Anti-Hyperglycemic Effect of Chebulagic Acid from the Fruits of Terminalia chebula Retz
Abstract
:1. Introduction
2. Results and Discussion
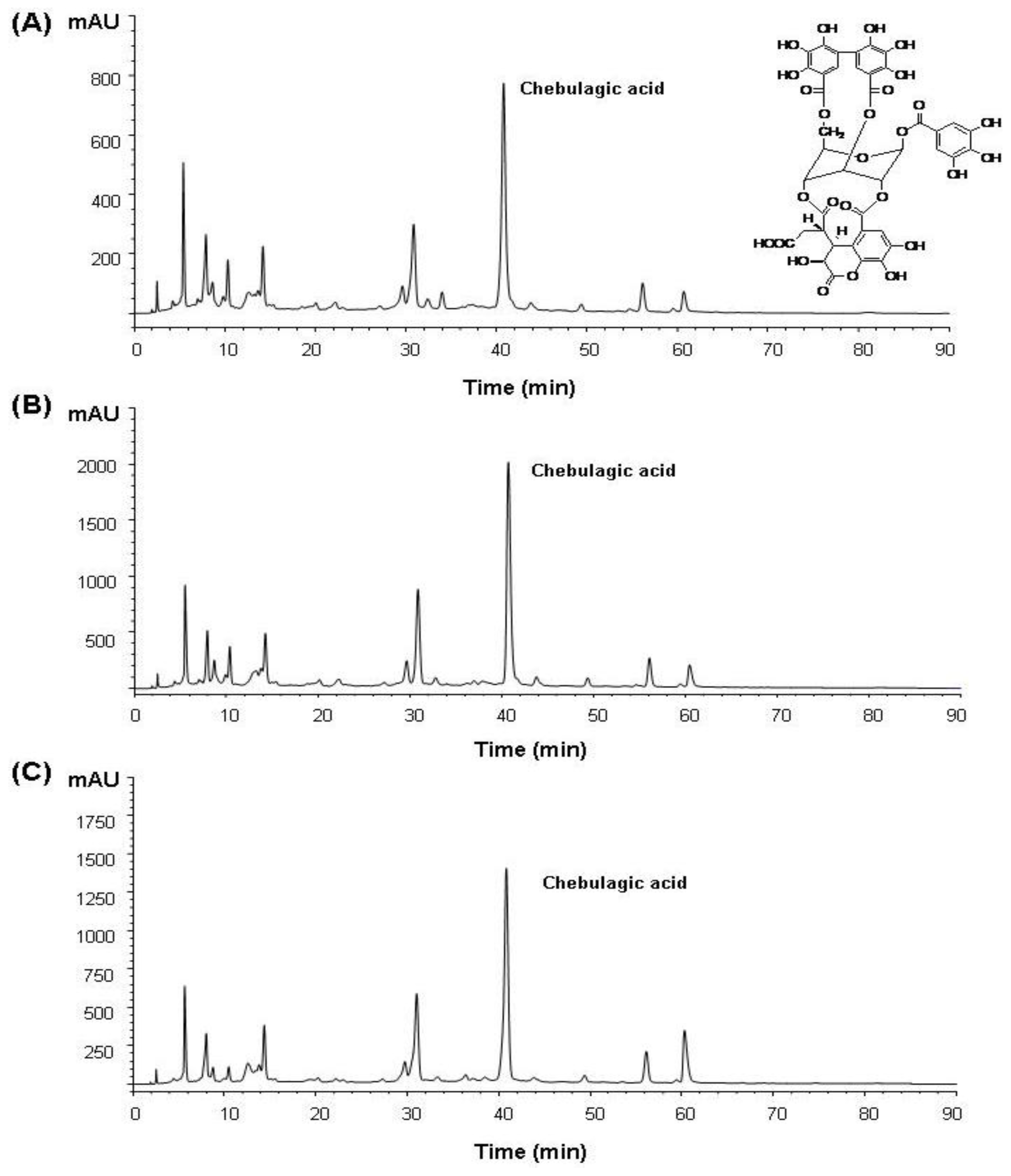
2.1. Isolation of Chebulagic Acid from T. chebula Fruits
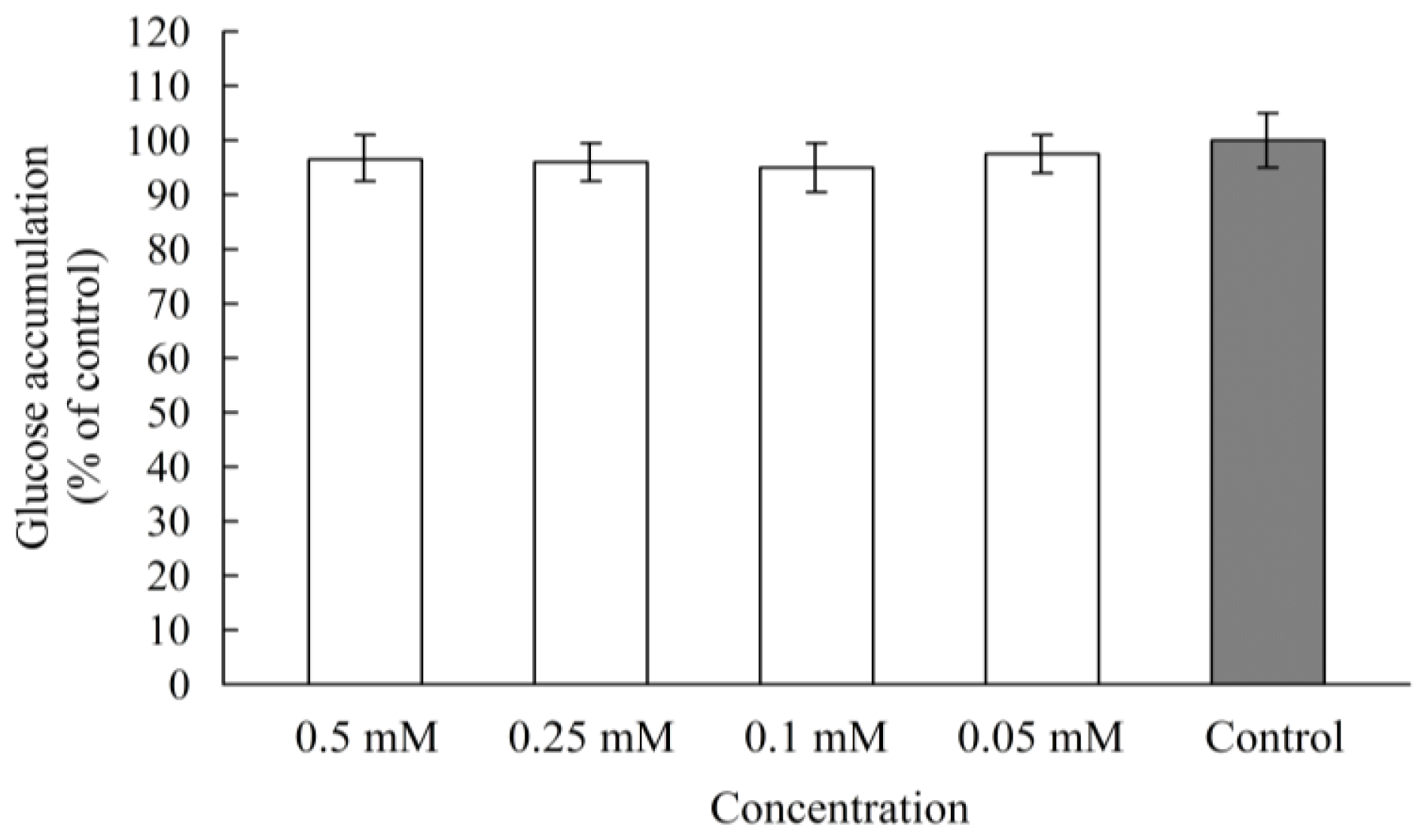
2.2. α-Glucosidase Inhibitory Activity in Caco-2 Cells
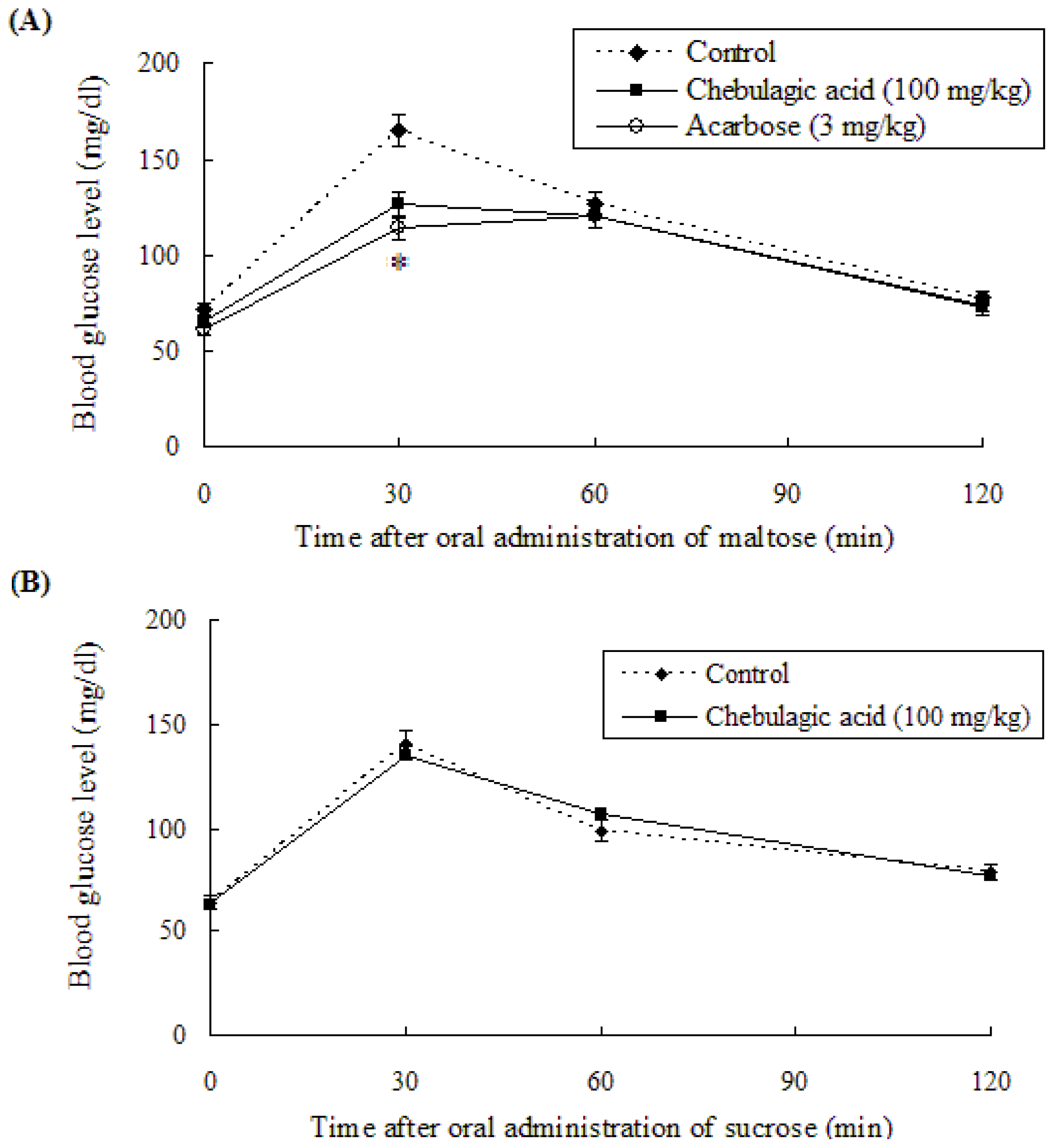
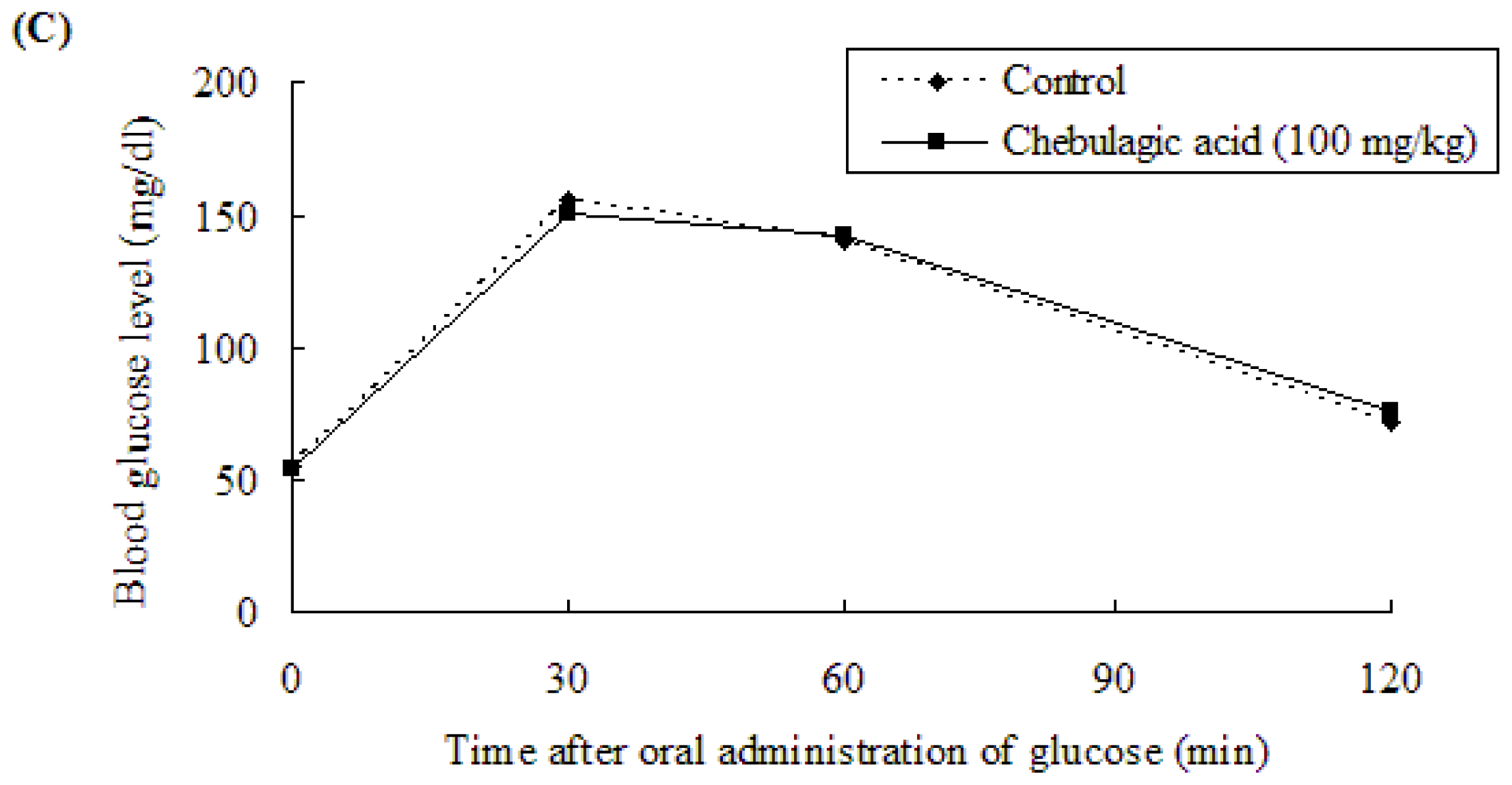
2.3. Effect of Chebulagic Acid on Blood Glucose Level in Sugar-Loaded SD-Rats
3. Materials and Methods
3.1. Materials
3.2. Preparation of Sample Extraction
3.3. Determination of Chebulagic Acid in Each Extract
3.4. Isolation of Chebulagic Acid
3.5. Intestinal α-Glucosidase Inhibitory Activity
3.6. Assay for the Caco-2 Cells Experiment
3.7. Animal Experimental
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Zhao, L.Y.; Lan, Q.J.; Huang, Z.C.; Ouyang, L.J.; Zeng, F.H. Antidiabetic effect of a newly identified component of Opuntia dillenii polysaccharides. Phytomedicine 2011, 18, 661–668. [Google Scholar]
- Rendell, M. The role of sulphonylureas in the management of type 2 diabetes mellitus. Drugs 2004, 64, 1339–1358. [Google Scholar]
- Lebovitz, H.E. alpha-Glucosidase inhibitors. Endocrinol. Metab. Clin. North Am 1997, 26, 539–551. [Google Scholar]
- Yao, Y.; Cheng, X.Z.; Wang, L.X.; Wang, S.H.; Ren, G.X. A determination of potential α-glucosidase inhibitors from azuki beans (Vigna angularis). Int. J. Mol. Sci 2011, 12, 6445–6451. [Google Scholar]
- Kim, S.H.; Jo, S.H.; Kwon, Y.I.; Kwang, J.K. Effects of onion (Allium cepa L.) extract administration on intestinal α-glucosidases activities and spikes in postprandial blood glucose levels in SD rats model. Int. J. Mol. Sci 2011, 12, 3757–3769. [Google Scholar]
- Gao, H.; Huang, Y.N.; Gao, B.; Xu, P.Y.; Inagaki, C.; Kawabata, J. α-Glucosidase inhibitory effect by the flower buds of Tussilago farfara L. Food Chem 2008, 106, 1195–1201. [Google Scholar]
- Srivastava, P.; Raut, H.N.; Wagh, R.S.; Puntambekar, H.M.; Kulkarni, M.J. Purification and characterization of an antioxidant protein (~16 kDa) from Terminalia chebula fruit. Food Chem 2012, 131, 141–148. [Google Scholar]
- Chang, C.L.; Lin, C.S. Phytochemical composition, antioxidant activity, and neuroprotective effect of Terminalia chebula Retzius extracts. Evid. Based Complement. Altern. Med 2012, 2012. [Google Scholar] [CrossRef]
- Saleem, A.; Husheem, M.; Härkönen, P.; Pihlajia, K. Inhibition of cancer cell growth by crude extract and the phenolics of Terminalia chebula Retz. fruit. J. Ethnopharmacol 2002, 81, 327–336. [Google Scholar]
- Malekzadeh, F.; Ehsanifar, H.; Shahamat, M.; Levin, M.; Colwell, R.R. Antibacterial activity of black myrobalan (Terminalia chebula Retz) against Helicobacter pylori. Int. J. Antimicrob. Agents 2011, 18, 85–88. [Google Scholar]
- Sabu, M.C.; Kuttan, R. Anti-diabetic activity of medicinal plants and its relationship with their antioxidant property. J. Ethnopharmacol 2002, 81, 155–160. [Google Scholar]
- Rao, N.K.; Nammi, S. Antidiabetic and renoprotective effects of the chloroform extract of Terminalia chebula Retz. seeds in streptozotocin-induced diabetic rats. BMC Complement. Altern. Med 2006, 6, 17–22. [Google Scholar]
- Murali, Y.K.; Anand, P.; Tandon, V.; Singh, R.; Chandra, R.; Murthy, P.S. Long-term effects of Terminalia chebula Retz. on hyperglycemia and associated hyperlipidemia, tissue glycogen content and in vitro release of insulin in streptozotocin induced diabetic rats. Exp. Clin. Endocrinol. Diabetes 2007, 115, 641–646. [Google Scholar]
- Pfundstein, B.; Ei Desouky, S.K.; Hull, W.E.; Haubner, R.; Erben, G.; Owen, R.W. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): Characterization, quantitation and determination of antioxidant capacities. Phytochemistry 2010, 71, 1132–1148. [Google Scholar]
- Kaur, S.; Grover, I.S.; Singh, M.; Kaur, S. Antimutagenicity of hydrolyzable tannins from Terminalia chebula in Salmonella typhimurium. Mutat. Res 1998, 419, 169–179. [Google Scholar]
- Kundu, A.P.; Mahato, S.B. Triterpenoids and their glycosides from Terminalia chebula. Phytochemistry 1993, 32, 999–1002. [Google Scholar]
- Gao, H.; Huang, Y.N.; Xu, P.Y.; Kawabata, J. Inhibitory effect on α-glucosidase by the fruits of Terminalia chebula Retz. Food Chem 2007, 105, 628–634. [Google Scholar]
- Gao, H.; Huang, Y.N.; Gao, B.; Kawabata, J. Chebulagic acid is a potent α-glucosidase inhibitor. Biosci. Biotechnol. Biochem 2008, 72, 601–603. [Google Scholar]
- Tubtimdee, C.; Shotipruk, A. Extraction of phenolics from Terminalia chebula Retz with water-ethanol and water-propylene glycol and sugaring-out concentration of extracts. Sep. Purif. Technol 2011, 77, 339–346. [Google Scholar]
- Palanisamy, U.; Manaharan, T.; Teng, L.L.; Radhakrishnan, A.K.C.; Subramaniam, T.; Masilamani, T. Rambutan rind in the management of hyperglycemia. Food Res. Int 2011, 44, 2278–2282. [Google Scholar]
- Mahraoui, L.; Rodolosse, A.; Barbat, A.; Dussaulx, E.; Zweibaum, A.; Rousset, M. Presence and differential expression of SGLT1, GLUT1, GLUT2, GLUT3 and GLUT5 hexose-transporter mRNAs in Caco-2 cell clones in relation to cell growth and glucose consumption. Biochem. J 1994, 298, 629–633. [Google Scholar]
- Toda, M.; Kawabata, J.; Kasai, T. α-Glucosidase inhibitors from Clove (Syzgium aromaticum). Biosci. Biotechnol. Biochem 2000, 64, 294–298. [Google Scholar]
- Hansawasdi, C.; Kawabata, J. α-Glucosidase inhibitory effect of mulberry (Morus alba) leaves on Caco-2. Fitoterapia 2006, 77, 568–573. [Google Scholar]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, K.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci 2010, 11, 1365–1402. [Google Scholar]
- Johnston, K.; Sharp, P.; Clifford, M.; Morgan, L. Dietary polyphenols decrease glucose uptake by human intestinal Caco-2 cells. FEBS Lett 2005, 579, 1653–1657. [Google Scholar]
- Jing, Z.; Zeng, W.C.; Luo, J.W.; Ye, H.X.; Huang, Y.N.; Gao, H. In vitro and in vivo inhibitory effect of methanol extract from Terminalia chebula Retz. fruits on α-glucosidase (In Chinsese). Food Sci 2010, 31, 284–287. [Google Scholar]
- Abesundara, K.J.M.; Matsui, T.; Matsumoto, K. α-Glucosidae inhibitory activity of Sri Lanka plant extracts, one of which, Cassia auriculata, exerts a strong antihyperglycemic effect in rats comparable to the therapeutic drug acrabose. J. Agric. Food Chem 2004, 52, 2541–2545. [Google Scholar]
- Huang, Y.N.; Zhao, Y.L.; Gao, X.L.; Zhao, Z.F.; Jing, Z.; Zeng, W.C.; Yang, R.; Peng, R.; Tong, T.; Wang, L.F.; Cen, J.Q.; Gao, H. Intestinal α-glucosidase inhibitory activity and toxicological evaluation of Nymphaea stellata flowers extract. J. Ethnopharmacol 2010, 131, 306–312. [Google Scholar]




© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huang, Y.-N.; Zhao, D.-D.; Gao, B.; Zhong, K.; Zhu, R.-X.; Zhang, Y.; Xie, W.-J.; Jia, L.-R.; Gao, H. Anti-Hyperglycemic Effect of Chebulagic Acid from the Fruits of Terminalia chebula Retz. Int. J. Mol. Sci. 2012, 13, 6320-6333. https://doi.org/10.3390/ijms13056320
Huang Y-N, Zhao D-D, Gao B, Zhong K, Zhu R-X, Zhang Y, Xie W-J, Jia L-R, Gao H. Anti-Hyperglycemic Effect of Chebulagic Acid from the Fruits of Terminalia chebula Retz. International Journal of Molecular Sciences. 2012; 13(5):6320-6333. https://doi.org/10.3390/ijms13056320
Chicago/Turabian StyleHuang, Yi-Na, Dong-Dong Zhao, Bo Gao, Kai Zhong, Rui-Xue Zhu, Yan Zhang, Wang-Jun Xie, Li-Rong Jia, and Hong Gao. 2012. "Anti-Hyperglycemic Effect of Chebulagic Acid from the Fruits of Terminalia chebula Retz" International Journal of Molecular Sciences 13, no. 5: 6320-6333. https://doi.org/10.3390/ijms13056320
APA StyleHuang, Y.-N., Zhao, D.-D., Gao, B., Zhong, K., Zhu, R.-X., Zhang, Y., Xie, W.-J., Jia, L.-R., & Gao, H. (2012). Anti-Hyperglycemic Effect of Chebulagic Acid from the Fruits of Terminalia chebula Retz. International Journal of Molecular Sciences, 13(5), 6320-6333. https://doi.org/10.3390/ijms13056320




