Evaluation of Antioxidant and Antiproliferative Properties of Three Actinidia (Actinidia kolomikta, Actinidia arguta, Actinidia chinensis) Extracts in Vitro
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Total Phenolic, Total Flavonoids and Vitamin C Contents
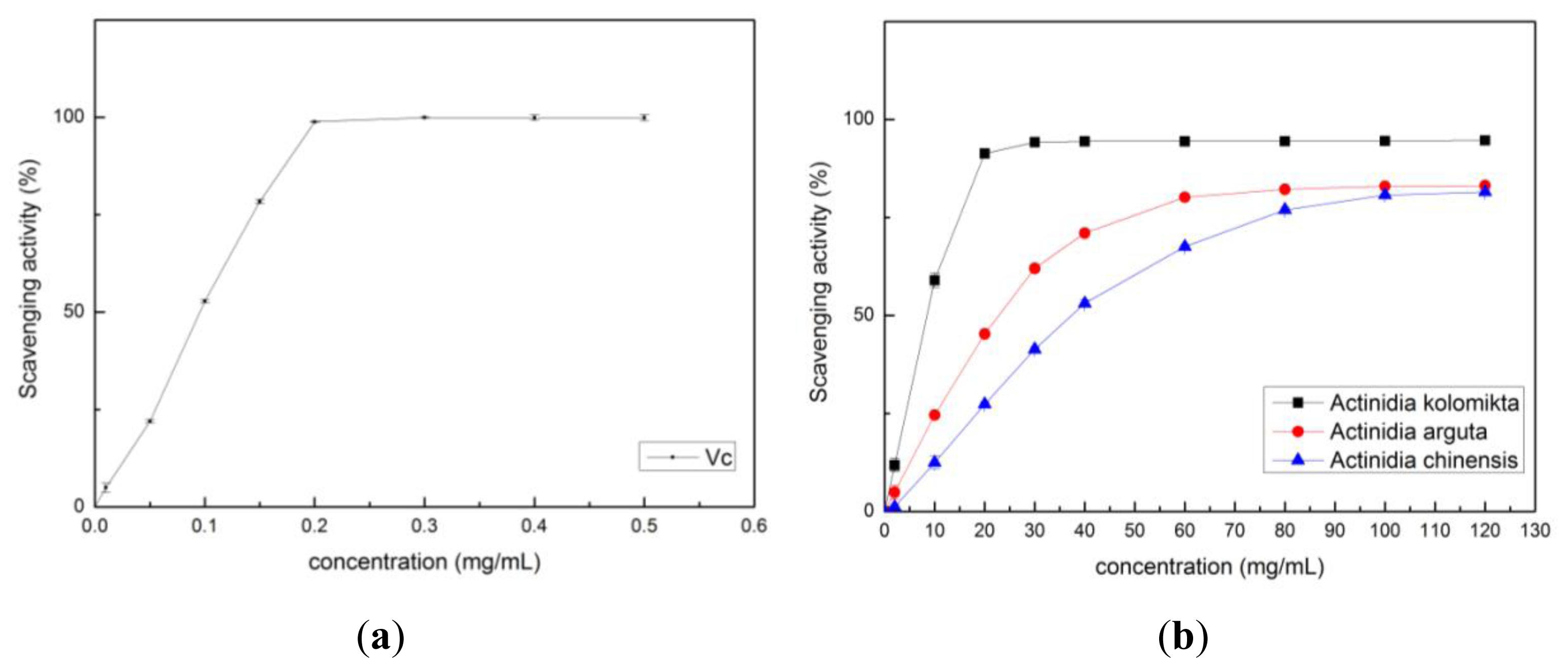
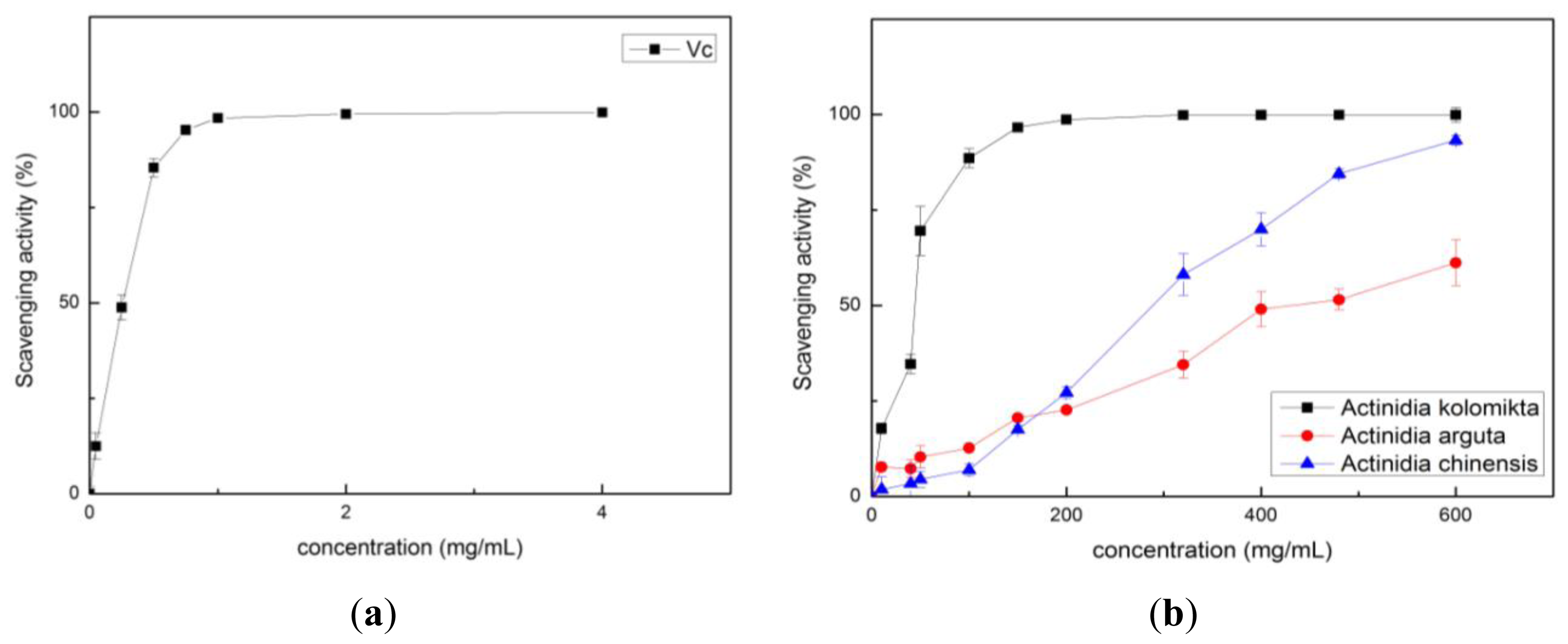
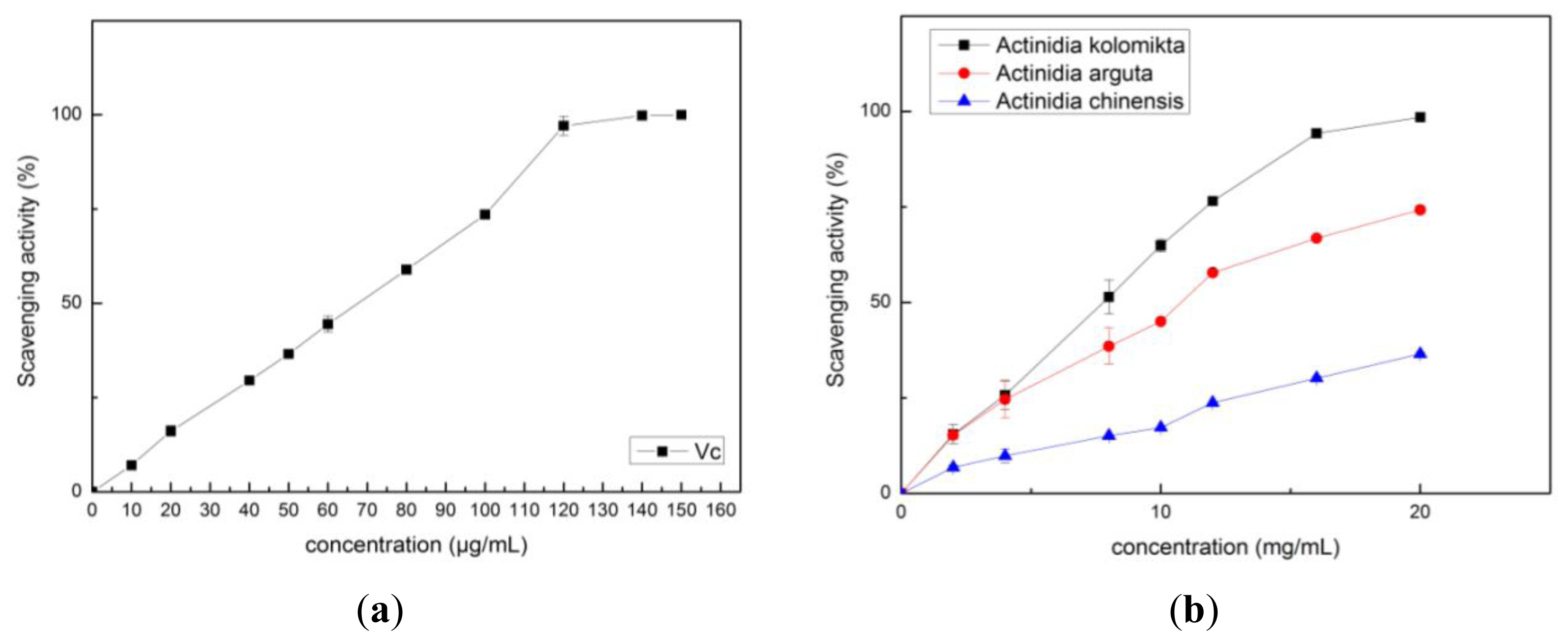
2.2. Antioxidant Activity
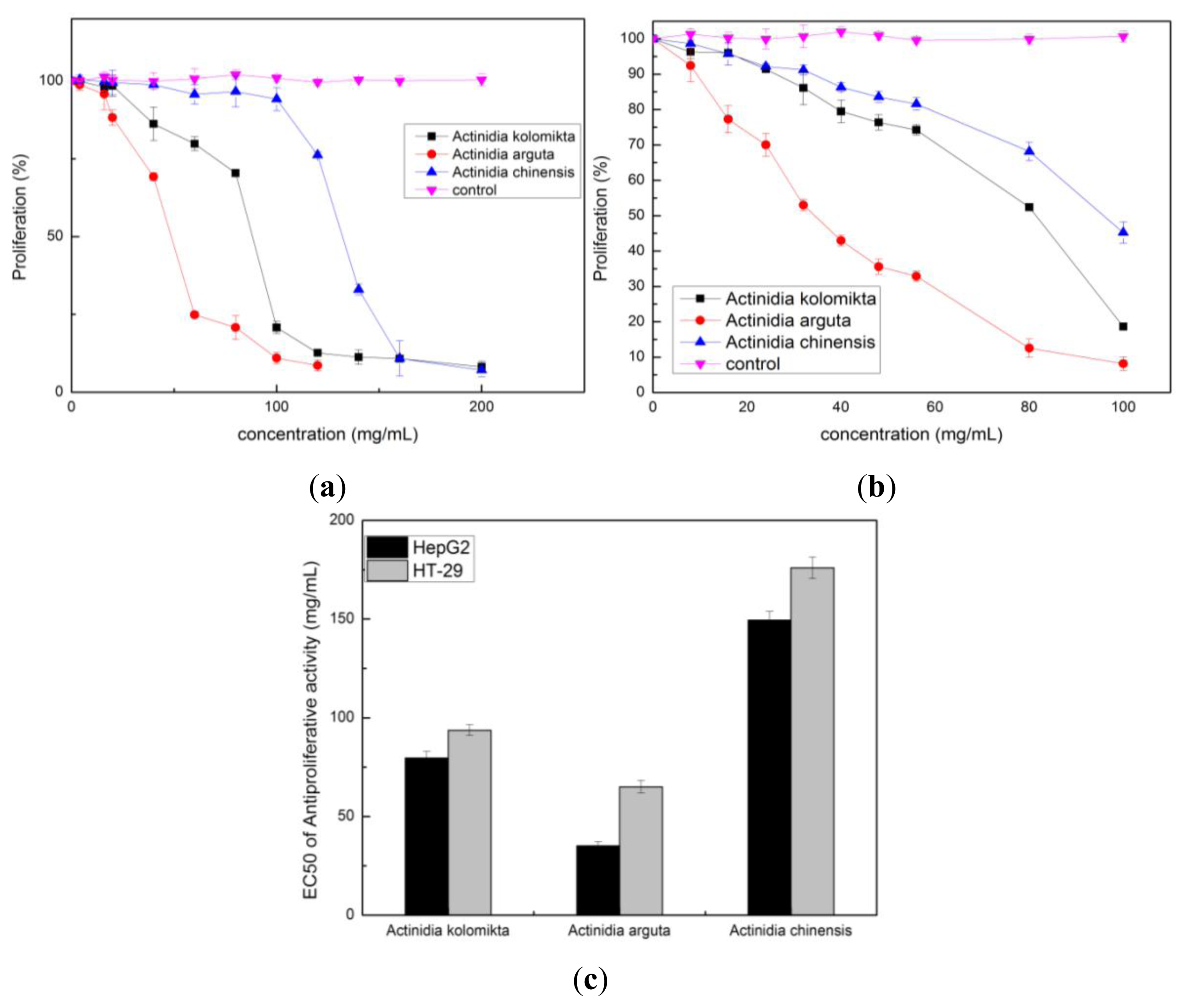
2.3. Effect of Actinidia Extracts on Cancer Cell Proliferation
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Sample Preparation
3.3. Determination of Total Phenolic Content
3.4. Determination of the Total Flavonoid Content
3.5. Determination of Vitamin C
3.6. Hydroxyl Radical Scavenging Activity
3.7. The O2− Scavenging Assay
3.8. DPPH Radical Scavenging Activity Assay
3.9. The ABTS+ Method
3.10. Determination of the Antiproliferative Activity
4. Conclusions
Acknowledgments
References
- Lizcano, L.J.; Bakkali, F.; Ruiz-Larrea, M.B.; Ruiz-Sanz, J.I. Antioxidant activity and polyphenol content of aqueous extracts from Colombian Amazonian plants with medicinal use. Food Chem 2010, 119, 1566–1570. [Google Scholar]
- Chedea, V.S.; Braicu, C.; Socaciu, C. Antioxidant/prooxidant activity of a polyphenolic grape seed extract. Food Chem 2010, 121, 132–139. [Google Scholar]
- Sinha, D.; Roy, S.; Roy, M. Antioxidant potential of tea reduces arsenite induced oxidative stress in Swiss albino mice. Food Chem. Toxicol 2010, 48, 1032–1039. [Google Scholar]
- Bellion, P.; Digles, J.; Will, F.; Dietrich, H.; Baum, M.; Eisenbrand, G.; Janzowski, C. Polyphenolic apple extracts: Effects of raw material and production method on antioxidant effectiveness and reduction of DNA damage in Caco-2 cells. J. Agric. Food Chem 2010, 58, 6636–6642. [Google Scholar]
- Tang, X.Z.; Dong, Y.X.; Wei, S.Q.; Zhang, X.S.; Yin, Y.P. Antioxidant activity of pigment extracted from green-wheat-bran. Agric. Sci. China 2010, 9, 825–832. [Google Scholar]
- Wei, S.-D.; Zhou, H.-C.; Lin, Y.-M. Antioxidant activities of extract and fractions from the hypocotyls of the mangrove plant Kandelia candel. Int. J. Mol. Sci 2010, 11, 4080–4093. [Google Scholar]
- Liu, Y.; Liu, M.; Li, B.; Zhao, J.-L.; Zhang, C.-P.; Lin, L.-Q.; Chen, H.-S.; Zhang, S.-J.; Jin, J.-C.; Wang, L.; et al. Fresh raspberry phytochemical extract inhibits hepatic lesion in a Wistar rat model. Nutr. Metab 2010, 7, 1–8. [Google Scholar]
- Liu, J.-R.; Dong, H.-W.; Chen, B.-Q.; Zhao, P.; Liu, R.H. Fresh apples suppress mammary carcinogenesis and proliferative activity and induce apoptosis in mammary tumors of the Sprague–Dawley rat. J. Agric. Food Chem 2008, 57, 297–304. [Google Scholar]
- Chen, H.-S.; Liu, M.; Shi, L.-J.; Zhao, J.-L.; Zhang, C.-P.; Lin, L.-Q.; Liu, Y.; Zhang, S.-J.; Jin, J.-C.; Wang, L.; et al. Effects of raspberry phytochemical extract on cell proliferation, apoptosis, and serum proteomics in a rat model. J. Food Sci 2011, 76, T192–T198. [Google Scholar]
- Liu, M.; Liu, R.H.; Song, B.B.; Li, C.F.; Lin, L.Q.; Zhang, C.P.; Zhao, J.L.; Liu, J.R. Antiangiogenetic effects of 4 varieties of grapes in vitro. J. Food Sci 2010, 75, T99–T104. [Google Scholar]
- Wang, H.; Cao, G.H.; Prior, R.L. Total antioxidant capacity of fruits. J. Agric. Food Chem 1996, 44, 701–705. [Google Scholar]
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem 2009, 114, 310–316. [Google Scholar]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process 2011, 89, 217–233. [Google Scholar]
- Atawodi, S.E.; Atawodi, J.C.; Idakwo, G.A.; Pfundstein, B.; Haubner, R.; Wurtele, G.; Bartsch, H.; Owen, R.W. Evaluation of the polyphenol content and antioxidant properties of methanol extracts of the leaves, stem, and root barks of Moringa oleifera lam. J. Med. Food 2010, 13, 710–716. [Google Scholar]
- Lee, J.-Y.; Hwang, W.-I.; Lim, S.-T. Antioxidant and anticancer activities of organic extracts from Platycodon grandiflorum A. De Candolle roots. J. Ethnopharmacol 2004, 93, 409–415. [Google Scholar]
- Du, G.R.; Li, M.J.; Ma, F.W.; Liang, D. Antioxidant capacity and the relationship with polyphenol and vitamin C in Actinidia fruits. Food Chem 2009, 113, 557–562. [Google Scholar]
- Rassam, M.; Laing, W. Variation in ascorbic acid and oxalate levels in the fruit of Actinidia chinensis tissues and genotypes. J. Agric. Food Chem 2005, 53, 2322–2326. [Google Scholar]
- Laur, L.M.; Tian, L. Provitamin A and vitamin C contents in selected California-grown cantaloupe and honeydew melons and imported melons. J. Food Compos. Anal 2011, 24, 194–201. [Google Scholar]
- Ismail, H.I.; Chan, K.W.; Mariod, A.A.; Ismail, M. Phenolic content and antioxidant activity of cantaloupe (Cucumis melo) methanolic extracts. Food Chem 2010, 119, 643–647. [Google Scholar]
- Su, X.-Y.; Wang, Z.-Y.; Liu, J.-R. In vitro and in vivo antioxidant activity of Pinus koraiensis seed extract containing phenolic compounds. Food Chem 2009, 117, 681–686. [Google Scholar]
- Prasad, K.N.; Hao, J.; Shi, J.; Liu, T.; Li, J.; Wei, X.; Qiu, S.; Xue, S.; Jiang, Y. Antioxidant and anticancer activities of high pressure-assisted extract of longan (Dimocarpus longan Lour.) fruit pericarp. Innovatuve Food Sci. Emerging Technol 2009, 10, 413–419. [Google Scholar]
- Xu, C.M.; Zhang, Y.L.; Cao, L.; Lu, J. Phenolic compounds and antioxidant properties of different grape cultivars grown in China. Food Chem 2010, 119, 1557–1565. [Google Scholar]
- Tzulker, R.; Glazer, I.; Bar-Ilan, I.; Holland, D.; Aviram, M.; Amir, R. Antioxidant activity, polyphenol content, and related compounds in different fruit juices and homogenates prepared from 29 different pomegranate accessions. J. Agric. Food Chem 2007, 55, 9559–9570. [Google Scholar]
- D’Angelo, S.; Cimmino, A.; Raimo, M.; Salvatore, A.; Zappia, V.; Galletti, P. Effect of reddening-ripening on the antioxidant activity of polyphenol extracts from cv. ‘Annurca’ apple fruits. J. Agric. Food Chem 2007, 55, 9977–9985. [Google Scholar]
- Bi, S.; Liu, J.R.; Li, Y.; Wang, Q.; Liu, H.K.; Yan, Y.G.; Chen, B.Q.; Sun, W.G. Gamma-tocotrienol modulates the paracrine secretion of VEGF induced by cobalt(II) chloride via ERK signaling pathway in gastric adenocarcinoma SGC-7901 cell line. Toxicology 2010, 274, 27–33. [Google Scholar]
- Castañeda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem 2009, 113, 859–871. [Google Scholar]
- Fan, Z.-L.; Wang, Z.-Y.; Liu, J.-R. Cold-field fruit extracts exert different antioxidant and antiproliferative activities in vitro. Food Chem 2011, 129, 402–407. [Google Scholar]
- Luo, W.; Zhao, M.; Yang, B.; Ren, J.; Shen, G.; Rao, G. Antioxidant and antiproliferative capacities of phenolics purified from Phyllanthus emblica L. fruit. Food Chem 2011, 126, 277–282. [Google Scholar]
- Liu, J.R.; Dong, H.W.; Chen, B.Q.; Zhao, P.; Liu, R.H. Fresh apples suppress mammary carcinogenesis and proliferative activity and induce apoptosis in mammary tumors of the Sprague–Dawley rat. J. Agric. Food Chem 2009, 57, 297–304. [Google Scholar]
- Wang, M.; Liu, J.R.; Gao, J.M.; Parry, J.W.; Wei, Y.M. Antioxidant activity of Tartary buckwheat bran extract and its effect on the lipid profile of hyperlipidemic rats. J. Agric. Food Chem 2009, 57, 5106–5112. [Google Scholar]
- Song, F.-L.; Gan, R.-Y.; Zhang, Y.; Xiao, Q.; Kuang, L.; Li, H.-B. Total phenolic contents and antioxidant capacities of selected Chinese medicinal plants. Int. J. Mol. Sci 2010, 11, 2362–2372. [Google Scholar]
- Kampfenkel, K.; Vanmontagu, M.; Inze, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem 1995, 225, 165–167. [Google Scholar]
- Reddy, C.V.K.; Sreeramulu, D.; Raghunath, M. Antioxidant activity of fresh and dry fruits commonly consumed in India. Food Res. Int 2010, 43, 285–288. [Google Scholar]
- Locatelli, M.; Travaglia, F.; Coisson, J.D.; Martelli, A.; Stevigny, C.; Arlorio, M. Total antioxidant activity of hazelnut skin (Nocciola Piemonte PGI): Impact of different roasting conditions. Food Chem 2010, 119, 1647–1655. [Google Scholar]
- Gorinstein, S.; Park, Y.S.; Heo, B.G.; Namiesnik, J.; Leontowicz, H.; Leontowicz, M.; Ham, K.S.; Cho, J.Y.; Kang, S.G. A comparative study of phenolic compounds and antioxidant and antiproliferative activities in frequently consumed raw vegetables. Eur. Food Res. Technol 2009, 228, 903–911. [Google Scholar]
- Teixeira, D.M.; Canelas, V.C.; do Canto, A.M.; Teixeira, J.M.G.; Dias, C.B. HPLC-DAD quantification of phenolic compounds contributing to the antioxidant activity of Maclura pomifera, Ficus carica and Ficus elastica extracts. Anal. Lett 2009, 42, 2986–3003. [Google Scholar]


| Variety of Actinidia | Total Phenolic Content (mg GAE/100 g FW) | Total Flavonoid Content (mg CE/100 g FW) | Vitamin C Content (mg ACE/100 g FW) |
|---|---|---|---|
| Actinidia kolomikta | 430.03 ± 21.85 | 69.05 ± 0.75 | 211.12 ± 7.91 |
| Actinidia arguta | 362.18 ± 19.87 | 188.43 ± 3.65 | 26.97 ± 5.64 |
| Actinidia chinensis | 115.76 ± 8.97 | 67.63 ± 0.68 | 42.28 ± 0.77 |
| Phenol | Flavone | Vitamin C | OH− | O2− | DPPH | ABTS | |
|---|---|---|---|---|---|---|---|
| Phenol | 1 | - | - | - | - | - | - |
| Flavone | 0.321 | 1 | - | - | - | - | - |
| Vitamin C | 0.609 | −0.555 | 1 | - | - | - | - |
| OH− | 0.844 | −0.236 | 0.939 | 1 | - | - | - |
| O2− | 0.712 | −0.436 | 0.991 | 0.978 | 1 | - | - |
| DPPH | 0.787 | −0.332 | 0.969 | 0.995 | 0.994 | 1 | - |
| ABTS | 0.975 | 0.105 | 0.769 | 0.941 | 0.849 | 0.903 | 1 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zuo, L.-L.; Wang, Z.-Y.; Fan, Z.-L.; Tian, S.-Q.; Liu, J.-R. Evaluation of Antioxidant and Antiproliferative Properties of Three Actinidia (Actinidia kolomikta, Actinidia arguta, Actinidia chinensis) Extracts in Vitro. Int. J. Mol. Sci. 2012, 13, 5506-5518. https://doi.org/10.3390/ijms13055506
Zuo L-L, Wang Z-Y, Fan Z-L, Tian S-Q, Liu J-R. Evaluation of Antioxidant and Antiproliferative Properties of Three Actinidia (Actinidia kolomikta, Actinidia arguta, Actinidia chinensis) Extracts in Vitro. International Journal of Molecular Sciences. 2012; 13(5):5506-5518. https://doi.org/10.3390/ijms13055506
Chicago/Turabian StyleZuo, Li-Li, Zhen-Yu Wang, Zi-Luan Fan, Shuang-Qi Tian, and Jia-Ren Liu. 2012. "Evaluation of Antioxidant and Antiproliferative Properties of Three Actinidia (Actinidia kolomikta, Actinidia arguta, Actinidia chinensis) Extracts in Vitro" International Journal of Molecular Sciences 13, no. 5: 5506-5518. https://doi.org/10.3390/ijms13055506
APA StyleZuo, L.-L., Wang, Z.-Y., Fan, Z.-L., Tian, S.-Q., & Liu, J.-R. (2012). Evaluation of Antioxidant and Antiproliferative Properties of Three Actinidia (Actinidia kolomikta, Actinidia arguta, Actinidia chinensis) Extracts in Vitro. International Journal of Molecular Sciences, 13(5), 5506-5518. https://doi.org/10.3390/ijms13055506




