Sericin Enhances the Bioperformance of Collagen-Based Matrices Preseeded with Human-Adipose Derived Stem Cells (hADSCs)
Abstract
1. Introduction
2. Results and Discussion
2.1. Biocompatibility Assessment of Coll and Coll-SS Biomatrices in Contact with hADSCs
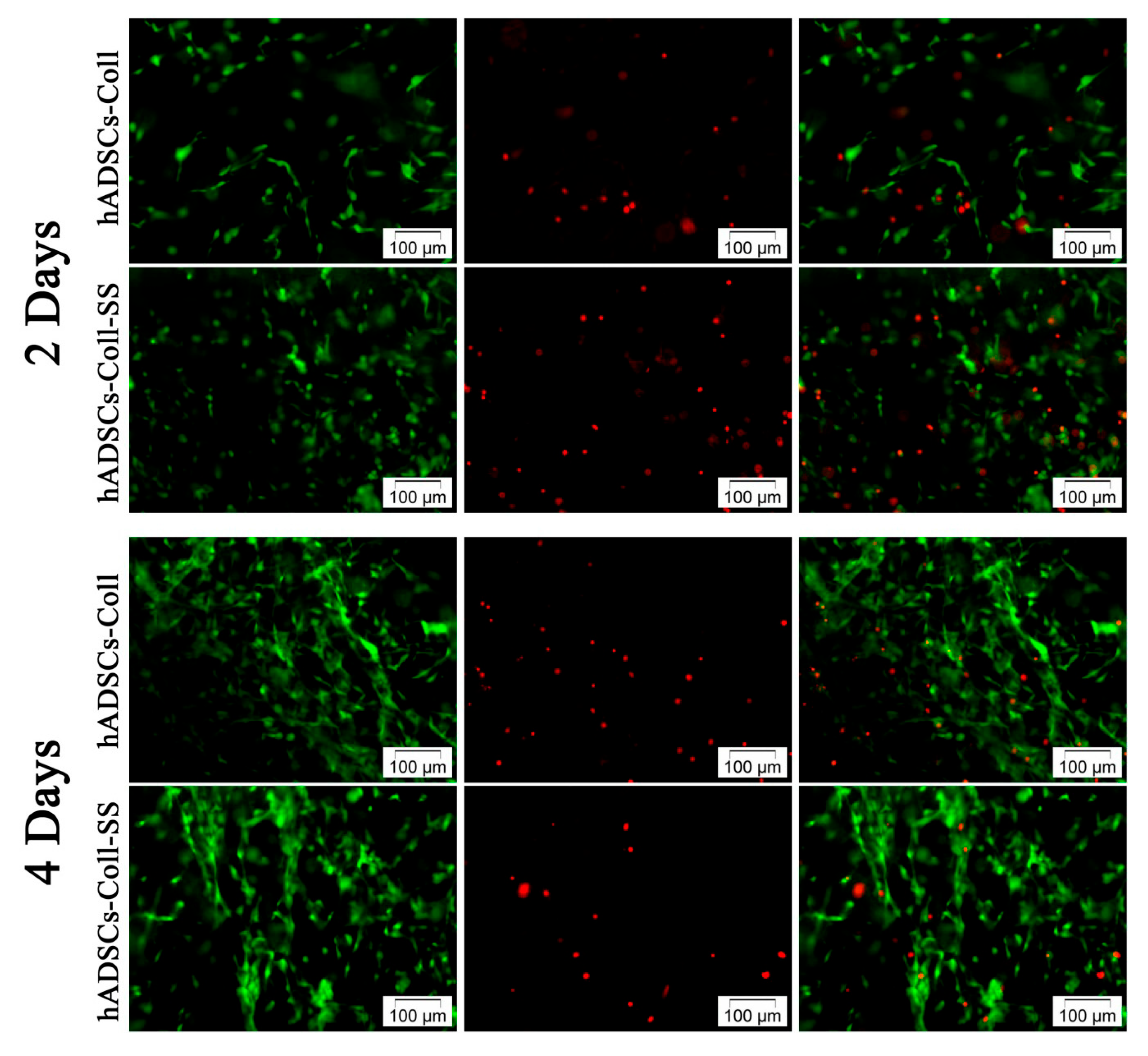
2.1.1. Qualitative Evaluation of hADSCs’ Viability and Proliferation Potential in Coll and Coll-SS
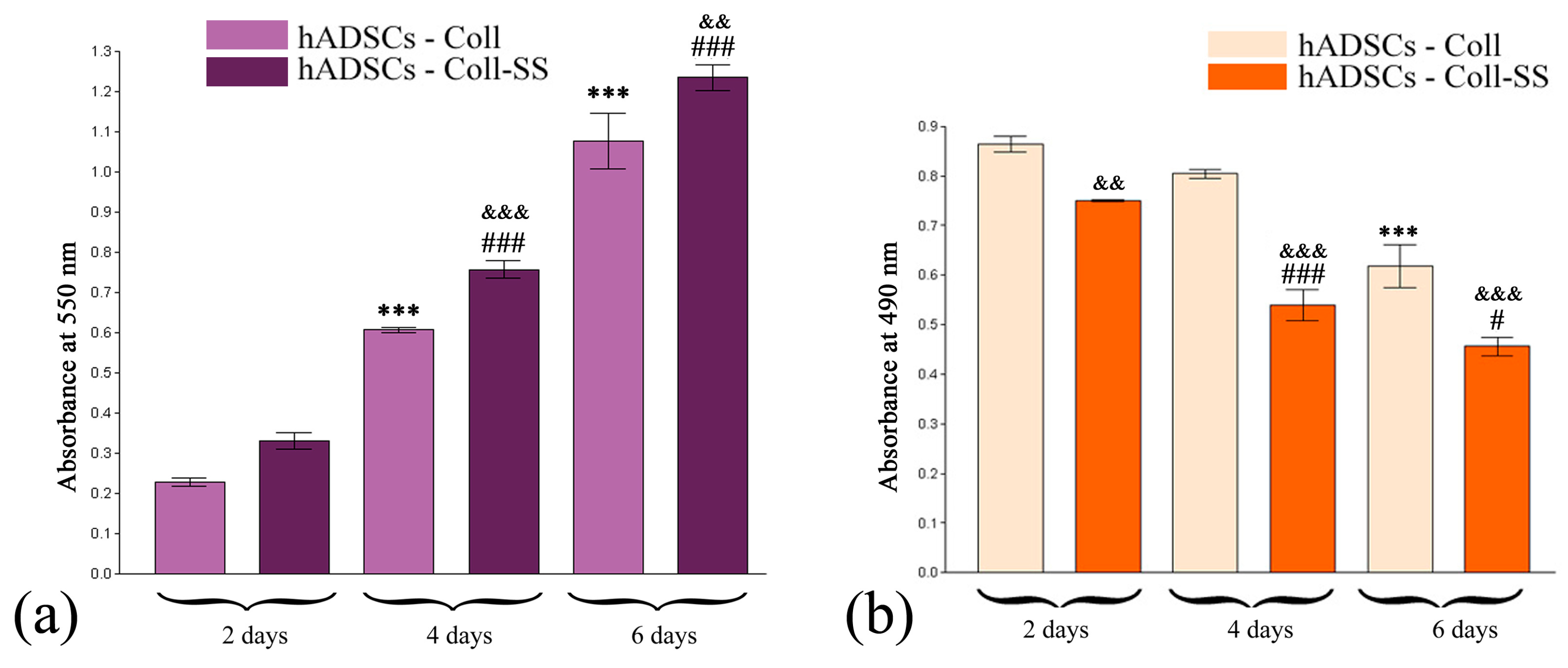
2.1.2. Quantitative Evaluation of hADSCs’ Viability and Proliferation Potential in Coll and Coll-SS
2.1.3. Cytotoxic Potential of Coll and Coll-SS on hADSCs
2.2. Assessment of hADSCs Adipogenic Differentiation Potential in Coll and Coll-SS Biomatrices
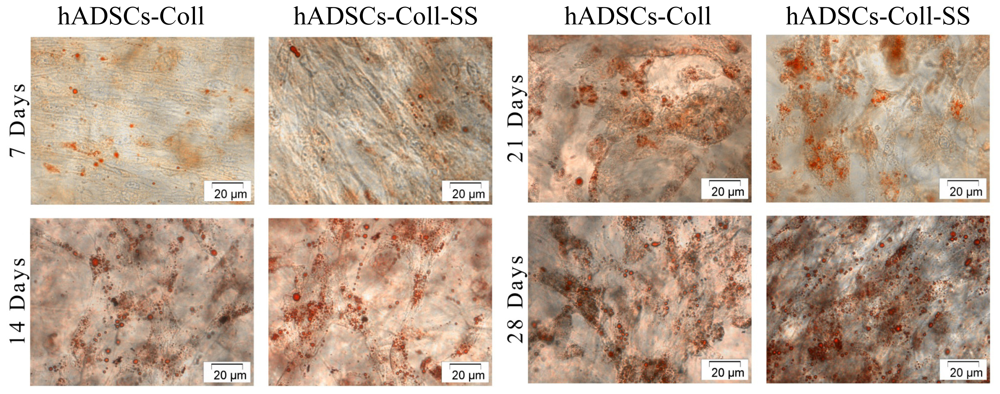
2.2.1. Morphological Evaluation of hADSCs-Scaffold Bioconstructs during Adipogenesis
2.2.2. Evaluation of the Intracellular Lipid Droplet Accumulation
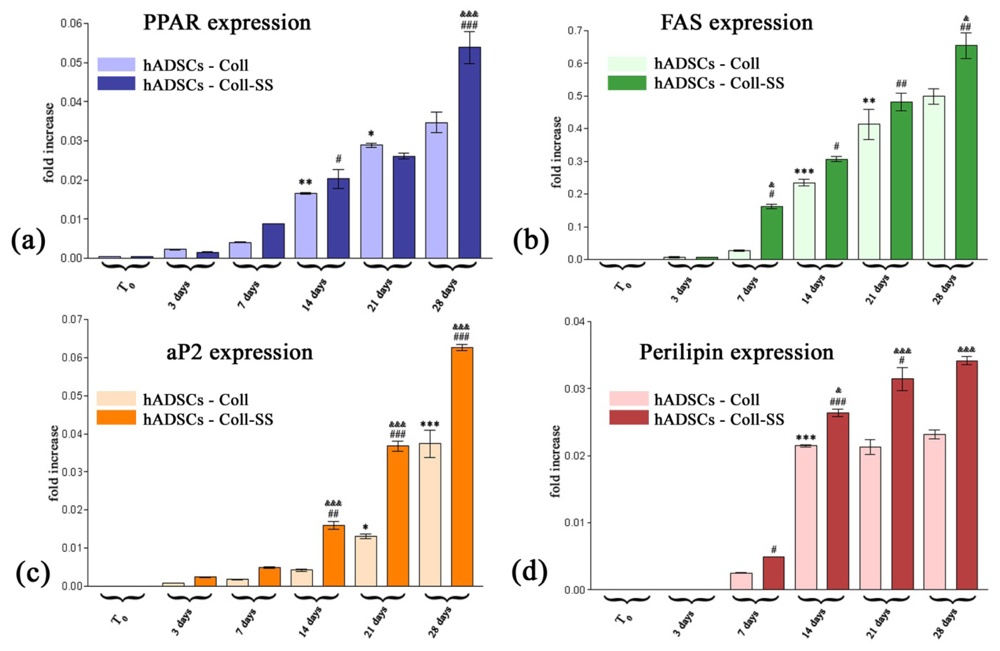
2.2.3. PPARγ2, FAS, aP2 and Perilipin Gene Expression Profiles
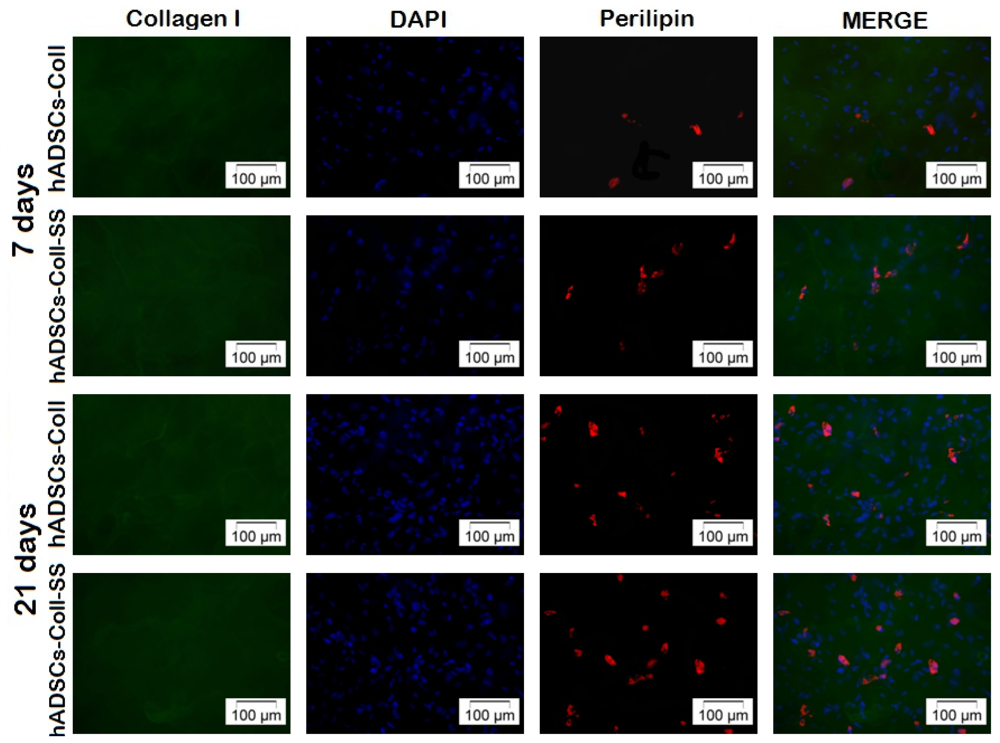
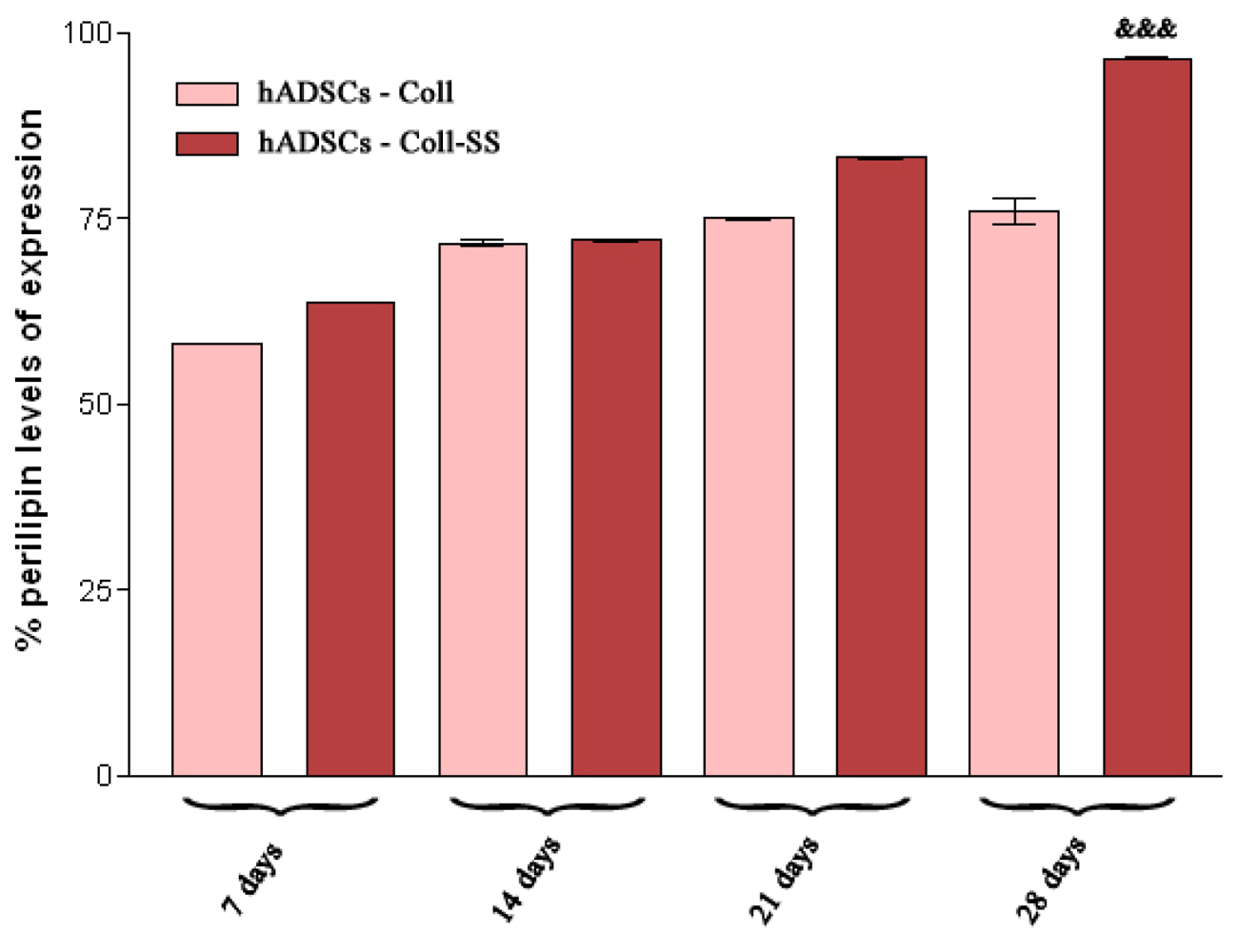
2.2.4. Late Adipogenic Marker Perilipin Protein Expression during Adipogenesis in hADSCs-Coll and hADSCs-Coll-SS Bioconstructs
3. Experimental Section
3.1. Cell Culture Model
3.2. Preparation of 3D hADSC Cultures within Coll and Coll-SS Biomatrices
3.3. Biocompatibility Assessment
3.3.1. Live/Dead Fluorescence Microscopy Assay
3.3.2. MTT Spectrophotometric Test
3.3.3. LDH Spectrophotometric Assay
3.4. hADSCs Adipogenic Potential in Coll and Coll-SS Biomatrices
3.4.1. SEM of Collagen-Based Scaffolds Populated with hADSCs during Adipogenesis
3.4.2. Oil Red O Staining for Lipid Droplet Accumulation
3.4.3. RealTime Quantification for PPARγ2, FAS, aP2 and Perilipin Adipogenic Markers
3.4.4. Qualitative and Quantitative Detection of Perilipin Late Adipogenic Marker
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Gomillion, C.T.; Burg, K.J.L. Stem cells and adipose tissue engineering. Biomaterials 2006, 27, 6052–6063. [Google Scholar]
- Weiser, B.; Neubauer, M.; Goepferich, A.; Blunk, T. Tissue Engineering, Fat. In Encyclopedia of Biomaterials and Biomedical Engineering, 2nd ed; Bowlin, G.L., Wnek, G.E., Eds.; Marcel Dekker Inc: New York, NY, USA, 2005; Volume 4, pp. 2725–2736. [Google Scholar]
- Patrick, C.W., Jr. Adipose tissue engineering: The future of breast and soft tissue reconstruction following tumor resection. Semin. Surg. Oncol. 2000, 19, 302–311. [Google Scholar]
- Tanzi, M.C.; Farè, S. Adipose tissue engineering: State of the art, recent advances and innovative approaches. Expert Rev. Med. Devices 2009, 6, 533–551. [Google Scholar]
- Katz, A.J.; Llull, R.; Hedrick, M.H.; Futrell, J.W. Emerging approaches to the tissue engineering of fat. Clin. Plast. Surg 1999, 26, 587–603. [Google Scholar]
- Patrick, C.W., Jr. Tissue engineering strategies for adipose tissue repair. Anat. Rec. 2001, 263, 361–366. [Google Scholar]
- Ebrahimian, T.G.; Pouzoulet, F.; Squiban, C.; Buard, V.; André, M.; Cousin, B.; Gourmelon, P.; Benderitter, M.; Casteilla, L.; Tamarat, R. Cell therapy based on adipose tissue-derived stromal cells promotes physiological and pathological wound healing. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 503–510. [Google Scholar]
- Blanton, M.W.; Hadad, I.; Johnstone, B.H.; Mund, J.A.; Rogers, P.I.; Eppley, B.L.; March, K.L. Adipose stromal cells and platelet-rich plasma therapies synergistically increase revascularization during wound healing. Plast. Reconstr. Surg 2009, 123, 56S–64S. [Google Scholar]
- Kim, W.S.; Park, B.S.; Sung, J.H.; Yang, J.M.; Park, S.B.; Kwak, S.J.; Park, J.S. Wound healing effect of adipose-derived stem cells: A critical role of secretory factors on human dermal fibroblasts. J. Dermatol. Sci 2007, 48, 15–24. [Google Scholar]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004, 109, 1292–1298. [Google Scholar]
- Badillo, A.T.; Redden, R.A.; Zhang, L.; Doolin, E.J.; Liechty, K.W. Treatment of diabetic wounds with fetal murine mesenchymal stromal cells enhances wound closure. Cell Tissue Res 2007, 329, 301–311. [Google Scholar]
- Glowacki, J.; Mizuno, S. Collagen scaffolds for tissue engineering. Biopolymers 2008, 89, 338–344. [Google Scholar]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar]
- Terada, S.; Nishimura, T.; Sasaki, M.; Yamada, H.; Miki, M. Sericin, a protein derived from silkworms, accelerates the proliferation of several mammalian cell lines including a hybridoma. Cytotechnology 2002, 40, 3–12. [Google Scholar]
- Tsubouchi, K.; Igarashi, Y.; Takasu, Y.; Yamada, H. Sericin enhances attachment of cultured human skin fibroblasts. Biosci. Biotechnol. Biochem 2005, 69, 403–405. [Google Scholar]
- Terada, S.; Sasaki, M.; Yanagihara, K.; Yamada, H. Preparation of silk protein sericin as mitogenic factor for better mammalian cell culture. J. Biosci. Bioeng 2005, 100, 667–671. [Google Scholar]
- Aramwit, P.; Kanokpanont, S.; De-Eknamkul, W.; Kamei, K.; Srichana, T. The effect of sericin with variable amino-acid content from different silk strains on the production of collagen and nitric oxide. J. Biomater. Sci. Polym. Ed 2009, 20, 1295–1306. [Google Scholar]
- Aramwit, P.; Sangcakul, A. The effects of sericin cream on wound healing in rats. Biosci. Biotechnol. Biochem 2007, 71, 2473–2477. [Google Scholar]
- Aramwit, P.; Kanokpanont, S.; Nakpheng, T.; Srichana, T. The effects of sericin from various extraction methods on cell viability and collagen production. Int. J. Mol. Sci 2010, 11, 2200–2211. [Google Scholar]
- Nazarov, R.; Jin, H.J.; Kaplan, D.L. Porous 3-D scaffolds from regenerated silk fibroin. Biomacromolecules 2004, 5, 718–726. [Google Scholar]
- Zhang, Y.Q.; Ma, Y.; Xia, Y.Y.; Shen, W.D.; Mao, J.P.; Xue, R.Y. Silk sericin-insulin bioconjugates: Synthesis, characterization and biological activity. J. Control. Release 2006, 115, 307–315. [Google Scholar]
- Zhang, Y.Q.; Tao, M.L.; Shen, W.D.; Mao, J.P.; Chen, Y.H. Synthesis of silk sericin peptides-L-asparaginase (SS-ASNase) bioconjugates and their characterization. J. Chem. Technol. Biotechnol 2006, 81, 136–145. [Google Scholar]
- Aramwit, P.; Siritientong, T.; Kanokpanont, S.; Srichana, T. Formulation and characterization of silk sericin-PVA scaffold crosslinked with genipin. Int. J. Biol. Macromol 2010, 47, 668–675. [Google Scholar]
- Mandal, B.B.; Priya, A.S.; Kundu, S.C. Novel silk sericin/gelatin 3-D scaffolds and 2-D films: Fabrication and characterization for potential tissue engineering applications. Acta Biomater 2009, 5, 3007–3020. [Google Scholar]
- Lungu, A.; Albu, M.G.; Stancu, I.C.; Florea, N.M.; Vasile, E.; Iovu, H. Superporous collagen-sericin scaffolds. J. Appl. Polym. Sci. 2012. [Google Scholar] [CrossRef]
- Siritientong, T.; Srichana, T.; Aramwit, P. The effect of sterilization methods on the physical properties of silk sericin scaffolds. AAPS PharmSciTech 2011, 12, 771–781. [Google Scholar]
- Spiegelman, B.M. PPARγ: Adipogenic regulator and thiazolidinedione receptor. Diabetes 1998, 4, 507–514. [Google Scholar]
- Rosen, E.D.; Spiegelman, B.M. PPARγ: A nuclear regulator of metabolism, differentiation, and cell growth. J. Biol. Chem 2001, 276, 37731–37734. [Google Scholar]
- Fajas, L.; Fruchart, J.C.; Auwerx, J. Transcriptional control of adipogenesis. Curr. Opin. Cell Biol 1998, 10, 165–173. [Google Scholar]
- Galateanu, B.; Dinescu, S.; Cimpean, A.; Dinischiotu, A.; Costache, M. Modulation of adipogenic conditions for prospective use of hADSCs in adipose tissue engineering. Int. J. Mol. Sci. 2012, 13, 15881–15900. [Google Scholar]
- Kundu, B.; Kundu, S.C. Silk sericin/polyacrylamide in situ forming hydrogels for dermal reconstruction. Biomaterials 2012, 33, 7456–7467. [Google Scholar]
- Ma, J.; Holden, K.; Zhu, J.; Pan, H.; Li, Y. The application of three-dimensional collagen-scaffolds seeded with myoblasts to repair skeletal muscle defects. J. Biomed. Biotechnol 2011, 2011, 812135. [Google Scholar]
- Nagura, M.; Ohnishi, R.; Gitoh, Y.; Ohkoshi, Y. Structures and physical properties of crosslinked sericin membranes. J. Insect Biotechnol. Sericol 2001, 70, 149–153. [Google Scholar]
- Archer, H.G.; Barnett, S.; Irving, S.; Middleton, K.R.; Seal, D.V. A controlled model of moist wound healing: Comparison between semi-permeable film, antiseptics and sugar paste. J. Exp. Pathol. 1990, 71, 155–170. [Google Scholar]
- Gnecchi, M.; Zhang, Z.; Ni, A.; Dzau, V.J. Paracrine mechanisms in adult stem cell signaling and therapy. Circ. Res 2008, 103, 1204–1219. [Google Scholar]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar]
- Galateanu, B.; Dimonie, D.; Vasile, E.; Nae, S.; Cimpean, A.; Costache, M. Layer-shaped alginate hydrogels enhance the biological performance of human adipose-derived stem cells. BMC Biotechnol 2012, 12, 35. [Google Scholar]
- Albu, M.G. Collagen Gels and Matrices for Biomedical Applications, 1st ed; Lambert Academic Publishing: Saarbrücken, Germany, 2011. [Google Scholar]


| Target | Nucleotidic sequence | Fragment length |
|---|---|---|
| PPARγ2 | F 5′-TTACACAATGCTGGCCTCCTT-3′ | 99 bp |
| PPARγ2 | R 5′-AGGCTTTCGCAGGCTCTTTAG-3′ | |
| aP2 | F 5′-ATGGGATGGAAAATCAACCA-3′ | 104 bp |
| aP2 | R 5′-GTGGAAGTGACGCCTTTCAT-3′ | |
| Perilipin F | 5′-ATGCTTCCAGAAGACCTACA-3′ | 224 bp |
| Perilipin R | 5′-CAGCTCAGAAGCAATCTTTT-3′ | |
| FAS F | 5′-GCTGGAAGTCACCTATGAAG-3′ | 205 bp |
| FAS R | 5′-TGAAGTCGAAGAAGAAGGAG-3′ | |
| GAPDH F | 5′-AAGGTCGGAGTCAACGGATT-3′ | 224 bp |
| GAPDH R | 5′-CTCCTGGAAGATGGTGATGG-3′ | |
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dinescu, S.; Galateanu, B.; Albu, M.; Cimpean, A.; Dinischiotu, A.; Costache, M. Sericin Enhances the Bioperformance of Collagen-Based Matrices Preseeded with Human-Adipose Derived Stem Cells (hADSCs). Int. J. Mol. Sci. 2013, 14, 1870-1889. https://doi.org/10.3390/ijms14011870
Dinescu S, Galateanu B, Albu M, Cimpean A, Dinischiotu A, Costache M. Sericin Enhances the Bioperformance of Collagen-Based Matrices Preseeded with Human-Adipose Derived Stem Cells (hADSCs). International Journal of Molecular Sciences. 2013; 14(1):1870-1889. https://doi.org/10.3390/ijms14011870
Chicago/Turabian StyleDinescu, Sorina, Bianca Galateanu, Madalina Albu, Anisoara Cimpean, Anca Dinischiotu, and Marieta Costache. 2013. "Sericin Enhances the Bioperformance of Collagen-Based Matrices Preseeded with Human-Adipose Derived Stem Cells (hADSCs)" International Journal of Molecular Sciences 14, no. 1: 1870-1889. https://doi.org/10.3390/ijms14011870
APA StyleDinescu, S., Galateanu, B., Albu, M., Cimpean, A., Dinischiotu, A., & Costache, M. (2013). Sericin Enhances the Bioperformance of Collagen-Based Matrices Preseeded with Human-Adipose Derived Stem Cells (hADSCs). International Journal of Molecular Sciences, 14(1), 1870-1889. https://doi.org/10.3390/ijms14011870









