Perforin Expression by CD4+ Regulatory T Cells Increases at Multiple Sclerosis Relapse: Sex Differences
Abstract
:1. Introduction
2. Results and Discussion
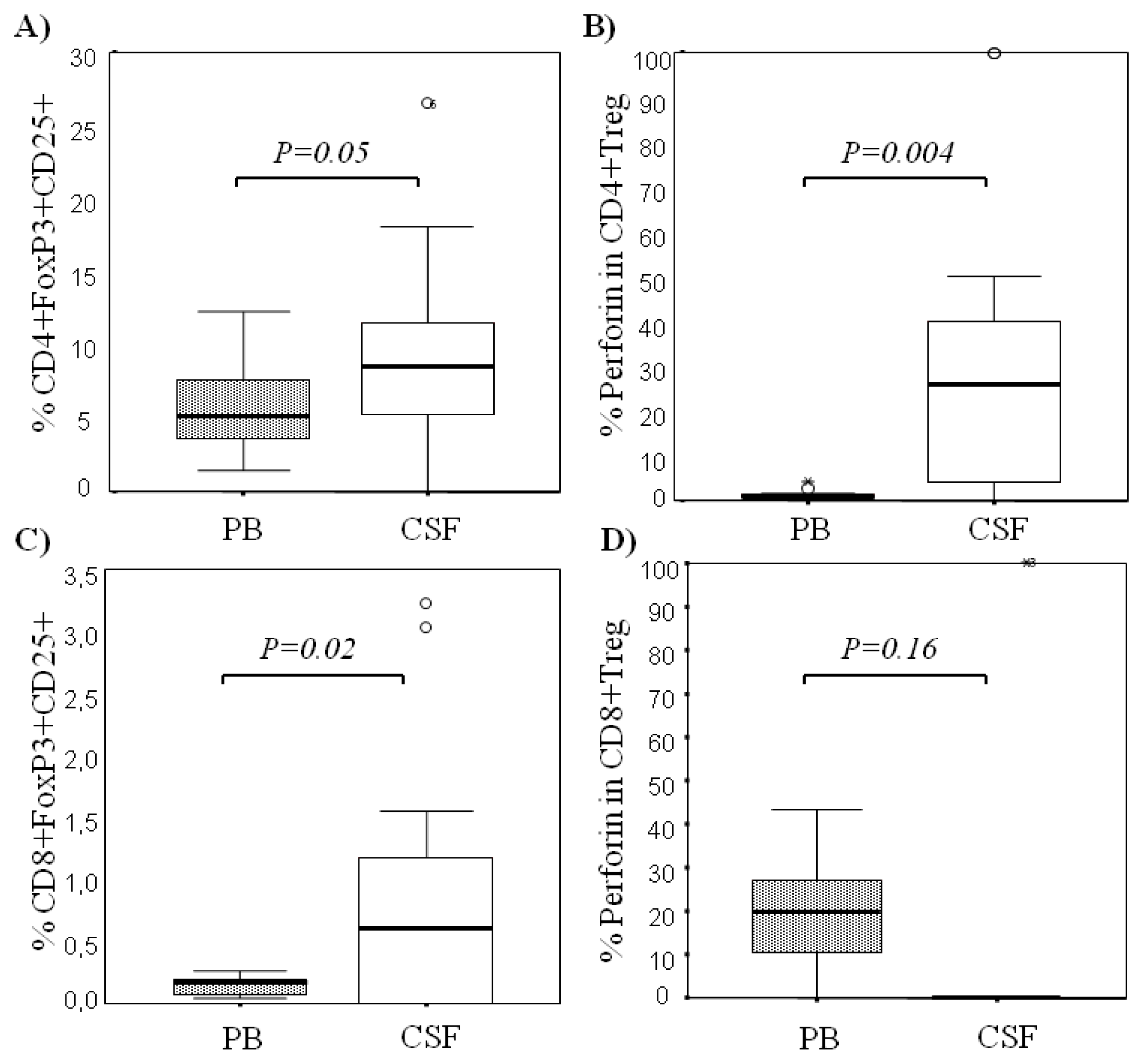
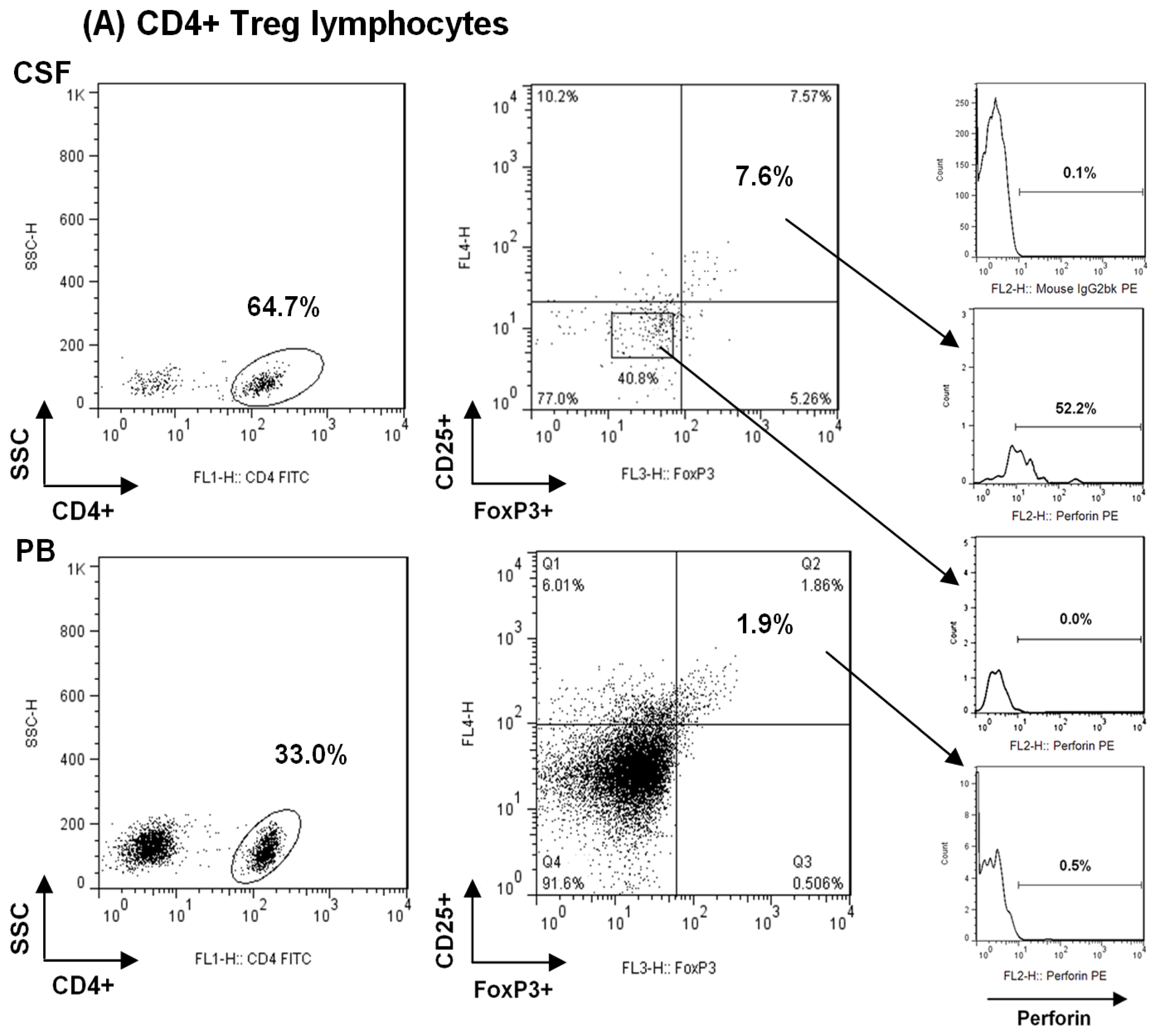
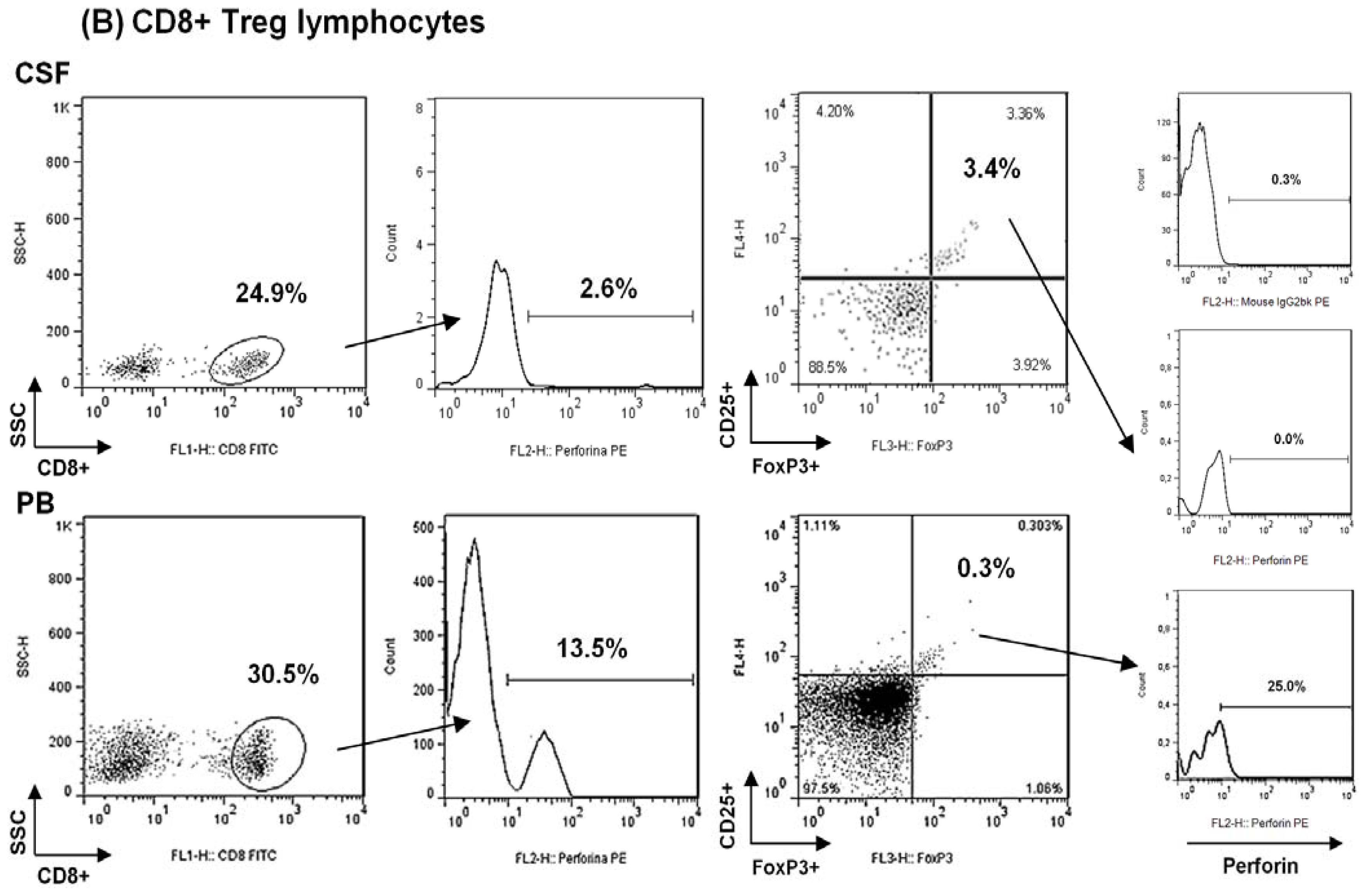
2.1. Comparison among Proportions of Regulatory CD4+ and CD8+ T-Cells Subsets in CSF and Peripheral Blood in MS Patients at First Clinical Relapse
2.2. Comparison of Regulatory T-Cells and Their Content of Perforin in Peripheral Blood between MS Patients and Healthy Controls
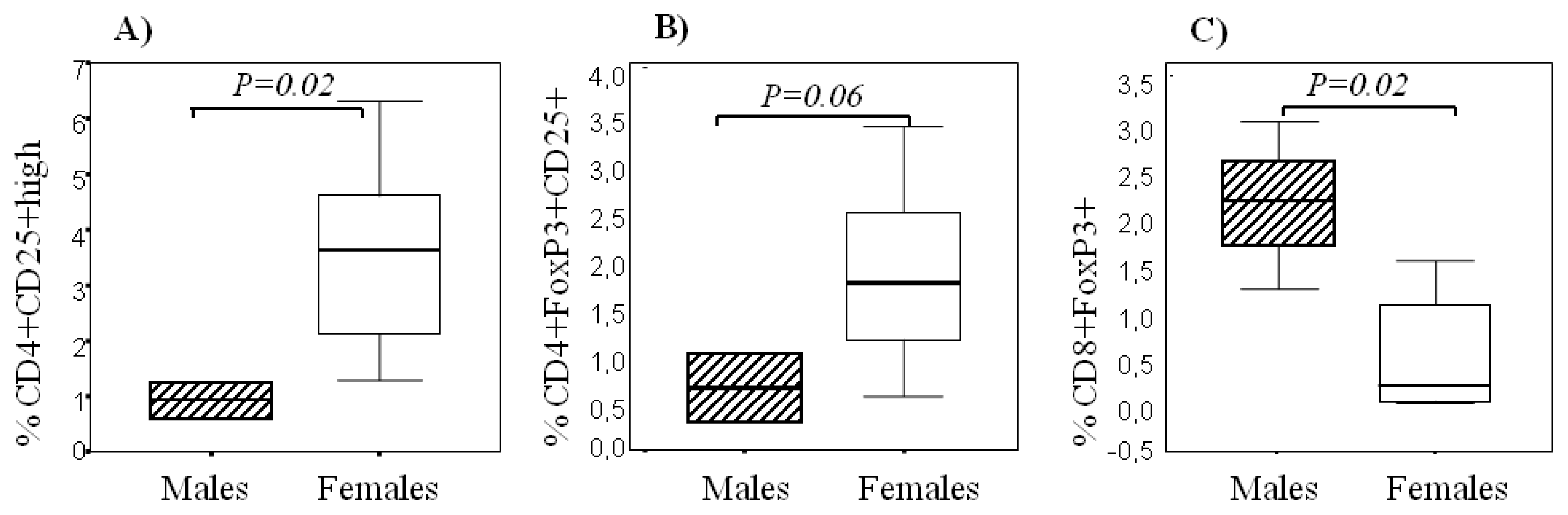
2.3. Sexual Dimorphism in Regulatory T Cells
2.4. Sex Hormones and Cytokine Levels
3. Experimental Section
3.1. Patients
3.2. Blood and CSF Sampling
3.3. Characterization and Quantitative Analysis of Regulatory CD4+ T Lymphocytes
Analysis of Regulatory CD8+ T Lymphocytes
3.4. Sex Hormones and Cytokine Detection
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
References
- McFarlin, D.E.; McFarland, H.F. Multiple sclerosis (second of two parts). N. Engl. J. Med 1982, 307, 1246–1251. [Google Scholar]
- Cvetanovich, G.L.; Hafler, D.A. Human regulatory T cells in autoimmune diseases. Curr. Opin. Immunol 2010, 22, 753–760. [Google Scholar]
- De Andres, C.; Aristimuno, C.; de Las Heras, V.; Martinez-Gines, M.L.; Bartolome, M.; Arroyo, R.; Navarro, J.; Gimenez-Roldan, S.; Fernandez-Cruz, E.; Sanchez-Ramon, S. Interferon beta-1a therapy enhances CD4+ regulatory T-cell function: An ex vivo and in vitro longitudinal study in relapsing-remitting multiple sclerosis. J. Neuroimmunol 2007, 182, 204–211. [Google Scholar]
- Haas, J.; Hug, A.; Viehover, A.; Fritzsching, B.; Falk, C.S.; Filser, A.; Vetter, T.; Milkova, L.; Korporal, M.; Fritz, B.; et al. Reduced suppressive effect of CD4+CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur. J. Immunol 2005, 35, 3343–3352. [Google Scholar]
- Viglietta, V.; Baecher-Allan, C.; Weiner, H.L.; Hafler, D.A. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J. Exp. Med 2004, 199, 971–979. [Google Scholar]
- Maggi, E.; Cosmi, L.; Liotta, F.; Romagnani, P.; Romagnani, S.; Annunziato, F. Thymic regulatory T cells. Autoimmun. Rev 2005, 4, 579–586. [Google Scholar]
- Baecher-Allan, C.; Viglietta, V.; Hafler, D.A. Human CD4+CD25+ regulatory T cells. Semin. Immunol 2004, 16, 89–98. [Google Scholar]
- Green, D.R.; Flood, P.M.; Gershon, R.K. Immunoregulatory T-cell pathways. Annu. Rev. Immunol 1983, 1, 439–463. [Google Scholar]
- Cosmi, L.; Liotta, F.; Lazzeri, E.; Francalanci, M.; Angeli, R.; Mazzinghi, B.; Santarlasci, V.; Manetti, R.; Vanini, V.; Romagnani, P.; et al. Human CD8+CD25+ thymocytes share phenotypic and functional features with CD4+CD25+ regulatory thymocytes. Blood 2003, 102, 4107–4114. [Google Scholar]
- Vlad, G.; Suciu-Foca, N. Resurgence or emergence of CD8+ Ts. Hum. Immunol. 2008, 69, 679–680. [Google Scholar]
- Aristimuno, C.; de Andres, C.; Bartolome, M.; de las Heras, V.; Martinez-Gines, M.L.; Arroyo, R.; Fernandez-Cruz, E.; Sanchez-Ramon, S. IFNbeta-1a therapy for multiple sclerosis expands regulatory CD8+ T cells and decreases memory CD8+ subset: a longitudinal 1-year study. Clin. Immunol 2009, 134, 148–157. [Google Scholar]
- Aristimuño, C.; Navarro, J.; de Andres, C.; Martinez-Gines, L.; Gimenez-Roldan, S.; Fernandez-Cruz, E.; Sanchez-Ramon, S. Expansion of regulatory CD8+ T-lymphocytes and fall of activated CD8+ T-lymphocytes after i.v. methyl-prednisolone for multiple sclerosis relapse. J. Neuroimmunol 2008, 204, 131–135. [Google Scholar]
- Tennakoon, D.K.; Mehta, R.S.; Ortega, S.B.; Bhoj, V.; Racke, M.K.; Karandikar, N.J. Therapeutic induction of regulatory, cytotoxic CD8+ T cells in multiple sclerosis. J. Immunol 2006, 176, 7119–7129. [Google Scholar]
- Feger, U.; Luther, C.; Poeschel, S.; Melms, A.; Tolosa, E.; Wiendl, H. Increased frequency of CD4+ CD25+ regulatory T cells in the cerebrospinal fluid but not in the blood of multiple sclerosis patients. Clin. Exp. Immunol 2007, 147, 412–418. [Google Scholar]
- Fritzsching, B.; Haas, J.; Konig, F.; Kunz, P.; Fritzsching, E.; Poschl, J.; Krammer, P.H.; Bruck, W.; Suri-Payer, E.; Wildemann, B. Intracerebral human regulatory T cells: Analysis of CD4+ CD25+ FOXP3+ T cells in brain lesions and cerebrospinal fluid of multiple sclerosis patients. PLoS One 2011, 6. [Google Scholar] [CrossRef]
- Correale, J.; Villa, A. Role of CD8+ CD25+ Foxp3+ regulatory T cells in multiple sclerosis. Ann. Neurol 2010, 67, 625–638. [Google Scholar]
- Valor, L.; Teijeiro, R.; Aristimuno, C.; Faure, F.; Alonso, B.; de Andres, C.; Tejera, M.; Lopez-Lazareno, N.; Fernandez-Cruz, E.; Sanchez-Ramon, S. Estradiol-dependent perforin expression by human regulatory T-cells. Eur. J. Clin. Invest 2010, 41, 357–364. [Google Scholar]
- Grossman, W.J.; Verbsky, J.W.; Barchet, W.; Colonna, M.; Atkinson, J.P.; Ley, T.J. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity 2004, 21, 589–601. [Google Scholar]
- McCombe, P.A.; Greer, J.M.; Mackay, I.R. Sexual dimorphism in autoimmune disease. Curr. Mol. Med 2009, 9, 1058–1079. [Google Scholar]
- Sanchez-Ramon, S.; Navarro, A.J.; Aristimuno, C.; Rodriguez-Mahou, M.; Bellon, J.M.; Fernandez-Cruz, E.; de Andres, C. Pregnancy-induced expansion of regulatory T-lymphocytes may mediate protection to multiple sclerosis activity. Immunol. Lett 2005, 96, 195–201. [Google Scholar]
- Venken, K.; Hellings, N.; Thewissen, M.; Somers, V.; Hensen, K.; Rummens, J.L.; Medaer, R.; Hupperts, R.; Stinissen, P. Compromised CD4+ CD25(high) regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology 2008, 123, 79–89. [Google Scholar]
- Boissonnas, A.; Scholer-Dahirel, A.; Simon-Blancal, V.; Pace, L.; Valet, F.; Kissenpfennig, A.; Sparwasser, T.; Malissen, B.; Fetler, L.; Amigorena, S. Foxp3+ T cells induce perforin-dependent dendritic cell death in tumor-draining lymph nodes. Immunity 2010, 32, 266–278. [Google Scholar]
- Beeston, T.; Smith, T.R.; Maricic, I.; Tang, X.; Kumar, V. Involvement of IFN-γ and perforin, but not Fas/FasL interactions in regulatory T cell-mediated suppression of experimental autoimmune encephalomyelitis. J. Neuroimmunol 2010, 229, 91–97. [Google Scholar]
- Cappellano, G.; Orilieri, E.; Comi, C.; Chiocchetti, A.; Bocca, S.; Boggio, E.; Bernardone, I.S.; Cometa, A.; Clementi, R.; Barizzone, N.; et al. Variations of the perforin gene in patients with multiple sclerosis. Genes. Immun 2008, 9, 438–444. [Google Scholar]
- Camiña-Tato, M.; Morcillo-Suarez, C.; Bustamante, M.F.; Ortega, I.; Navarro, A.; Muntasell, A.; Lopez-Botet, M.; Sanchez, A.; Carmona, P.; Julia, E.; et al. Gender-associated differences of perforin polymorphisms in the susceptibility to multiple sclerosis. J. Immunol 2010, 185, 5392–5404. [Google Scholar]
- Lenschow, D.J.; Walunas, T.L.; Bluestone, J.A. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 1996, 14, 233–258. [Google Scholar]
- Aristimuno, C.; Teijeiro, R.; Valor, L.; Alonso, B.; Tejera-Alhambra, M.; de Andres, C.; Minarro, D.O.; Lopez-Lazareno, N.; Faure, F.; Sanchez-Ramon, S. Sex-hormone receptors pattern on regulatory T-cells: clinical implications for multiple sclerosis. Clin. Exp. Med 2012. [Google Scholar] [CrossRef]
- Berghofer, B.; Frommer, T.; Haley, G.; Fink, L.; Bein, G.; Hackstein, H. TLR7 ligands induce higher IFN-alpha production in females. J. Immunol 2006, 177, 2088–2096. [Google Scholar]
| CSF | PB | P | |
|---|---|---|---|
| % CD8+FoxP3+ T cells | 0.95 ± 1.00 0.99 (1.49) | 0.13 ± 0.08 0.14 (0.12) | 0.02 |
| % CD8+CD25+FoxP3+ T cells | 0.84 ± 1.05 0.60 (1.23) | 0.13 ± 0.07 0.16 (0.15) | 0.02 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tejera-Alhambra, M.; Alonso, B.; Teijeiro, R.; Ramos-Medina, R.; Aristimuño, C.; Valor, L.; De Andrés, C.; Sánchez-Ramón, S. Perforin Expression by CD4+ Regulatory T Cells Increases at Multiple Sclerosis Relapse: Sex Differences. Int. J. Mol. Sci. 2012, 13, 6698-6710. https://doi.org/10.3390/ijms13066698
Tejera-Alhambra M, Alonso B, Teijeiro R, Ramos-Medina R, Aristimuño C, Valor L, De Andrés C, Sánchez-Ramón S. Perforin Expression by CD4+ Regulatory T Cells Increases at Multiple Sclerosis Relapse: Sex Differences. International Journal of Molecular Sciences. 2012; 13(6):6698-6710. https://doi.org/10.3390/ijms13066698
Chicago/Turabian StyleTejera-Alhambra, Marta, Bárbara Alonso, Roseta Teijeiro, Rocío Ramos-Medina, Carol Aristimuño, Larissa Valor, Clara De Andrés, and Silvia Sánchez-Ramón. 2012. "Perforin Expression by CD4+ Regulatory T Cells Increases at Multiple Sclerosis Relapse: Sex Differences" International Journal of Molecular Sciences 13, no. 6: 6698-6710. https://doi.org/10.3390/ijms13066698
APA StyleTejera-Alhambra, M., Alonso, B., Teijeiro, R., Ramos-Medina, R., Aristimuño, C., Valor, L., De Andrés, C., & Sánchez-Ramón, S. (2012). Perforin Expression by CD4+ Regulatory T Cells Increases at Multiple Sclerosis Relapse: Sex Differences. International Journal of Molecular Sciences, 13(6), 6698-6710. https://doi.org/10.3390/ijms13066698




