Effect of Chain-Extenders on the Properties and Hydrolytic Degradation Behavior of the Poly(lactide)/ Poly(butylene adipate-co-terephthalate) Blends
Abstract
:1. Introduction
2. Results and Discussion
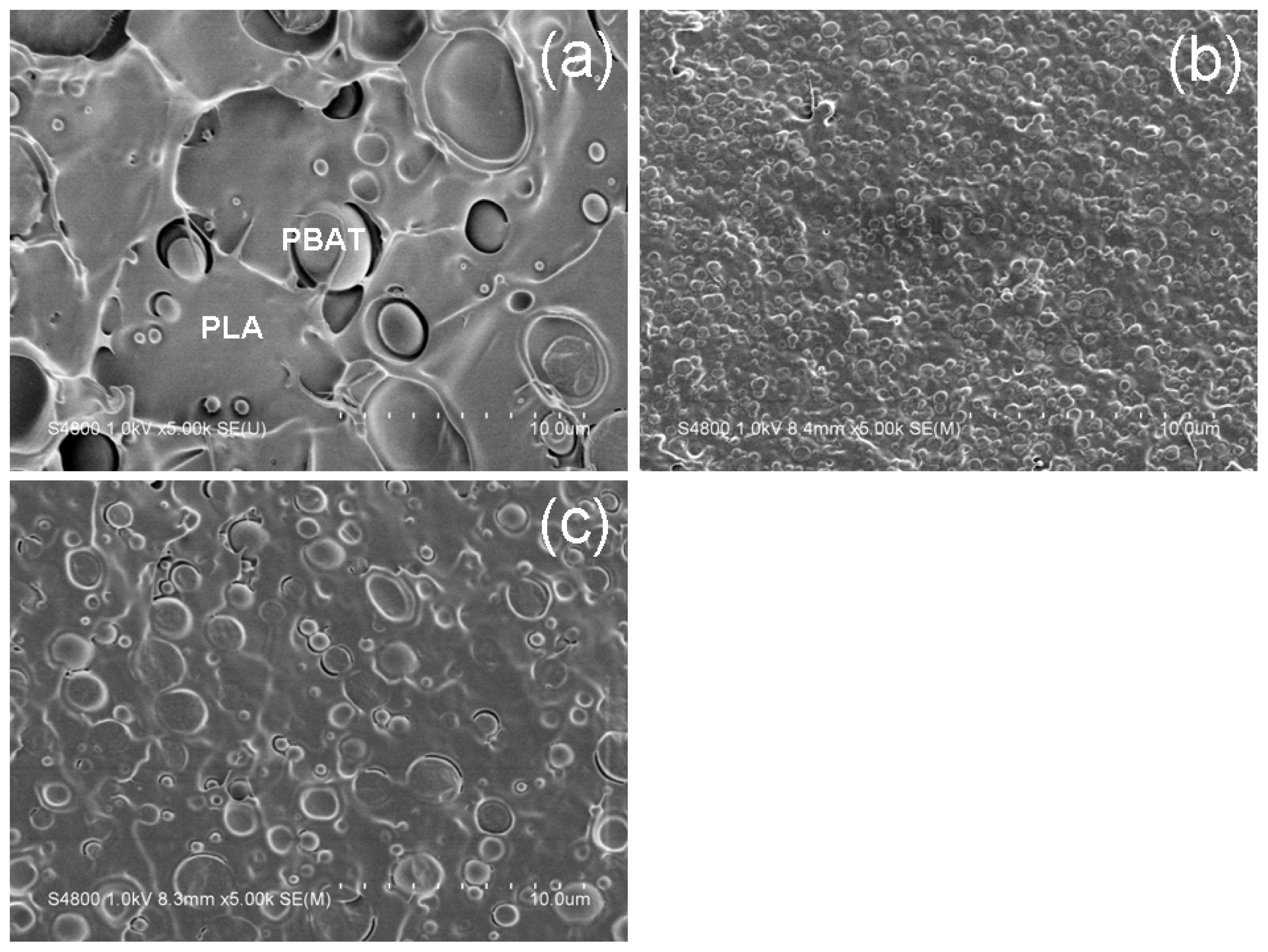
2.1. Compatibilization Effect of the Chain-Extenders on the PLA/PBAT Blends
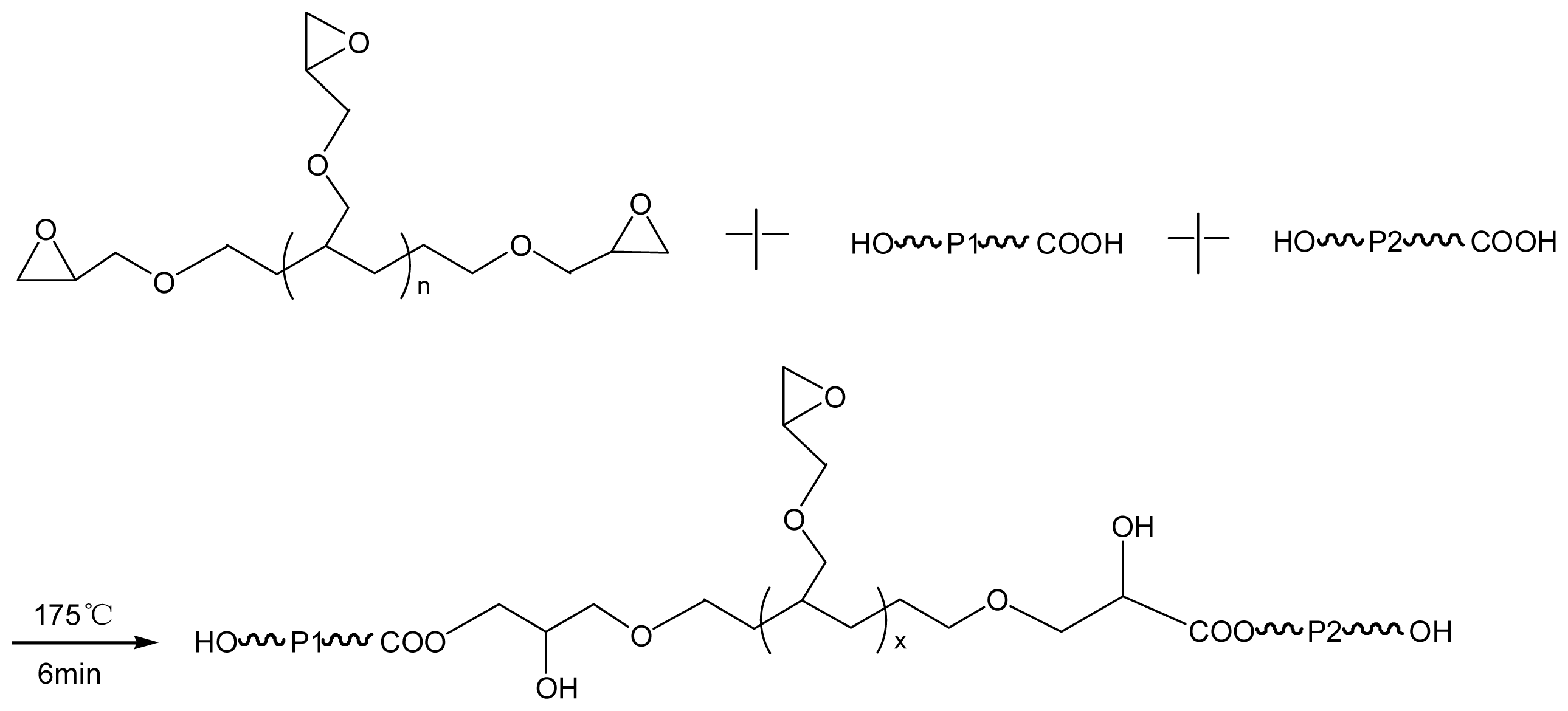
2.2. Compatibilization Mechanism of the Chain-Extenders
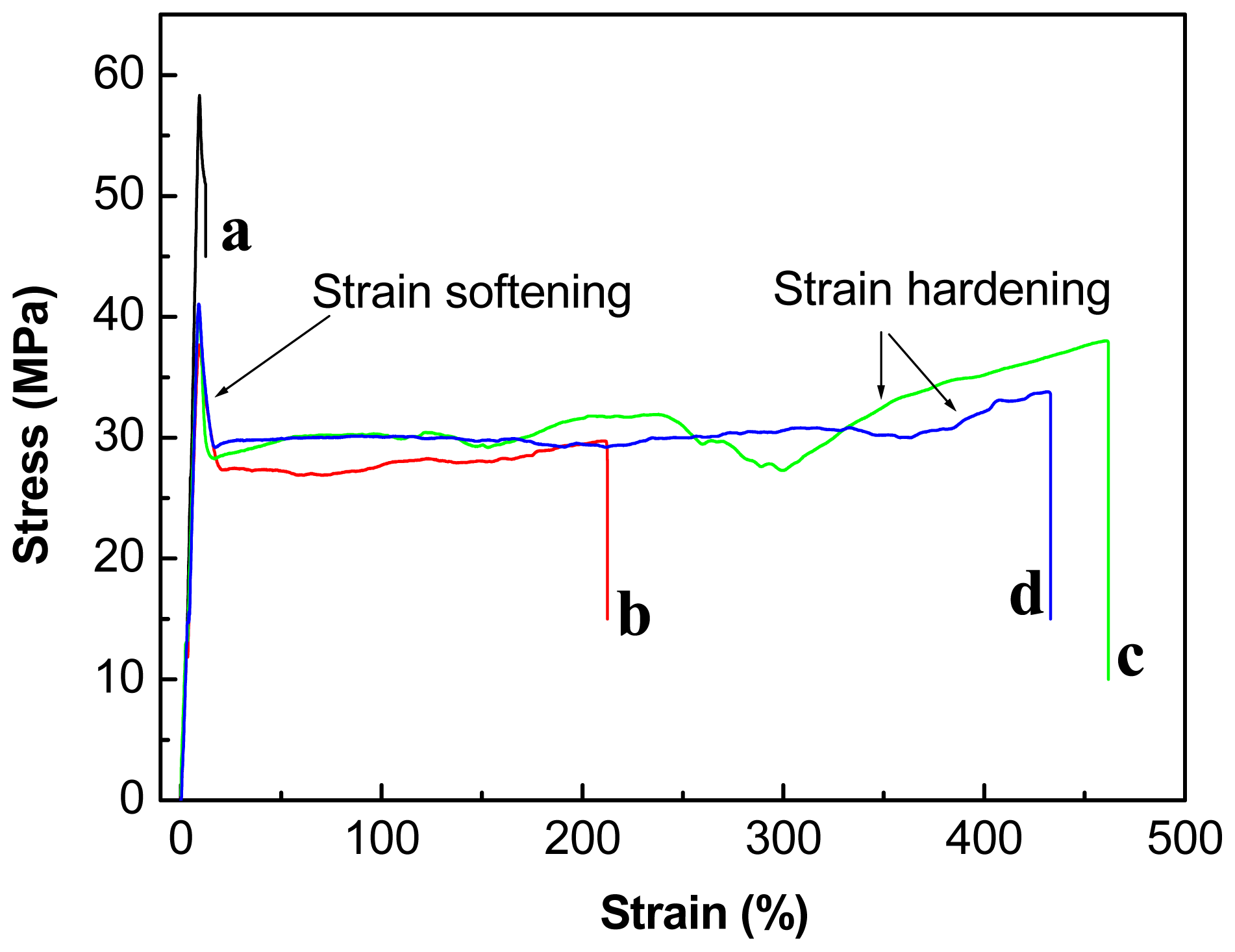
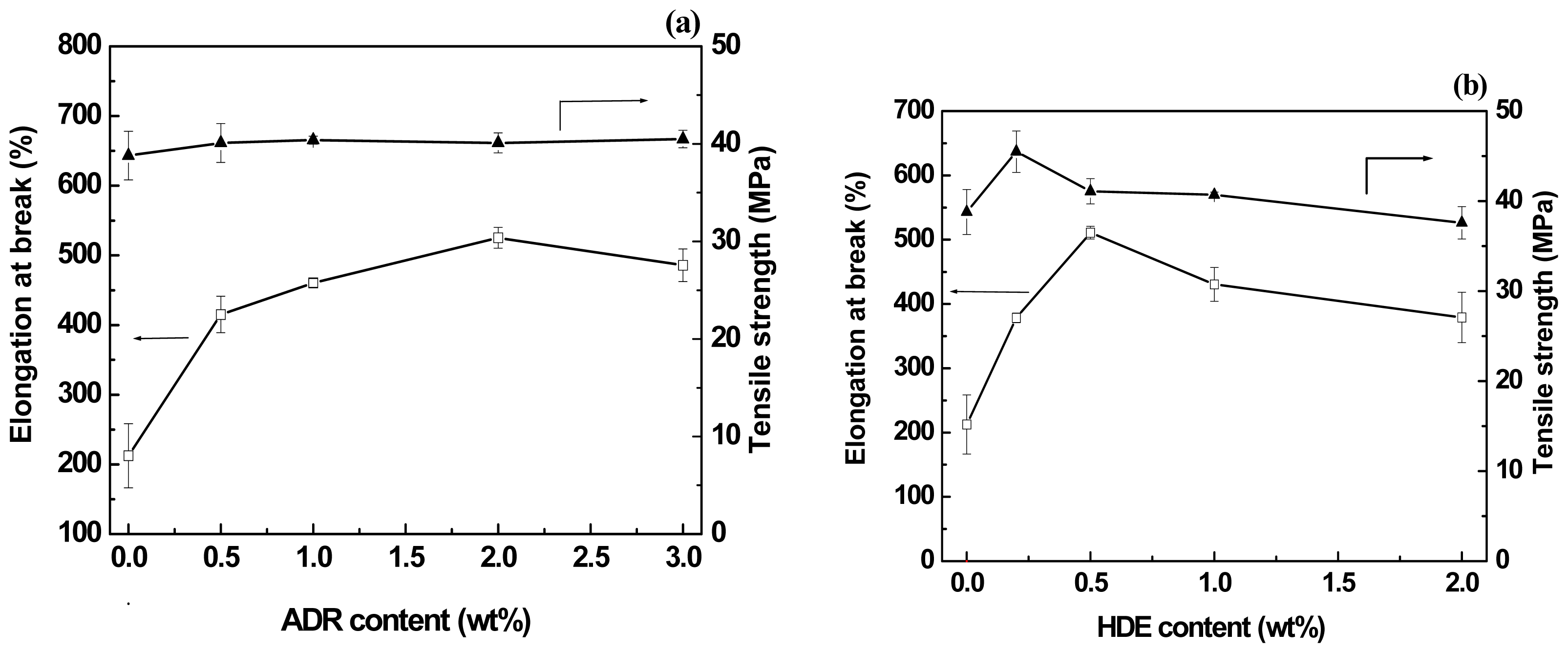
2.3. Effect of Chain-Extenders on the Mechanical Properties of the PLA/PBAT Blends
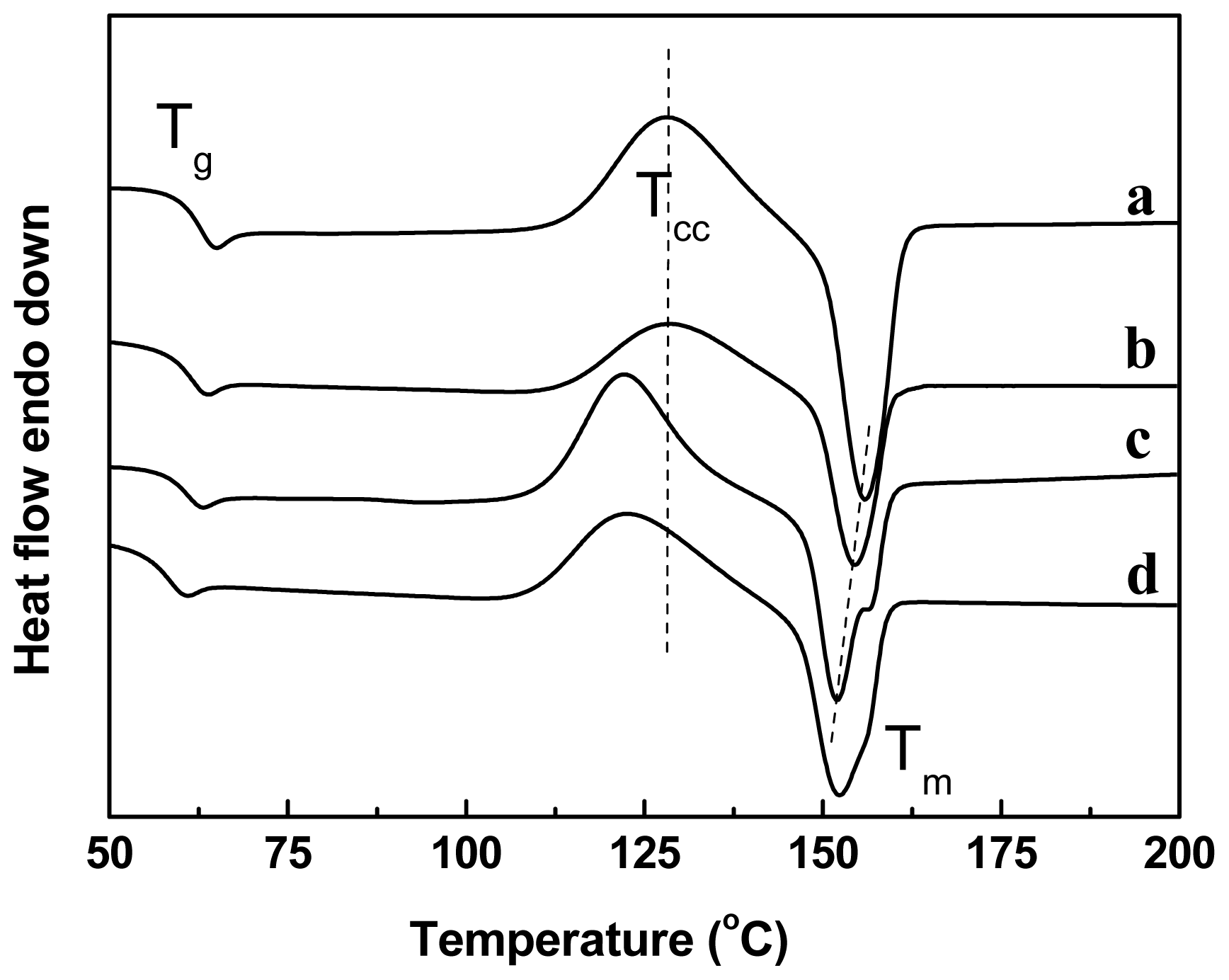
2.4. Effect of Chain-Extenders on the Thermal Behavior of the PLA/PBAT Blends
2.5. Hydrolytic Degradation Behavior of the PLA/PBAT Blends
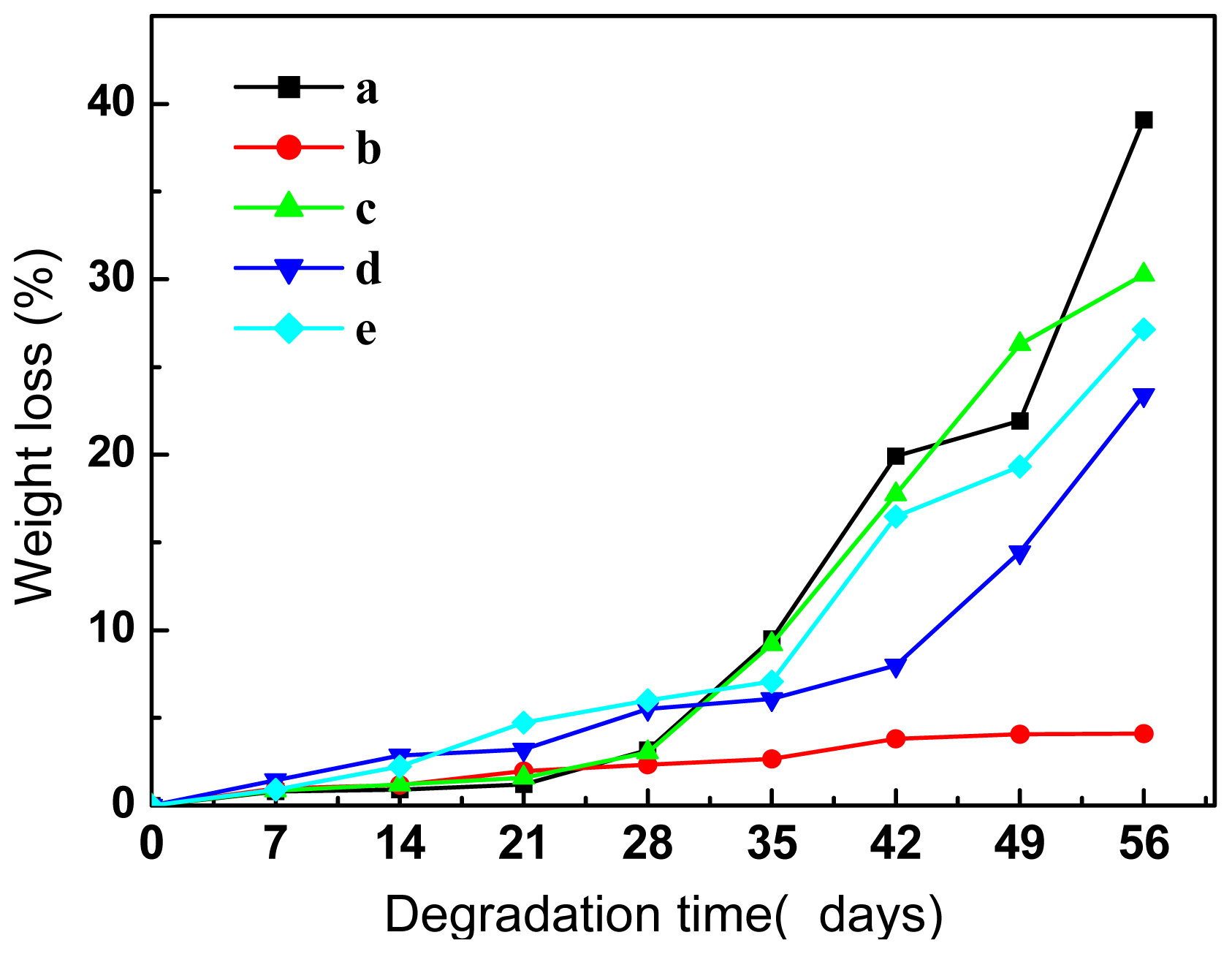
2.5.1. Morphological Analysis and Weight Loss
2.5.2. Molecular Weight Variation
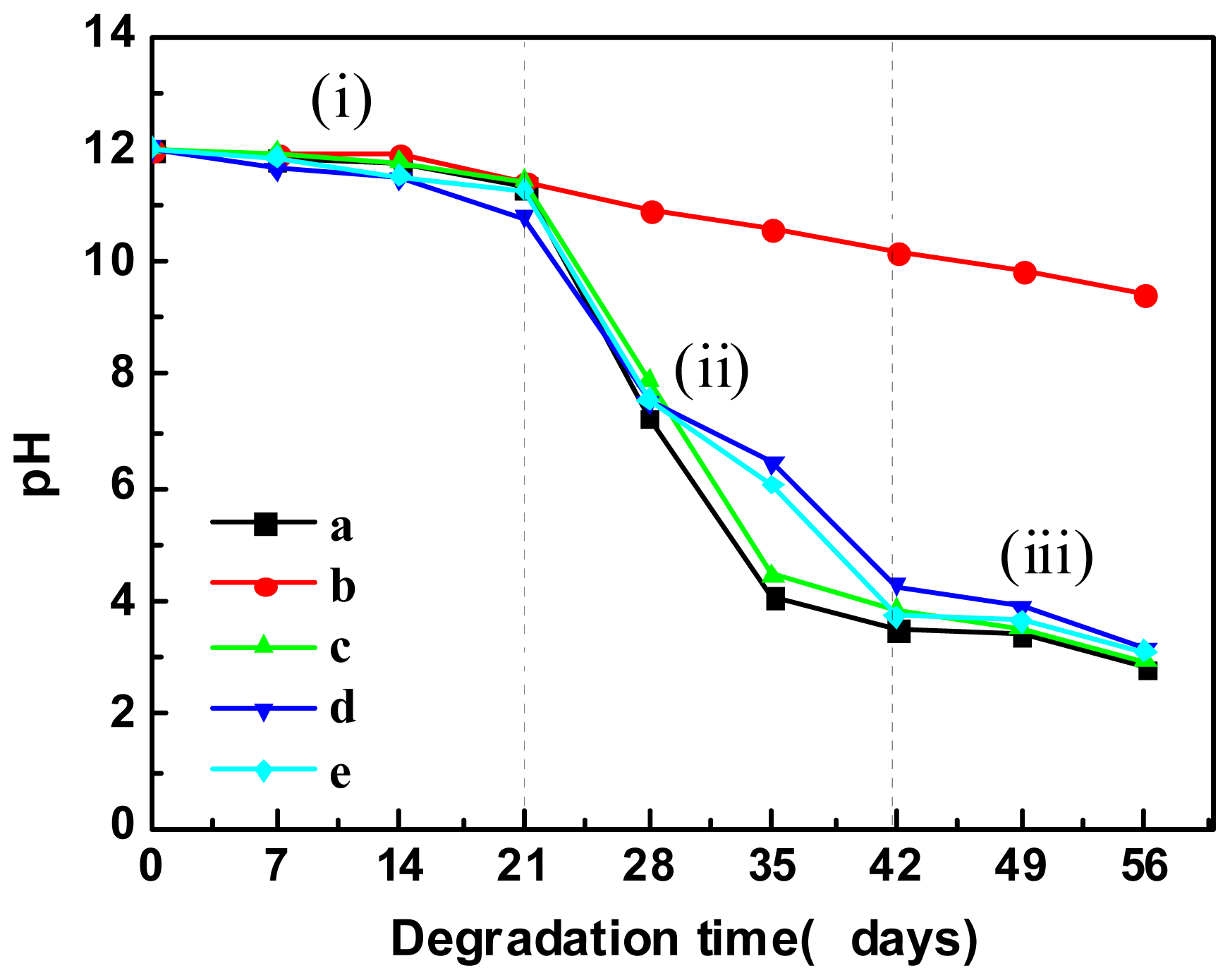
2.5.3. pH Variation
3. Experimental Section
3.1. Materials
3.2. Blend Preparation
3.3. Characterization
3.3.1. Tensile Properties
3.3.2. Scanning Electron Microscopy (SEM)
3.3.3. Gel Permeation Chromatography (GPC)
3.3.4. Differential Scanning Calorimeter (DSC)
3.3.5. Hydrolytic Degradation Behavior
4. Conclusions

| Samples | M̄n (kDa) | M̄w (kDa) | PDI a |
|---|---|---|---|
| Neat PLA | 90 | 150 | 1.7 |
| Neat PBAT | 30 | 65 | 2.2 |
| PLA/PBAT (80/20, w/w) | 60 | 130 | 2.2 |
| PLA/PBAT/ADR (80/20/1, w/w) | 100 | 330 | 3.3 |
| PLA/PBAT/HDE (80/20/1, w/w) | 70 | 160 | 2.3 |
| Sample | Tg (°C) | Tcc (°C) | Tm (°C) | ΔHm (J/g) b |
|---|---|---|---|---|
| PLA | 65 | 128 | 156 | 21 |
| PLA/PBAT (80/20, w/w) | 64 | 130 | 155 | 18 |
| PLA/PBAT/ADR (80/20/1, w/w) | 63 | 123 | 152/157 a | 28 |
| PLA/PBAT/HDE (80/20/1, w/w) | 61 | 123 | 152/156 a | 23 |
| Samples | Parameters | Days of degradation | ||
|---|---|---|---|---|
| 0 | 35 | 56 | ||
| PLA | M̄w (kDa) | 150 | 14/5 | 10/4 |
| PDI | 1.7 | 1.4/0.5 | 1.0/1.1 | |
| ratio (%) | 100 | 39/61 | 9/91 | |
| PBAT | M̄w (kDa) | 65 | 42 | 27/1 |
| PDI | 2.2 | 2.1 | 2.7/1.0 | |
| ratio (%) | 100 | 100 | 97/3 | |
| PLA/PBAT | M̄w (kDa) | 130 | 15/5 | 13/4 |
| PDI | 2.2 | 1.2/1.1 | 1.2/1.2 | |
| ratio (%) | 100 | 54/46 | 27/73 | |
| PLA/PBAT/ADR (80/20/1, w/w) | M̄w (kDa) | 330 | 67 | 13/5 |
| PDI | 3.3 | 3.1 | 1.1/1.1 | |
| ratio (%) | 100 | 100 | 26/74 | |
| PLA/PBAT/HDE (80/20/1, w/w) | M̄w (kDa) | 160 | 20/2 | 12/4 |
| PDI | 2.3 | 1.8/1.0 | 1.1/1.2 | |
| ratio (%) | 100 | 97/3 | 28/72 | |
Acknowledgments
Conflicts of Interest
References
- Gross, A.R.; Kalra, B. Biodegradable polymers for the environment. Science 2002, 297, 803–807. [Google Scholar]
- Velde, K.; Kiekens, P. Biopolymers: Overview of several properties and consequences on their applications. Polym. Test 2002, 21, 433–442. [Google Scholar]
- Anders, S.; Mikael, S. Properties of lactic acid based polymers and their correlation with composition. Prog. Polym. Sci 2002, 27, 1123–1163. [Google Scholar]
- Iannace, S.; Maffezzoli, A.; Leo, G.; Nicolais, L. Influence of crystal and amorphous phase morphology on hydrolytic degradation of PLLA subjected to different processing conditions. Polymer 2001, 42, 3799–3807. [Google Scholar]
- James, L. Large-scale production, properties and commercial applications of polylactic acid polymers. Polym. Degrad. Stab 1998, 59, 145–152. [Google Scholar]
- Martin, L.; Avérous, L. Poly(lactic acid): Plasticization and properties of biodegradable multiphase systems. Polymer 2001, 42, 6209–6219. [Google Scholar]
- Rahul, M.; Amol, V.; Douglas, E. Poly(lactic acid) modifications. Prog. Polym. Sci 2010, 35, 338–356. [Google Scholar]
- Grijpma, D.W.; van Hofslot, R.D.A.; Super, H.; Pennings, A.J. Rubber toughening of poly(lactide) by blending and block copolymerization. Polym. Eng. Sci 1994, 32, 1674–1684. [Google Scholar]
- Hiljanen, V.M.; Karjalainen, T.; Seppala, J. Biodegradable lactone copolymers. I. Characterization and mechanical behavior of ɛ-caprolactone and lactide copolymers. J. Appl. Polym. Sci 1996, 59, 1281–1288. [Google Scholar]
- Li, Y.; Shimizu, H. Toughening of polylactide by melt blending with a biodegradable poly(ether)urethane elastomer. Macromol. Biosci 2007, 7, 921–928. [Google Scholar]
- Anderson, K.S.; Hillmyer, M.A. The influence of block copolymer microstructure on the toughness of compatibilized polylactide/polyethylene blends. Polymer 2004, 45, 8809–8823. [Google Scholar]
- Oyama, H.T. Super-tough poly(lactic acid) materials: Reactive blending with ethylene copolymer. Polymer 2009, 50, 747–751. [Google Scholar]
- Afrifah, K.A.; Matuana, L.M. Impact modification of polylactide with a biodegradable ethylene/acrylate copolymer. Macromol. Mater. Eng 2010, 295, 802–811. [Google Scholar]
- Ma, P.; Lemstra, P.J. Toughening of poly(lactic acid) by ethylene-co-vinyl acetate copolymer with different vinyl acetate contents. Eur. Polym. J 2012, 48, 146–154. [Google Scholar]
- Ho, C.H.; Wang, C.H.; Lin, C.I.; Lee, Y.D. Synthesis and characterization of TPO-PLA copolymer and its behavior as compatibilizer for PLA/TPO blends. Polymer 2008, 49, 3902–3910. [Google Scholar]
- Ma, P.; Spoelstra, A.B.; Schmit, P.; Lemstra, P.J. Toughening of poly(lactic acid) by poly(hydroxyburate-co-hydroxyvalerate) with high hydroxyvalerate content. Eur. Polym. J 2013, 49, 1523–1531. [Google Scholar]
- Broz, M.E.; VanderHart, D.L.; Washburn, N.R. Structure and mechanical properties of poly(d,l-lactic acid)/poly(ɛ-caprolactone) blends. Biomaterials 2003, 24, 4181–4190. [Google Scholar]
- Chien, C.C.; Ju, Y.C.; How, T.; Haw, M.H.; Sheng, Y.L. Preparation and characterization of biodegradable PLA polymeric blends. Biomaterials 2003, 24, 1167–1173. [Google Scholar]
- Takeshi, S.; Kazuo, K.; Umaru, S.I.; Hiroyuki, H. The effect of crosslinking on the mechanical properties of polylactic acid/polycaprolactone blends. J. Appl. Polym. Sci 2006, 101, 1816–1825. [Google Scholar]
- Wang, R.; Wang, S.; Zhang, Y.; Wan, C.; Ma, P. Toughening modification of PLLA/PBS blends via in situ compatibilization. Polym. Eng. Sci 2009, 49, 26–33. [Google Scholar]
- Mitsuhiro, S.; Yusuke, I.; Masanao, M. Mechanical properties, morphology, and crystallization behavior of blends of poly(l-lactide) with poly(butylene succinate-co-l-lactate) and poly(butylene succinate). Polymer 2006, 47, 3557–3564. [Google Scholar]
- Long, J.; Michael, P.W.; Zhang, J. Study of biodegradable polylactide/poly(butylenes adipate-co-terephthalate) blends. Biomacromolecules 2006, 7, 199–207. [Google Scholar]
- Zhang, N.; Wang, Q.; Ren, J.; Wang, L. Preparation and properties of biodegradable poly(lactic acid)/poly(butylene adipate-co-terephthalate) blend with glycidyl methacrylate as reactive processing agent. J. Mater. Sci 2009, 44, 250–256. [Google Scholar]
- Ko, S.W.; Gupta, R.K.; Bhattacharya, S.N.; Choi, H.J. Rheology and physical characteristics of synthetic biodegradable aliphatic polymer blends dispersed with MWNTs. Macromol. Mater. Eng 2010, 295, 320–328. [Google Scholar]
- Kumar, M.; Mohanty, S.; Nayak, S.K.; Parvaiz, M.R. Effect of glycidy methacrylate (GMA) on the thermal mechanical and morphological property of biodegradable PLA/PBAT blend and its nanocomposites. Bioresour. Technol 2010, 101, 8406–8415. [Google Scholar]
- Racha, A.; Khalid, L.; Abderrahim, M. Improvement of thermal stability, rheological and mechanical properties of PLA, PBAT and their blends by reactive extrusion with functionalized epoxy. Polym. Degrad. Stab 2012, 97, 1898–1914. [Google Scholar]
- Villalobos, M.; Awojulu, A.; Greeley, T.; Turco, G.; Deeter, C. Oligomeric chain extenders for economic reprocessing and recycling of condensation plastics. Energy 2006, 31, 3227–3234. [Google Scholar]
- Gerds, N.; Katiyar, V.; Koch, C.B.; Hansen, H.C.B.; Plackett, D.; Larsen, E.H.; Risbo, J. Degradation of l-polylactide during melt processing with layered double hydroxides. Polym. Degrad. Stab 2012, 97, 2002–2009. [Google Scholar]
- Díaz, M.F.; Barbosa, S.E.; Capiati, N.J. Reactive compatibilization of PE/PS blends. Effect of copolymer chain length on interfacial adhesion and mechanical behavior. Polymer 2007, 48, 1058–1065. [Google Scholar]
- Wu, S. Phase structure and adhesion in polymer blends: A criterion for rubber toughening. Polymer 1985, 26, 1855–1863. [Google Scholar]
- Ma, P.M.; Wang, R.Y.; Wang, S.F.; Zhang, Y.; Zhang, Y.X.; Hristova, D. Effect of fumed silica on the crystallization behavior and thermal properties of poly(hydroxybutyrate-co-hydroxyvalerate). J. Appl. Polym. Sci 2008, 108, 1770–1777. [Google Scholar]
- Ping, S.; Schley, J.; Loy, B.; Lind, D.; Hobot, C.; Sparer, R.; Untereker, D. Kinetics and time-temperature equivalence of polymer degradation. Biomacromolecules 2007, 8, 2301–2310. [Google Scholar]
- Höglund, A.; Hakkarainen, M.; Albertsson, A.C. Migration and hydrolysis of hydrophobic polylactide plasticizer. Biomacromolecules 2010, 11, 277–283. [Google Scholar]
- Giuliana, G.; Roberto, P. Effect of PLA grades and morphologies on hydrolytic degradation at composting temperature: Assessment of structural modification and kinetic parameters. Polym. Degrad. Stab 2013, 98, 1006–1014. [Google Scholar]
- Siparsky, G.L.; Voorhees, K.J.; Miao, F.D. Hydrolysis of polylactic acid (PLA) and polycaprolactone (PCL) in aqueous acetonitrile solutions: Autocatalysis. J. Environ. Polym. Degrad 1998, 6, 31–41. [Google Scholar]
- Li, S.M.; Garreau, H.; Vert, M. Structure-property relationships in the case of the degradation of massive poly(α-hydroxy acids) in aqueous-media. J. Mater. Sci. Mater. Med 1990, 1, 198–206. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dong, W.; Zou, B.; Yan, Y.; Ma, P.; Chen, M. Effect of Chain-Extenders on the Properties and Hydrolytic Degradation Behavior of the Poly(lactide)/ Poly(butylene adipate-co-terephthalate) Blends. Int. J. Mol. Sci. 2013, 14, 20189-20203. https://doi.org/10.3390/ijms141020189
Dong W, Zou B, Yan Y, Ma P, Chen M. Effect of Chain-Extenders on the Properties and Hydrolytic Degradation Behavior of the Poly(lactide)/ Poly(butylene adipate-co-terephthalate) Blends. International Journal of Molecular Sciences. 2013; 14(10):20189-20203. https://doi.org/10.3390/ijms141020189
Chicago/Turabian StyleDong, Weifu, Benshu Zou, Yangyang Yan, Piming Ma, and Mingqing Chen. 2013. "Effect of Chain-Extenders on the Properties and Hydrolytic Degradation Behavior of the Poly(lactide)/ Poly(butylene adipate-co-terephthalate) Blends" International Journal of Molecular Sciences 14, no. 10: 20189-20203. https://doi.org/10.3390/ijms141020189
APA StyleDong, W., Zou, B., Yan, Y., Ma, P., & Chen, M. (2013). Effect of Chain-Extenders on the Properties and Hydrolytic Degradation Behavior of the Poly(lactide)/ Poly(butylene adipate-co-terephthalate) Blends. International Journal of Molecular Sciences, 14(10), 20189-20203. https://doi.org/10.3390/ijms141020189




