater 2009, 21, 743–756. [Google Scholar]
- Bahartan, K.; Gun, J.; Sladkevich, S.; Prikhodchenko, P.V.; Lev, O.; Alfonta, L. Encapsulation of yeast displaying glucose oxidase on their surface in graphene oxide hydrogel scaffolding and its bioactivation. Chem. Commun 2012, 48, 11957–11959. [Google Scholar]
- Holtz, J.H.; Asher, S.A. Polymerized colloidal crystal hydrogel films as intelligent chemical sensing materials. Nature 1997, 389, 829–832. [Google Scholar]
- Dong, L.; Jiang, H. Autonomous microfluidics with stimuli-responsive hydrogels. Soft Matter 2007, 3, 1223–1230. [Google Scholar]
- Place, E.S.; Evans, N.D.; Stevens, M.M. Complexity in biomaterials for tissue engineering. Nat. Mater 2009, 8, 457–470. [Google Scholar]
- Okumura, Y.; Ito, K. The polyrotaxane gel: A topological gel by figure-of-eight cross-links. Adv. Mater 2001, 13, 485–487. [Google Scholar]
- Haraguchi, K.; Takehisa, T. Nanocomposite hydrogels a unique organic-inorganic network structure with extraordinary mechanical, optical and swelling deswelling properties. Adv. Mater 2002, 14, 1120–1124. [Google Scholar]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater 2003, 15, 1155–1158. [Google Scholar]
- Malkoch, M.; Vestberg, R.; Gupta, N.; Mespouille, L.; Dubis, P.; Mason, A.F.; Hedrik, J.L.; Liao, Q.; Frank, C.W.; Kingsbury, K.; et al. Synthesis of well-defined hydrogel networks using click chemistry. Chem. Commun 2006, 2774–2776. [Google Scholar]
- Huang, T.; Xu, H.; Jiao, K.; Zhu, L.P.; Brown, H.R.; Wang, H.L. A novel hydrogel with high mechanical strength: A macromolecular microsphere composite hydrogel. Adv. Mater 2007, 19, 1622–1626. [Google Scholar]
- Jiang, G.Q.; Liu, C.; Liu, X.L.; Zhang, M.Y.; Liu, F.Q. Construction and properties of hydrophobic association hydrogels with high mechanical strength and reforming capability. Macromol. Mater. Eng 2009, 294, 815–820. [Google Scholar]
- Chen, Y.M.; Dong, K.; Liu, Z.Q.; Xu, F. Double network hydrogel with high mechanical strength: Serformance, progress and future perspective. Sci. China Technol. Sci 2012, 55, 2241–2254. [Google Scholar]
- Shi, S.R.; Bae, H.; Cha, J.M.; Mun, J.Y.; Chen, Y.C.; Tekin, H.; Shin, H.; Farshchi, S.; Dokmeci, M.R.; Tang, S.; et al. Carbon nanotube reinforced hybrid microgels as scaffold materials for cell encapsulation. ACS Nano 2012, 6, 362–372. [Google Scholar]
- Shi, S.R.; Jung, S.M.; Zalabany, M.; Kim, K.Y.; Zorlutuna, P.; Kim, S.B.; Nikkhah, M.; Khabiry, M.; Azize, M.; Kong, J.; et al. Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano 2013, 7, 2369–2380. [Google Scholar]
- Lian, C.X.; Lin, Z.M.; Tao, W.; Liu, X.X.; Tong, Z. Self-reinforcement of PNIPAm-Laponite nanocomposite gels investigated by atom force microscopy nanoindentation. Macromolecules 2012, 45, 7220–7227. [Google Scholar]
- Gaharwar, A.K.; Dammu, S.A.; Canter, J.M.; Wu, C.J.; Schmidt, G. Highly extensible, tough, and elastomeric nanocomposite hydrogels from poly(ethylene glycol) and hydroxyapatite nanoparticles. Biomacromolecules 2011, 12, 1641–1650. [Google Scholar]
- Rose, S.; Dizeux, A.; Narita, T.; Hourdet, D.; Marcellan, A. Time dependence of dissipative and recovery processes in nanohybrid hydrogels. Macromolecules 2013, 46, 4095–4104. [Google Scholar]
- Wang, Q.; Hou, R.X.; Cheng, Y.J.; Fu, J. Super-tough double-network hydrogels reinforced by covalently compositing with silica-nanoparticles. Soft Matter 2012, 8, 6048–6056. [Google Scholar]
- Li, Z.Q.; Shen, J.F.; Ma, H.G.; Lu, X.; Shi, M.; Li, N.; Ye, M.X. Preparation and characterization of pH- and temperature-responsive nanocomposite double network hydrogels. Mater. Sci. Eng. C 2013, 33, 1951–1957. [Google Scholar]
- Fei, R.C.; George, J.T.; Park, J.Y.; Grunlan, M.A. Thermoresponsive nanocomposite double network hydrogels. Soft Matter 2012, 8, 481–487. [Google Scholar]
- Lin, J.T.; Xu, S.M.; Shi, X.M.; Feng, S.; Wang, J. Synthesis and properties of a novel double network nanocomposite hydrogel. Polym. Adv. Technol 2009, 20, 645–649. [Google Scholar]
- Fei, X.; Xu, S.M.; Feng, S.; Lin, J.L.; Shi, X.M.; Wang, J. Mechanically strengthened double network composite hydrogels with high water content: A preliminary study. J. Polym. Res 2011, 18, 1131–1136. [Google Scholar]
- Fei, X.; Xu, S.M.; Lin, J.L.; Shi, X.M.; Xu, S.M. Synthesis and mechanical strength of a novel double network nanocomposite hydrogel with core-shell structure. Polym. Adv. Technol 2012, 23, 736–741. [Google Scholar]
- Nakajima, T.; Furukawa, H.; Tanaka, Y.; Kurokawa, T.; Gong, J.P. Effect of void structure on the toughness of double network hydrogels. J. Polym. Sci. Part B 2011, 49, 1246–1254. [Google Scholar]
- Dai, T.; Qing, X.; Zhou, H.; Shen, C.; Wang, J.; Lu, Y. Mechanically strong conducting hydrogels with special double-network structure. Synth. Metals 2010, 160, 791–796. [Google Scholar]
- Tsukeshiba, H.; Huang, M.; Na, Y.; Kurokawa, T.; Kurokawa, R.; Tanaka, Y.; Furukawa, H.; Osada, Y.; Gong, J.P. Effect of polymer entanglement on the toughening of double network hydrogels. J. Phys. Chem. B 2005, 109, 16304–16309. [Google Scholar]
- Huang, M.; Furukawa, H.; Tanaka, Y.; Nakajima, T.; Osada, Y.; Gong, J.P. Importance of entanglement between first and second components in high-strength double network gels. Macromolecules 2007, 40, 6658–6664. [Google Scholar]
- Nakajima, T.; Furukawa, H.; Tanaka, Y.; Nakajima, T.; Osada, Y.; Gong, J.P. True chemical structure of double network hydrogels. Macromolecules 2009, 42, 2184–2189. [Google Scholar]
- Webber, R.E.; Creton, C. Large strain hysteresis and mullins effect of tough double-network hydrogels. Macromolecules 2007, 40, 2919–2927. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dong, W.; Huang, C.; Wang, Y.; Sun, Y.; Ma, P.; Chen, M. Superior Mechanical Properties of Double-Network Hydrogels Reinforced by Carbon Nanotubes without Organic Modification. Int. J. Mol. Sci. 2013, 14, 22380-22394. https://doi.org/10.3390/ijms141122380
Dong W, Huang C, Wang Y, Sun Y, Ma P, Chen M. Superior Mechanical Properties of Double-Network Hydrogels Reinforced by Carbon Nanotubes without Organic Modification. International Journal of Molecular Sciences. 2013; 14(11):22380-22394. https://doi.org/10.3390/ijms141122380
Chicago/Turabian StyleDong, Weifu, Chiguang Huang, Yang Wang, Yujie Sun, Piming Ma, and Mingqing Chen. 2013. "Superior Mechanical Properties of Double-Network Hydrogels Reinforced by Carbon Nanotubes without Organic Modification" International Journal of Molecular Sciences 14, no. 11: 22380-22394. https://doi.org/10.3390/ijms141122380
APA StyleDong, W., Huang, C., Wang, Y., Sun, Y., Ma, P., & Chen, M. (2013). Superior Mechanical Properties of Double-Network Hydrogels Reinforced by Carbon Nanotubes without Organic Modification. International Journal of Molecular Sciences, 14(11), 22380-22394. https://doi.org/10.3390/ijms141122380




