Generation of a Highly Reactive Chicken-Derived Single-Chain Variable Fragment against Fusarium verticillioides by Phage Display
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antigen Preparation and Immunization
2.2. Selection of Phage Clones Reactive to Antigens by Phage Display
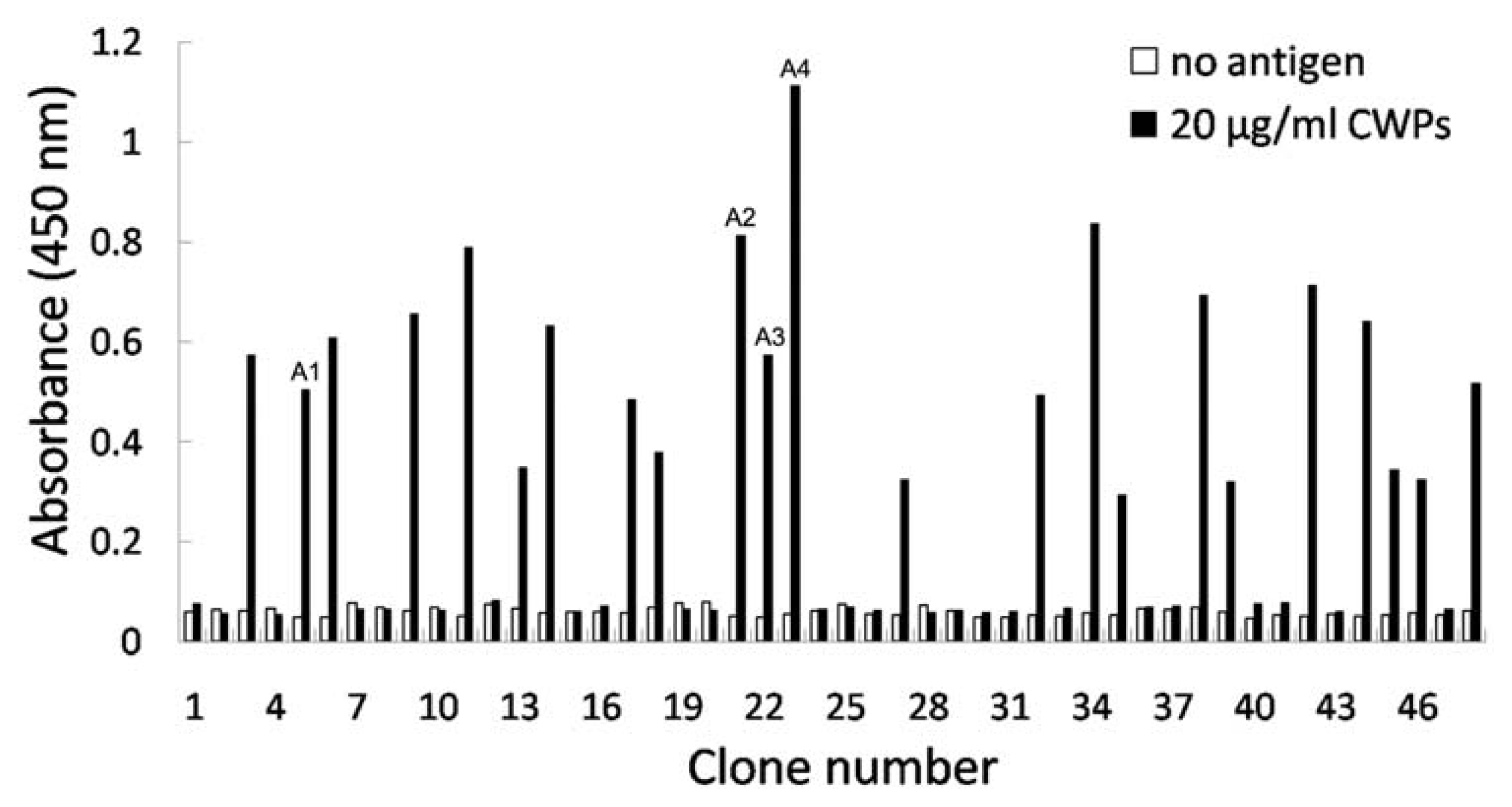
2.3. Identification of Phage Clones by Phage ELISA
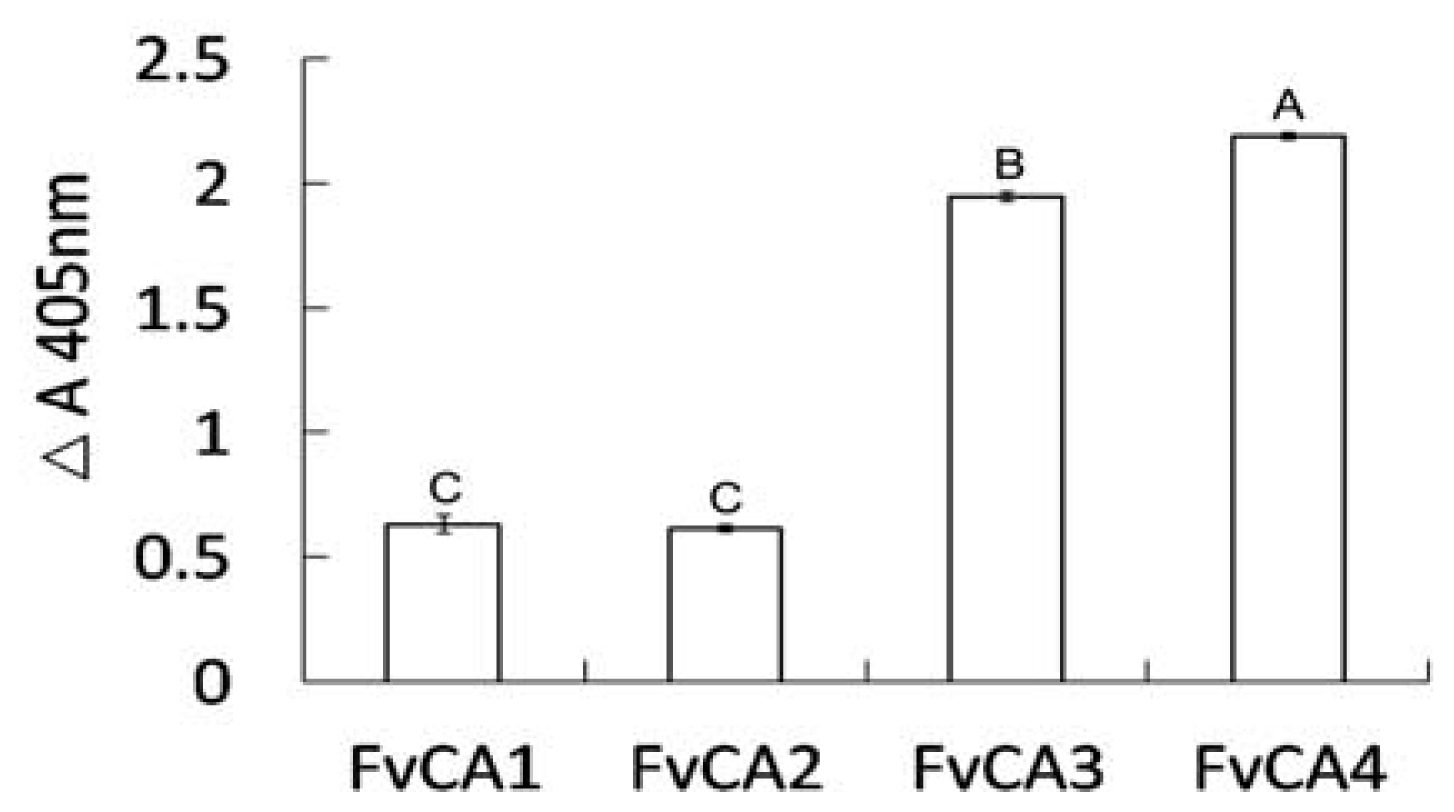
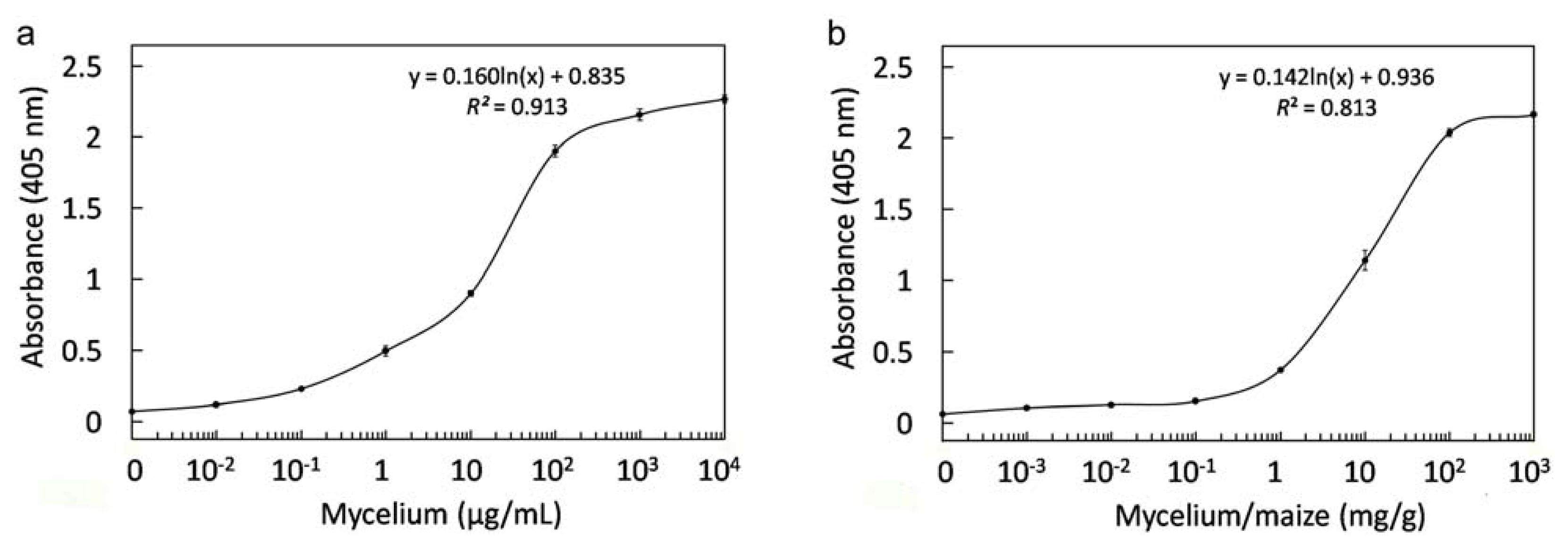
2.4. Antigen Binding of Soluble Antibodies
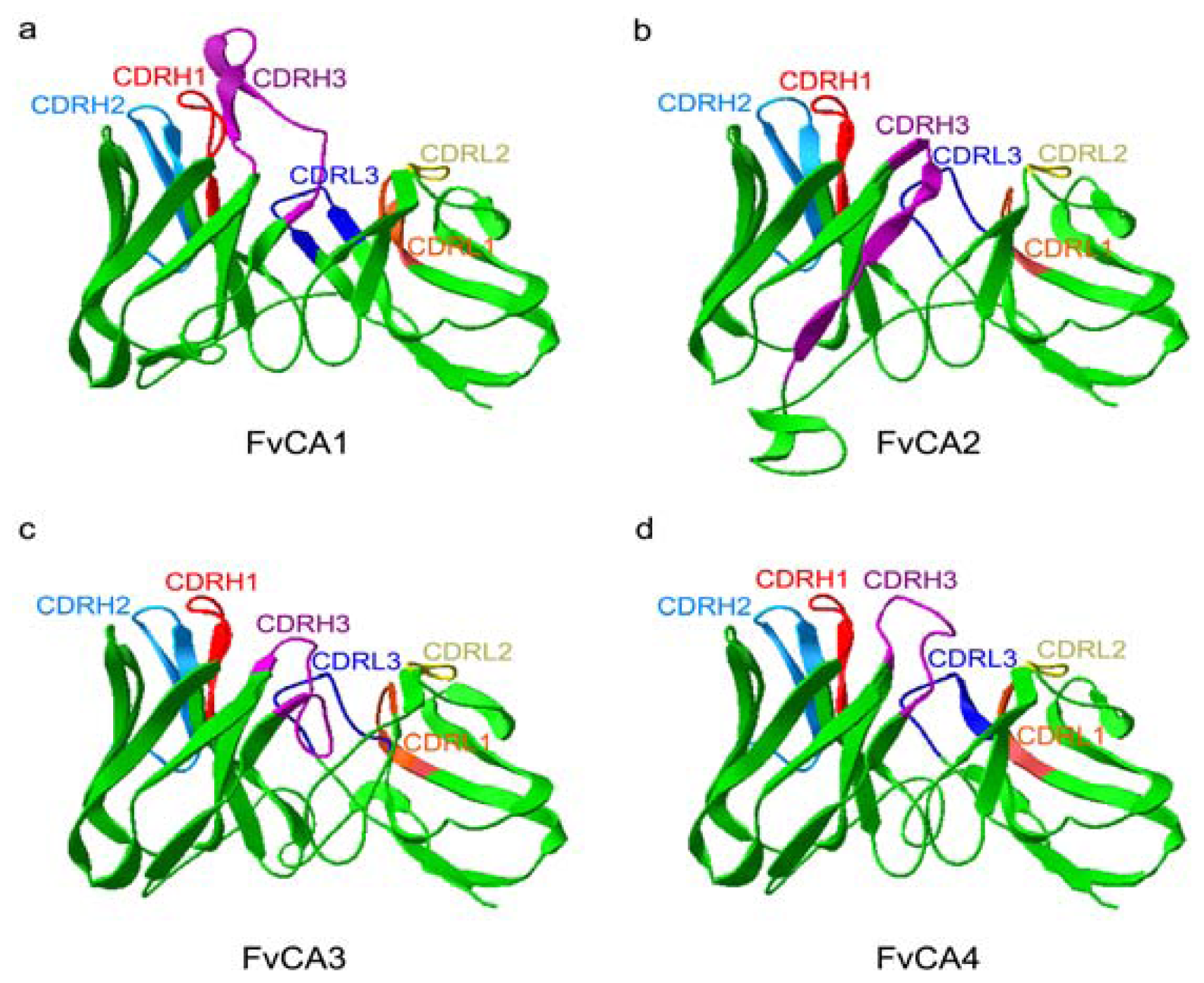
2.5. Three-Dimension Structures of scFv Antibodies
2.6. Characterization of FvCA4 Antibody
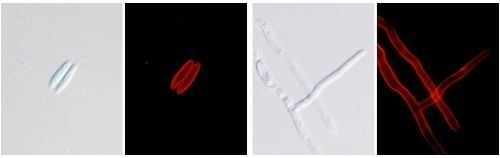
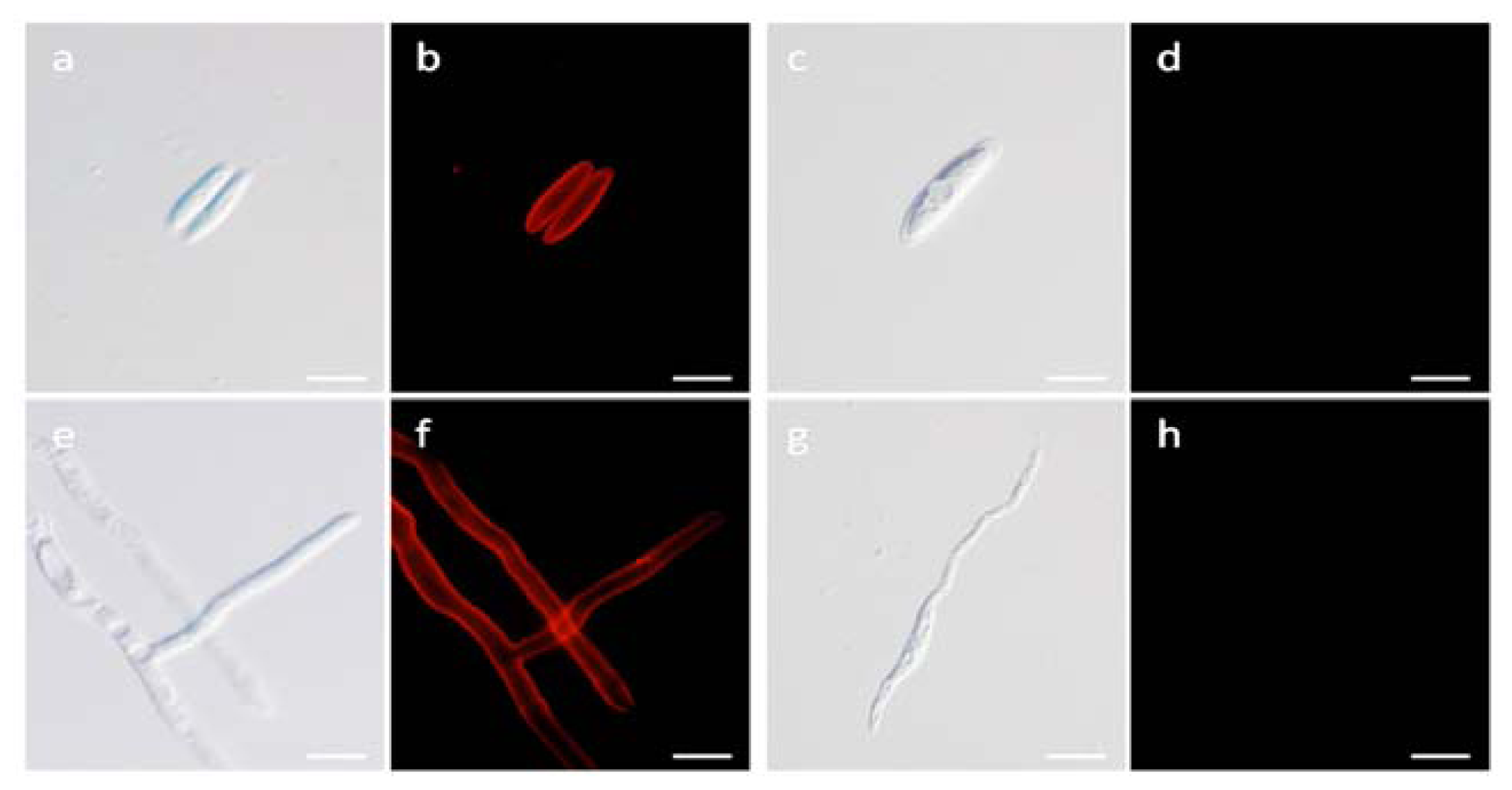
2.7. Localization Labeling of FvCA4 Antibody Binding
2.8. Immunological Detection of Mycelium and Contaminated Samples
3. Experimental Section
3.1. Antigen Preparation and Immunization
3.2. Library Construction
3.3. Panning
3.4. Phage ELISA
3.5. Sequencing
3.6. Expression and Purification
3.7. ELISA Assays
3.8. Immunoblot Analyses
3.9. Immunofluorescence Microscopy
3.10. Sample Preparation for Immunological Detection
4. Conclusions
Acknowledgments
References
- Bacon, C.W.; Williamson, J.W. Interactions of Fusarium moniliforme, its metabolites and bacteria with corn. Mycopathologia 1992, 117, 65–71. [Google Scholar]
- Desjardins, A.E. Gibberella from A (venaceae) to Z (eae). Annu. Rev. Phytopathol 2003, 41, 177–198. [Google Scholar]
- Nelson, P.E.; Desjardins, A.E.; Plattner, R.D. Fumonisins, mycotoxins produced by Fusarium species: Biology, chemistry, and significance. Annu. Rev. Phytopathol 1993, 31, 233–252. [Google Scholar]
- Wang, E.; Norred, W.P.; Bacon, C.W.; Riley, R.T.; Merrill, A.H., Jr. Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme. J. Biol. Chem. 1991, 266, 14486–14490. [Google Scholar]
- Yoo, H.S.; Norred, W.P.; Wang, E.; Merrill, A.H., Jr; Riley, R.T. Fumonisin inhibition of de novo sphingolipid biosynthesis and cytotoxicity are correlated in LLC-PK1 cells. Toxicol. Appl. Pharmacol. 1992, 114, 9–15. [Google Scholar]
- Voss, K.A.; Plattner, R.D.; Riley, R.T.; Meredith, F.I.; Norred, W.P. In vivo effects of fumonisin B1-producing and fumonisin B1-nonproducing Fusarium moniliforme isolates are similar: Fumonisins B2 and B3 cause hepato- and nephrotoxicity in rats. Mycopathologia 1998, 141, 45–58. [Google Scholar]
- Voss, K.A.; Riley, R.T.; Norred, W.P.; Bacon, C.W.; Meredith, F.I.; Howard, P.C.; Plattner, R.D.; Collins, T.F.; Hansen, D.K.; Porter, J.K. An overview of rodent toxicities: Liver and kidney effects of fumonisins and Fusarium moniliforme. Environ. Health Perspect 2001, 109, S259–S266. [Google Scholar]
- Odhav, B.; Adam, J.K.; Bhoola, K.D. Modulating effects of fumonisin B1 and ochratoxin A on leukocytes and messenger cytokines of the human immune system. Int. Immunopharmacol 2008, 8, 799–809. [Google Scholar]
- Stockmann-Juvala, H.; Alenius, H.; Savolainen, K. Effects of fumonisin B1 on the expression of cytokines and chemokines in human dendritic cells. Food Chem. Toxicol 2008, 46, 1444–1451. [Google Scholar]
- Carlson, D.B.; Williams, D.E.; Spitsbergen, J.M.; Ross, P.F.; Bacon, C.W.; Meredith, F.I.; Riley, R.T. Fumonisin B1 promotes aflatoxin B1 and N-methyl-N′-nitro-nitrosoguanidine-initiated liver tumors in rainbow trout. Toxicol. Appl. Pharmacol 2001, 172, 29–36. [Google Scholar]
- Sun, G.; Wang, S.; Hu, X.; Su, J.; Zhang, Y.; Xie, Y.; Zhang, H.; Tang, L.; Wang, J.S. Co-contamination of aflatoxin B1 and fumonisin B1 in food and human dietary exposure in three areas of China. Food Addit. Contam. A 2011, 28, 461–470. [Google Scholar]
- Rheeder, J.P.; Marasas, W.F.O.; Thiel, P.G.; Sydenham, E.W.; Shephard, G.S.; van Schalkwyk, D.J. Fusarium moniliforme and fumonisins in corn in relation to human esophageal cancer in Transkei. Phytopathology 1992, 82, 353–357. [Google Scholar]
- Myburg, R.B.; Dutton, M.F.; Chuturgoon, A.A. Cytotoxicity of fumonisin B1, diethylnitrosamine, and catechol on the SNO esophageal cancer cell line. Environ. Health Perspect 2002, 110, 813–815. [Google Scholar]
- Sun, G.; Wang, S.; Hu, X.; Su, J.; Huang, T.; Yu, J.; Tang, L.; Gao, W.; Wang, J.S. Fumonisin B1 contamination of home-grown corn in high-risk areas for esophageal and liver cancer in China. Food Addit. Contam 2007, 24, 181–185. [Google Scholar]
- Ueno, Y.; Iijima, K.; Wang, S.D.; Sugiura, Y.; Sekijima, M.; Tanaka, T.; Chen, C.; Yu, S.Z. Fumonisins as a possible contributory risk factor for primary liver cancer: A 3-year study of corn harvested in Haimen, China, by HPLC and ELISA. Food Chem. Toxicol 1997, 35, 1143–1150. [Google Scholar]
- Yang, H.Q.; Lu, F.Y.; Hao, Y.K.; Dong, B. Situation analysis and development strategy of maize industry in China. Chin. Agric. Sci. Bull 2011, 27, 368–373. [Google Scholar]
- Wang, Z.; Liu, X.; Cong, L.; Li, X.; Tong, Z.; Cheng, S.; Ge, S. Characteristics of producing-fumonisin and dimorphic fungus of Fusarium moniliforme. Chin. J. Prev. Med 2000, 34, 44–46. [Google Scholar]
- Gong, H.Z.; Ji, R.; Li, Y.X.; Zhang, H.Y.; Li, B.; Zhao, Y.; Sun, L.; Yu, F.; Yang, J. Occurrence of fumonisin B1 in corn from the main corn-producing areas of china. Mycopathologia 2009, 167, 31–36. [Google Scholar]
- Parsons, M.W.; Munkvold, G.P. Associations of planting date, drought stress, and insects with Fusarium ear rot and fumonisin B1 contamination in California maize. Food Addit. Contam. A 2010, 27, 1–17. [Google Scholar]
- Samapundo, S.; Devliehgere, F.; de Meulenaer, B.; Debevere, J. Effect of water activity and temperature on growth and the relationship between fumonisin production and the radial growth of Fusarium verticillioides and Fusarium proliferatum on corn. J. Food Prot 2005, 68, 1054–1059. [Google Scholar]
- Miller, J.D. Factors that affect the occurrence of fumonisin. Environ. Health Perspect 2001, 109, S321–S324. [Google Scholar]
- Notermans, S.; Heuvelman, C.J. Immunological detection of moulds in food by using the enzyme-linked immunosorbent assay (ELISA); preparation of antigens. Int. J. Food Microbiol 1985, 2, 247–258. [Google Scholar]
- Lin, H.H.; Cousin, M.A. Evaluation of enzyme-linked immunosorbent assay for detection of molds in foods. J. Food Sci 1987, 52. [Google Scholar]
- Iyer, M.S.; Cousin, M.A. Immunological detection of Fusarium species in cornmeal. J. Food Prot 2003, 66, 451–456. [Google Scholar]
- Biazon, L.; Meirelles, P.G.; Ono, M.A.; Itano, E.N.; Taniwaki, M.H.; Sugiura, Y.; Ueno, Y.; Hirooka, E.Y.; Ono, E.Y.S. Development of polyclonal antibodies against Fusarium verticillioides exoantigens. Food Agric. Immunol 2006, 17, 69–77. [Google Scholar]
- De Ruiter, G.A.; van Bruggen-van der Lugt, A.W.; Bos, W.; Notermans, S.H.; Rombouts, F.M.; Hofstra, H. The production and partial characterization of a monoclonal IgG antibody specific for moulds belonging to the order Mucorales. J. Gen. Microbiol 1993, 139, 1557–1564. [Google Scholar]
- Hiatt, E.E., III; Hill, N.S.; Hiatt, E.N. Monoclonal antibodies incorporated into Neotyphodium coenophialum fungal cultures: Inhibition of fungal growth and stability of antibodies. Fungal Genet. Biol. 2001, 33, 107–114. [Google Scholar]
- Kwak, B.Y.; Shon, D.H.; Kwon, B.J.; Kweon, C.H.; Lee, K.H. Detection of Aspergillus and Penicillium genera by enzyme-linked immunosorbent assay using a monoclonal antibody. J. Microbiol. Biotechnol 2001, 11, 21–28. [Google Scholar]
- Thornton, C.R.; Slaughter, D.C.; Davis, R.M. Detection of the sour-rot pathogen Geotrichum candidum in tomato fruit and juice by using a highly specific monoclonal antibody-based ELISA. Int. J. Food Microbiol 2010, 143, 166–172. [Google Scholar]
- Yajima, W.; Rahman, M.H.; Das, D.; Suresh, M.R.; Kav, N.N. Detection of Sclerotinia sclerotiorum using a monomeric and dimeric single-chain fragment variable (scFv) antibody. J. Agric. Food Chem 2008, 56, 9455–9463. [Google Scholar]
- Yang, B.; Yajima, W.; Das, D.; Suresh, M.R.; Kav, N.N. Isolation, expression and characterization of two single-chain variable fragment antibodies against an endo-polygalacturonase secreted by Sclerotinia sclerotiorum. Protein Expr. Purif 2009, 64, 237–243. [Google Scholar]
- Peschen, D.; Li, H.P.; Fischer, R.; Kreuzaler, F.; Liao, Y.C. Fusion proteins comprising a Fusarium-specific antibody linked to antifungal peptides protect plants against a fungal pathogen. Nat. Biotechnol 2004, 22, 732–738. [Google Scholar]
- Li, H.P.; Zhang, J.B.; Shi, R.P.; Huang, T.; Fischer, R.; Liao, Y.C. Engineering Fusarium head blight resistance in wheat by expression of a fusion protein containing a Fusarium-specific antibody and an antifungal peptide. Mol. Plant Microbe Interact 2008, 21, 1242–1248. [Google Scholar]
- Yajima, W.; Verma, S.S.; Shah, S.; Rahman, M.H.; Liang, Y.; Kav, N.N. Expression of anti-sclerotinia scFv in transgenic Brassica napus enhances tolerance against stem rot. N. Biotechnol 2010, 27, 816–821. [Google Scholar]
- Hu, Z.Q.; Li, H.P.; Zhang, J.B.; Glinka, E.; Liao, Y.C. Antibody-mediated prevention of Fusarium mycotoxins in the field. Int. J. Mol. Sci 2008, 9, 1915–1926. [Google Scholar]
- Safarnejad, M.R.; Jouzani, G.S.; Tabatabaie, M.; Twyman, R.M.; Schillberg, S. Antibody-mediated resistance against plant pathogens. Biotechnol. Adv 2011, 29, 961–971. [Google Scholar]
- Winter, G.; Griffiths, A.D.; Hawkins, R.E.; Hoogenboom, H.R. Making antibodies by phage display technology. Annu. Rev. Immunol 1994, 12, 433–455. [Google Scholar]
- Holliger, P.; Hudson, P.J. Engineered antibody fragments and the rise of single domains. Nat. Biotechnol 2005, 23, 1126–1136. [Google Scholar]
- Fukuda, I.; Kojoh, K.; Tabata, N.; Doi, N.; Takashima, H.; Miyamoto-Sato, E.; Yanagawa, H. In vitro evolution of single-chain antibodies using mRNA display. Nucleic Acids Res 2006, 34, e127. [Google Scholar]
- Liu, J.L.; Hu, Z.Q.; Xing, S.; Xue, S.; Li, H.P.; Zhang, J.B.; Liao, Y.C. Attainment of 15-fold higher affinity of a Fusarium-specific single-chain antibody by directed molecular evolution coupled to phage display. Mol. Biotechnol 2011. [Google Scholar] [CrossRef]
- Davies, E.L.; Smith, J.S.; Birkett, C.R.; Manser, J.M.; Anderson-Dear, D.V.; Young, J.R. Selection of specific phage-display antibodies using libraries derived from chicken immunoglobulin genes. J. Immunol. Methods 1995, 186, 125–135. [Google Scholar]
- Andris-Widhopf, J.; Rader, C.; Steinberger, P.; Fuller, R.; Barbas, C.F., III. Methods for the generation of chicken monoclonal antibody fragments by phage display. J. Immunol. Methods 2000, 242, 159–181. [Google Scholar]
- Yamanaka, H.I.; Inoue, T.; Ikeda-Tanaka, O. Chicken monoclonal antibody isolated by a phage display system. J. Immunol 1996, 157, 1156–1162. [Google Scholar]
- Foord, A.J.; Muller, J.D.; Yu, M.; Wang, L.F.; Heine, H.G. Production and application of recombinant antibodies to foot-and-mouth disease virus non-structural protein 3ABC. J. Immunol. Methods 2007, 321, 142–151. [Google Scholar]
- Reynaud, C.A.; Anquez, V.; Grimal, H.; Weill, J.C. A hyperconversion mechanism generates the chicken light chain preimmune repertoire. Cell 1987, 48, 379–388. [Google Scholar]
- Reynaud, C.A.; Dahan, A.; Anquez, V.; Weill, J.C. Somatic hyperconversion diversifies the single VH gene of the chicken with a high incidence in the D region. Cell 1989, 59, 171–183. [Google Scholar]
- McCormack, W.T.; Tjoelker, L.W.; Thompson, C.B. Immunoglobulin gene diversification by gene conversion. Prog. Nucleic Acid Res. Mol. Biol 1993, 45, 27–45. [Google Scholar]
- Woolley, J.A.; Landon, J. Comparison of antibody production to human interleukin-6 (IL-6) by sheep and chickens. J. Immunol. Methods 1995, 178, 253–265. [Google Scholar]
- Svendsen Bollen, L.; Crowley, A.; Stodulski, G.; Hau, J. Antibody production in rabbits and chickens immunized with human IgG. A comparison of titre and avidity development in rabbit serum, chicken serum and egg yolk using three different adjuvants. J. Immunol. Methods 1996, 191, 113–120. [Google Scholar]
- CPHmodels 3.2 Server. Available online: http://www.cbs.dtu.dk/services/CPHmodels/ accessed on 27 February 2012.
- Duquesnoy, R.J. A structurally based approach to determine HLA compatibility at the humoral immune level. Hum. Immunol 2006, 67, 847–862. [Google Scholar]
- Ohno, S.; Mori, N.; Matsunaga, T. Antigen-binding specificities of antibodies are primarily determined by seven residues of VH. Proc. Natl. Acad. Sci. USA 1985, 82, 2945–2949. [Google Scholar]
- Wu, T.T.; Johnson, G.; Kabat, E.A. Length distribution of CDRH3 in antibodies. Proteins 1993, 16, 1–7. [Google Scholar]
- Barrios, Y.; Jirholt, P.; Ohlin, M. Length of the antibody heavy chain complementarity determining region 3 as a specificity-determining factor. J. Mol. Recognit 2004, 17, 332–338. [Google Scholar]
- Kim, M.; Sun, Z.Y.; Rand, K.D.; Shi, X.; Song, L.; Cheng, Y.; Fahmy, A.F.; Majumdar, S.; Ofek, G.; Yang, Y.; et al. Antibody mechanics on a membrane-bound HIV segment essential for GP41-targeted viral neutralization. Nat. Struct. Mol. Biol 2011, 18, 1235–1243. [Google Scholar]
- Nuttall, S.D.; Irving, R.A.; Hudson, P.J. Immunoglobulin VH domains and beyond: Design and selection of single-domain binding and targeting reagents. Curr. Pharm. Biotechnol 2000, 1, 253–263. [Google Scholar]
- Jirholt, P.; Ohlin, M.; Borrebaeck, C.A.; Soderlind, E. Exploiting sequence space: Shuffling in vivo formed complementarity determining regions into a master framework. Gene 1998, 215, 471–476. [Google Scholar]
- Jirholt, P.; Strandberg, L.; Jansson, B.; Krambovitis, E.; Soderlind, E.; Borrebaeck, C.A.; Carlsson, R.; Danielsson, L.; Ohlin, M. A central core structure in an antibody variable domain determines antigen specificity. Protein Eng 2001, 14, 67–74. [Google Scholar]
- Schoonbroodt, S.; Steukers, M.; Viswanathan, M.; Frans, N.; Timmermans, M.; Wehnert, A.; Nguyen, M.; Ladner, R.C.; Hoet, R.M. Engineering antibody heavy chain CDR3 to create a phage display Fab library rich in antibodies that bind charged carbohydrates. J. Immunol 2008, 181, 6213–6221. [Google Scholar]
- Kang, A.S.; Jones, T.M.; Burton, D.R. Antibody redesign by chain shuffling from random combinatorial immunoglobulin libraries. Proc. Natl. Acad. Sci. USA 1991, 88, 11120–11123. [Google Scholar]
- Kathuria, S.; Sriraman, R.; Nath, R.; Sack, M.; Pal, R.; Artsaenko, O.; Talwar, G.P.; Fischer, R.; Finnern, R. Efficacy of plant-produced recombinant antibodies against HCG. Hum. Reprod 2002, 17, 2054–2061. [Google Scholar]
- Rodoni, B.C.; Dale, J.L.; Harding, R.M. Characterization and expression of the coat protein-coding region of banana bract mosaic potyvirus, development of diagnostic assays and detection of the virus in banana plants from five countries in southeast Asia. Arch. Virol 1999, 144, 1725–1737. [Google Scholar]
- Patiño, B.; Mirete, S.; González-Jaén, M.T.; Mulé, G.; Rodríguez, M.T.; Vázquez, C. PCR detection assay of fumonisin-producing Fusarium verticillioides strains. J. Food. Prot 2004, 67, 1278–1283. [Google Scholar]
- Schoffelmeer, E.A.; Klis, F.M.; Sietsma, J.H.; Cornelissen, B.J. The cell wall of Fusarium oxysporum. Fungal Genet. Biol 1999, 27, 275–282. [Google Scholar]
- Kirsch, M.; Zaman, M.; Meier, D.; Dubel, S.; Hust, M. Parameters affecting the display of antibodies on phage. J. Immunol. Methods 2005, 301, 173–185. [Google Scholar]
- Nossal, N.G.; Heppel, L.A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J. Biol. Chem 1966, 241, 3055–3062. [Google Scholar]



| Round | Input (pfu) | Output (pfu) | Ratio (%) |
|---|---|---|---|
| 1 | 2.6 × 1012 | 1.5 × 104 | 5.8 × 10−9 |
| 2 | 4.5 × 1012 | 6.0 × 104 | 1.3 × 10−8 |
| 3 | 5.4 × 1012 | 1.2 × 106 | 2.2 × 10−7 |
| Species | Host | Reactivity of FvCA4 |
|---|---|---|
| F. verticillioides | maize | +++ |
| F. verticillioides | rice | +++ |
| F. proliferatum | maize | +++ |
| F. proliferatum | potato | +++ |
| F. proliferatum | wheat | +++ |
| F. oxysporum f.sp. matthiolae | stock | +++ |
| F. oxysporum f.sp. zingiberi | turmeric | +++ |
| F. oxysporum f.sp. niveum | watermelon | +++ |
| F. poae | wheat | +++ |
| F. culmorum | wheat | +++ |
| F. asiaticum | wheat | ++ |
| F. solani | mung bean | +++ |
| F. tricinctum | wheat | +++ |
| A. flavus | peanut | − |
| S. sclerotiorum | rapeseed | − |
| R. cerealis | wheat | − |
| M. fructicola | peach | − |
| V. dahliae | cotton | − |
| Name | Nucleotide Sequence |
|---|---|
| VH-cDNA | 5’-CGGTGGGGGACATCTGAGTGGG-3’ |
| VL-cDNA | 5’-AGGGGTGGAGGACCTGCACCTC-3’ |
| CPDVHF | 5’-ATCTAGGCATCCCTTGGCCCAGCCGGCCATGGCTGCCGTGACGTTGGACGAGTCC-3’ |
| CHIC-Gly | 5’-GCCAGAGCCACCTCCGCCTGAACCGCCTCCACCTGAGGAGACGATGACCTCGGTCCC-3’ |
| CHIC-Ser | 5’-GGCGGAGGTGGCTCTGGCGGTGGCGGATCGGCGCTGACTCAGCCGTCCTCGGTG-3’ |
| CPDVLB | 5’-TGACCTGCGAGGATGCGCGGCCGCGTCGACGGGCTGGCCTAGGACGGTCAG-3’ |
| pHENpel | 5’-GCAGCCGCTGGATTGTTATTACTCGC-3’ |
| pHENmyc | 5’-ATTCAGATCCTCTTCTGAGATGAG-3’ |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hu, Z.-Q.; Liu, J.-L.; Li, H.-P.; Xing, S.; Xue, S.; Zhang, J.-B.; Wang, J.-H.; Nölke, G.; Liao, Y.-C. Generation of a Highly Reactive Chicken-Derived Single-Chain Variable Fragment against Fusarium verticillioides by Phage Display. Int. J. Mol. Sci. 2012, 13, 7038-7056. https://doi.org/10.3390/ijms13067038
Hu Z-Q, Liu J-L, Li H-P, Xing S, Xue S, Zhang J-B, Wang J-H, Nölke G, Liao Y-C. Generation of a Highly Reactive Chicken-Derived Single-Chain Variable Fragment against Fusarium verticillioides by Phage Display. International Journal of Molecular Sciences. 2012; 13(6):7038-7056. https://doi.org/10.3390/ijms13067038
Chicago/Turabian StyleHu, Zu-Quan, Jin-Long Liu, He-Ping Li, Shu Xing, Sheng Xue, Jing-Bo Zhang, Jian-Hua Wang, Greta Nölke, and Yu-Cai Liao. 2012. "Generation of a Highly Reactive Chicken-Derived Single-Chain Variable Fragment against Fusarium verticillioides by Phage Display" International Journal of Molecular Sciences 13, no. 6: 7038-7056. https://doi.org/10.3390/ijms13067038
APA StyleHu, Z.-Q., Liu, J.-L., Li, H.-P., Xing, S., Xue, S., Zhang, J.-B., Wang, J.-H., Nölke, G., & Liao, Y.-C. (2012). Generation of a Highly Reactive Chicken-Derived Single-Chain Variable Fragment against Fusarium verticillioides by Phage Display. International Journal of Molecular Sciences, 13(6), 7038-7056. https://doi.org/10.3390/ijms13067038