Lipid Oxidation Inhibitory Effects and Phenolic Composition of Aqueous Extracts from Medicinal Plants of Colombian Amazonia
Abstract
:1. Introduction
2. Results and Discussion
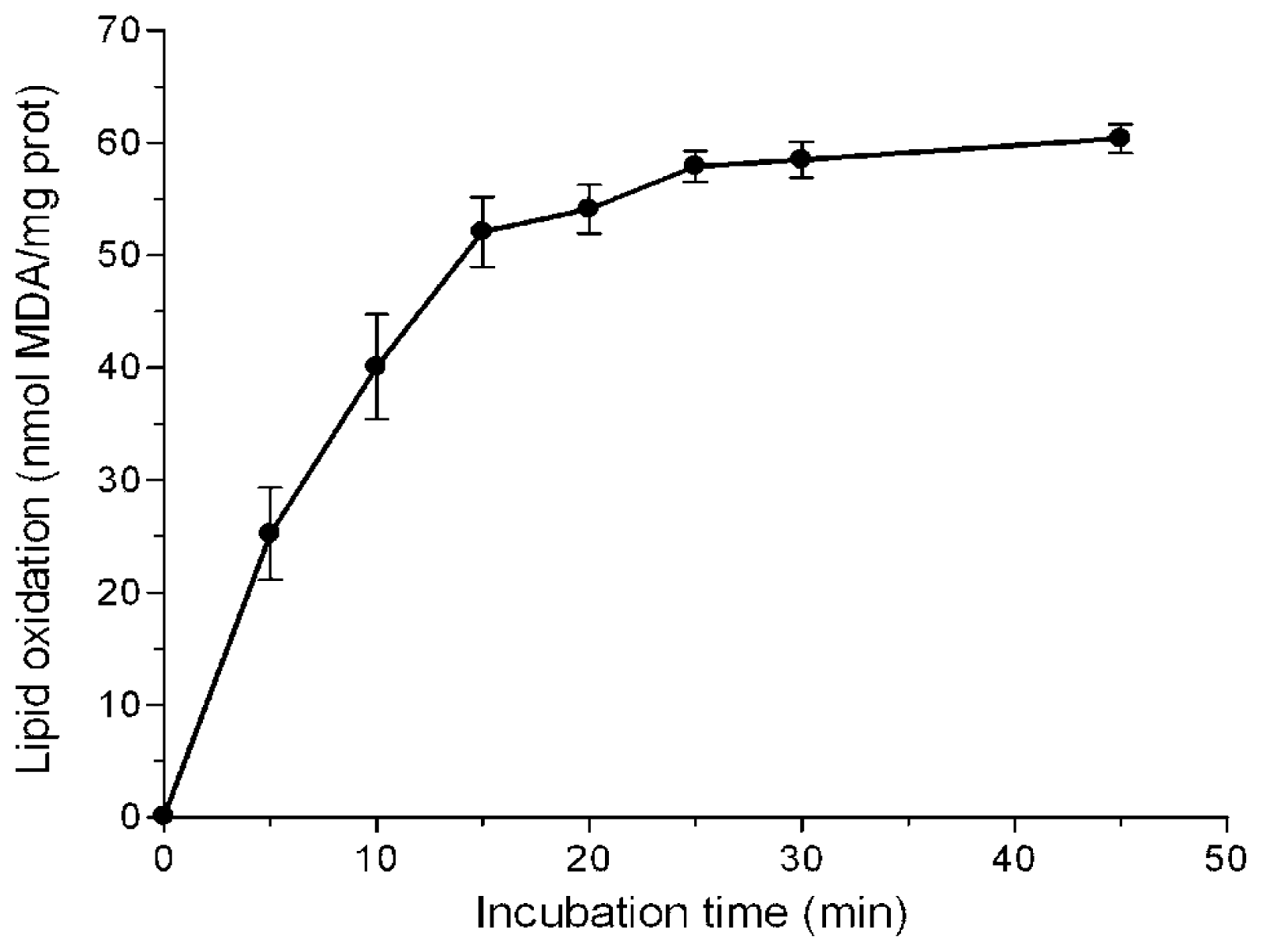
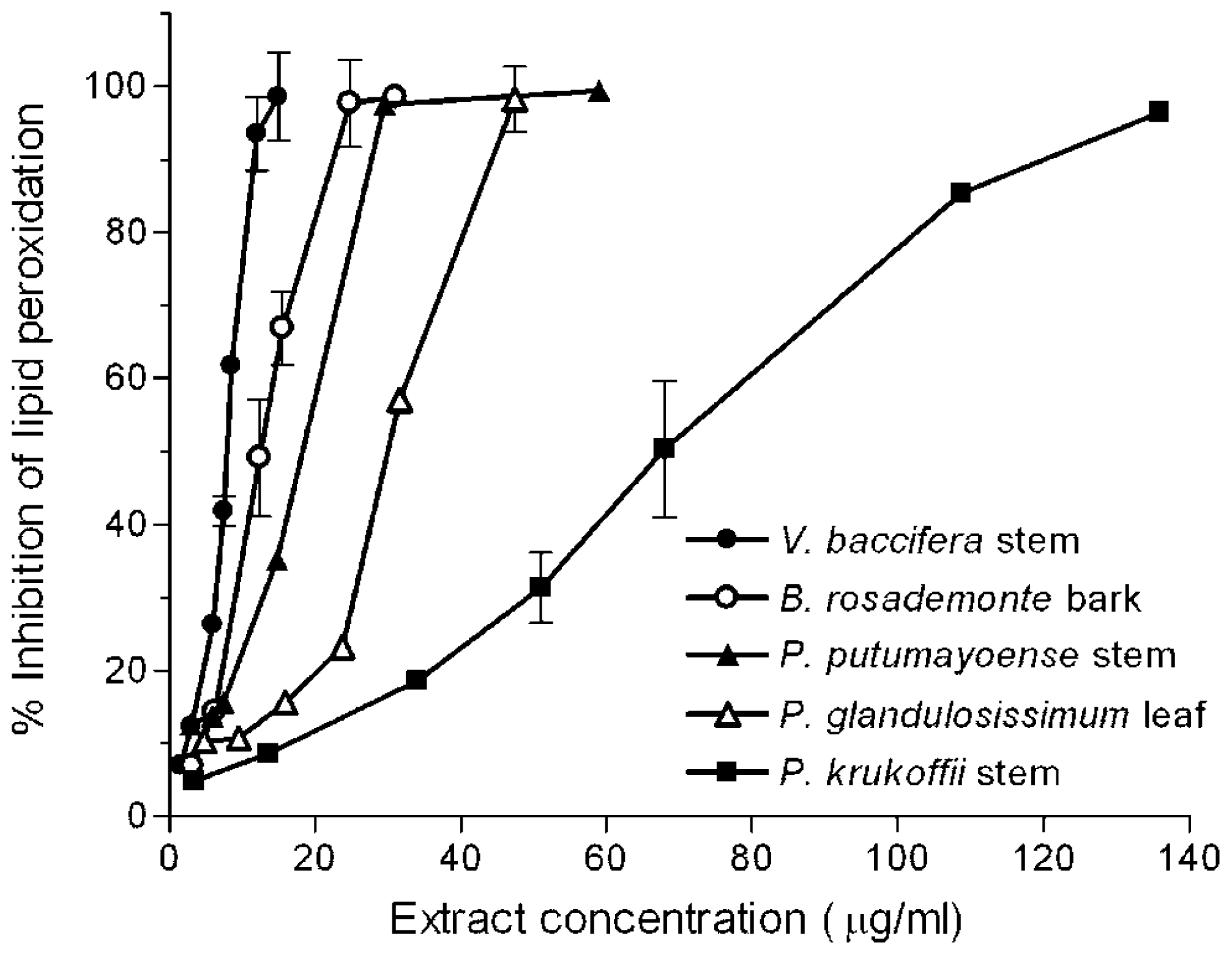
2.1. Inhibition of Lipid Peroxidation
2.2. Antimicrobial Activity
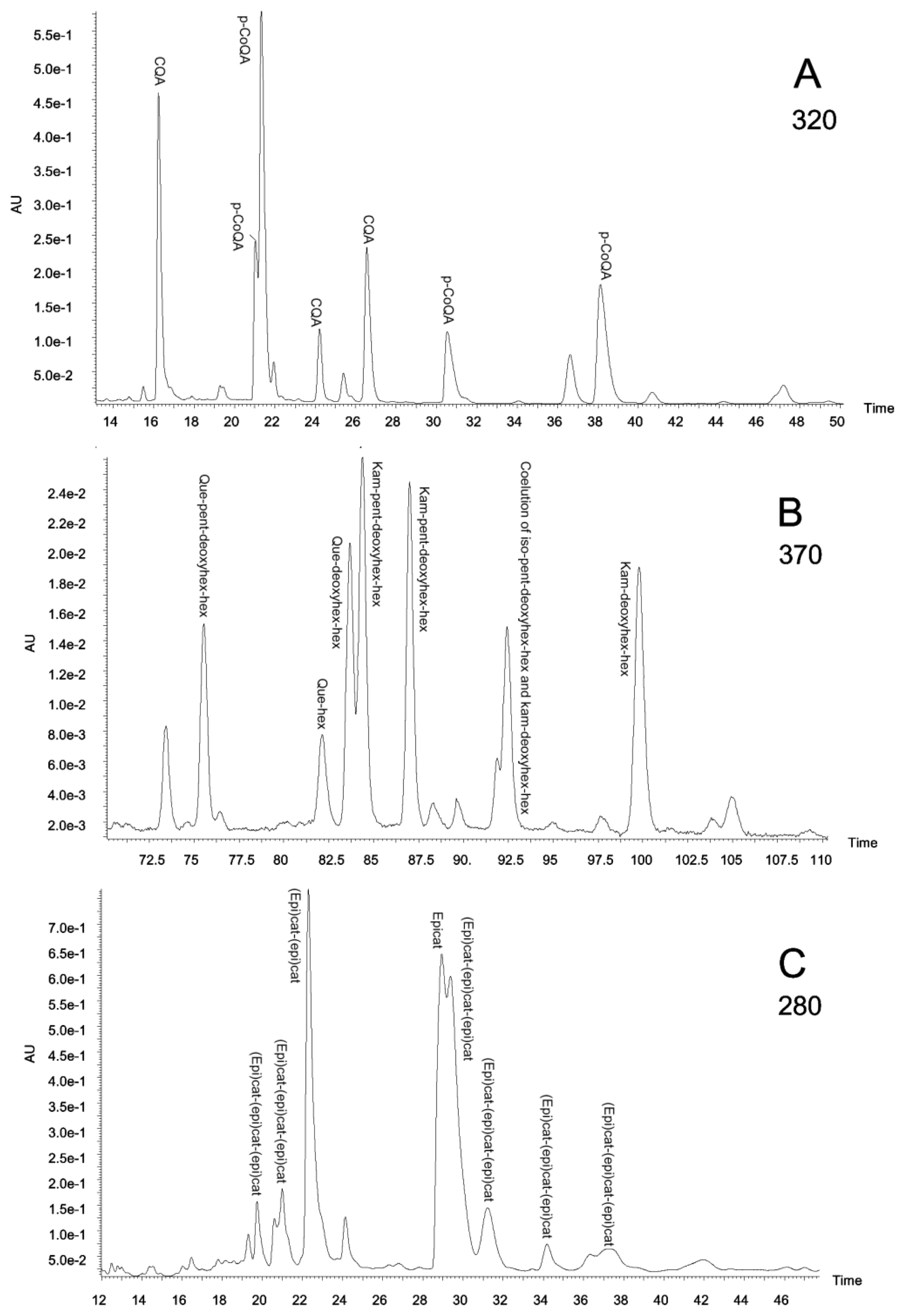
2.3. Polyphenolic Composition by HPLC-DAD and MS/MS Analysis
3. Experimental Section
3.1. Chemicals and Standards
3.2. Plant Samples
3.3. Preparation of Plant Extracts
3.4. Inhibition of Lipid Peroxidation
3.5. Antimicrobial Activity
3.6. HPLC-DAD and MS/MS Analysis
3.7. Statistical Analysis
4. Conclusions
Acknowledgements
- Conflict of InterestThe authors declare that they have no conflicts of interest. The authors alone are responsible for the content and writing the paper.
References
- Renner, U.D.; Oertel, R.; Kirch, W. Pharmacokinetics and pharmacodynamics in clinical use of scopolamine. Ther. Drug Monit 2005, 27, 655–665. [Google Scholar]
- Kintzel, P.E.; Chase, S.L.; Thomas, W.; Vancamp, D.M.; Clements, E.A. Anticholinergic medications for managing noisy respirations in adult hospice patients. Am. J. Health Syst. Pharm 2009, 66, 458–464. [Google Scholar]
- Vandebroek, I.; van Damme, P.; van Puyvelde, L.; Arrazola, S.; de Kimpe, N. A comparison of traditional healers’ medicinal plant knowledge in the Bolivian Andes and Amazon. Soc. Sci. Med 2004, 59, 837–849. [Google Scholar]
- Schultes, R.E. Amazonia as a source of new economic plants. Econ. Bot 1979, 33, 259–266. [Google Scholar]
- Schultes, R.E. Amazonian ethnobotany and the search for new drugs. Ciba Found. Symp 1994, 185, 106–112. [Google Scholar]
- Otero, R.; Nuñez, V.; Barona, J.; Fonnegra, R.; Jimenez, S.L.; Osorio, R.G.; Saldarriaga, M.; Diaz, A. Snakebites and ethnobotany in the northwest region of Colombia Part III: Neutralization of the haemorrhagic effect of Bothrops atrox venom. J. Ethnopharmacol 2000, 73, 233–241. [Google Scholar]
- Nuñez, V.; Otero, R.; Barona, J.; Saldarriaga, M.; Osorio, R.G.; Fonnegra, R.; Jimenez, S.L.; Diaz, A.; Quintana, J.C. Neutralization of the edema-forming, defibrinating and coagulant effects of Bothrops asper venom by extracts of plants used by healers in Colombia. Braz. J. Med. Biol. Res 2004, 37, 969–977. [Google Scholar]
- De Angelis Pereira, M.C.; Carvalho, J.C.; Lima, L.M.; Caputo, L.R.; Ferreira, L.R.; Fiorini, J.E.; Bastos, J.K. Toxicity of a subchronic treatment with hydroalcoholic crude extract from Solanum grandiflorum (Ruiz et Pav) in rats. J. Ethnopharmacol 2003, 89, 97–99. [Google Scholar]
- Sanz-Biset, J.; Campos-de-la-Cruz, J.; Epiquien-Rivera, M.A.; Canigueral, S. A first survey on the medicinal plants of the Chazuta valley (Peruvian Amazon). J. Ethnopharmacol 2009, 122, 333–362. [Google Scholar]
- Lopez, A.; Hudson, J.B.; Towers, G.H.N. Antiviral and antimicrobial activities of Colombian medicinal plants. J. Ethnopharmacol 2001, 77, 189–196. [Google Scholar]
- Mbwambo, Z.H.; Apers, S.; Moshi, M.J.; Kapingu, M.C.; van Miert, S.; Claeys, M.; Brun, R.; Cos, P.; Pieters, L.; Vlietinck, A. Anthranoid compounds with antiprotozoal activity from Vismia orientalis. Planta Med 2004, 70, 706–710. [Google Scholar]
- Nguemeving, J.R.; Azebaze, A.G.B.; Kuete, V.; Carly, N.N.E.; Beng, V.P.; Meyer, M.; Blond, A.; Bodo, B.; Nkengfack, A.E. Laurentixanthones A and B, antimicrobial xanthones from Vismia laurentii. Phytochemistry 2006, 67, 1341–1346. [Google Scholar]
- Menan, H.; Banzouzi, J.T.; Hocquette, A.; Pelissier, Y.; Blache, Y.; Kone, M.; Mallie, M.; Assi, L.A.; Valentin, A. Antiplasmodial activity and cytotoxicity of plants used in West African traditional medicine for the treatment of malaria. J. Ethnopharmacol 2006, 105, 131–136. [Google Scholar]
- Suffredini, I.B.; Paciencia, M.L.B.; Varella, A.D.; Younes, R.N. In vitro cytotoxic activity of Brazilian plant extracts against human lung, colon and CNS solid cancers and leukemia. Fitoterapia 2007, 78, 223–226. [Google Scholar]
- Estevez, Y.; Castillo, D.; Pisango, M.T.; Arevalo, J.; Rojas, R.; Alban, J.; Deharo, E.; Bourdy, G.; Sauvain, M. Evaluation of the leishmanicidal activity of plants used by Peruvian Chayahuita ethnic group. J. Ethnopharmacol 2007, 114, 254–259. [Google Scholar]
- Hussein, A.A.; Bozzi, B.; Correa, M.; Capson, T.L.; Kursar, T.A.; Coley, P.D.; Solis, P.N.; Gupta, M.P. Bioactive constituents from three Vismia species. J. Nat. Prod 2003, 66, 858–860. [Google Scholar]
- Fuller, R.W.; Westergaard, C.K.; Collins, J.W.; Cardellina, J.H.I.I.; Boyd, M.R. Vismiaphenones D–G, new prenylated benzophenones from Vismia cayennensis. J. Nat. Prod 1999, 62, 67–69. [Google Scholar]
- Kuete, V.; Nguemeving, J.R.; Beng, V.P.; Azebaze, A.G.B.; Etoa, F.X.; Meyer, M.; Bodo, B.; Nkengfack, A.E. Antimicrobial activity of the methanolic extracts and compounds from Vismia laurentii De Wild (Guttiferae). J. Ethnopharmacol 2007, 109, 372–379. [Google Scholar]
- Croft, K.D. The chemistry and biological effects of flavonoids and phenolic acids. Ann. N. Y. Acad. Sci 1998, 854, 435–442. [Google Scholar]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod 2000, 63, 1035–1042. [Google Scholar]
- Lizcano, L.J.; Bakkali, F.; Ruiz-Larrea, M.B.; Ruiz-Sanz, J.I. Antioxidant activity and polyphenol content of aqueous extracts from Colombian Amazonian plants with medicinal use. Food Chem 2010, 119, 1566–1570. [Google Scholar]
- Ruiz-Larrea, M.B.; Leal, A.M.; Liza, M.; Lacort, M.; de Groot, H. Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids 1994, 59, 383–388. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem 1976, 72, 248–254. [Google Scholar]
- Fee, J.A.; Teitelbaum, H.D. Evidence that superoxide dismutase plays a role in protecting red blood cells against peroxidative hemolysis. Biochem. Biophys. Res. Commun 1972, 49, 150–158. [Google Scholar]
- Abad-Garcia, B.; Berrueta, L.A.; Garmon-Lobato, S.; Gallo, B.; Vicente, F. A general analytical strategy for the characterization of phenolic compounds in fruit juices by high-performance liquid chromatography with diode array detection coupled to electrospray ionization and triple quadrupole mass spectrometry. J. Chromatogr. A 2009, 1216, 5398–5415. [Google Scholar]
- Sokmen, A.; Jones, B.M.; Erturk, M. The in vitro antibacterial activity of Turkish medicinal plants. J. Ethnopharmacol 1999, 67, 79–86. [Google Scholar]
- Candan, F.; Unlu, M.; Tepe, B.; Daferera, D.; Polissiou, M.; Sokmen, A.; Akpulat, H.A. Antioxidant and antimicrobial activity of the essential oil and methanol extracts of Achillea millefolium subsp millefolium Afan. (Asteraceae). J. Ethnopharmacol 2003, 87, 215–220. [Google Scholar]
| IC50 | ||||
|---|---|---|---|---|
| Species | Plant Part | Concentration (μg/mL) | Total Phenols (μg GAE a/mL) | Total Flavonoids (μg CE b/mL) |
| B. rosademonte | bark | 12.4 | 4.9 | 1.6 |
| P. glandulosissimum | leaf | 30.0 | 3.7 | 1.4 |
| P. glandulosissimum | stem | ND | ND | ND |
| P. krukoffii | leaf | 75.0 | 46.3 | 23.9 |
| P. krukoffii | stem | 68.0 | 34.0 | 17.2 |
| P. putumayoense | leaf | 17.9 | 8.7 | 4.5 |
| P. putumayoense | stem | 18.4 | 6.7 | 3.0 |
| S. grandiflorum | leaf | 97.7 | 22.4 | 5.5 |
| S. grandiflorum | stem | 24.1 | 4.5 | 2.0 |
| V. baccifera | leaf | 5.5 | 2.2 | 1.2 |
| V. baccifera | stem | 7.9 | 2.6 | 1.0 |
| Caffeic acid | 38.0 | |||
| Catechin | 3.0 | |||
| Gallic acid | 8.8 | |||
| Major Polyphenols and Sums of the Different Families Found | Rt (min) | B. rosademonte | P. glandulosissimum | P. glandulosissimum | S. grandiflorum | S. grandiflorum | V. baccifera | V. baccifera |
|---|---|---|---|---|---|---|---|---|
| Bark | Leaf | Stem | Leaf | Stem | Leaf | Stem | ||
| ∑FA | 171730 | 32296 | 7637 | n.d. | n.d. | 1971026 | 257339 | |
| (epi)cat-(epi)cat-(epi)cat | 9.10 | n.d. | 9344 | 1369 | n.d. | n.d. | n.d. | n.d. |
| (epi)cat-(epi)cat | 14.85 | 15104 | 3094 | n.d. | n.d. | n.d. | n.d. | n.d. |
| (epi)cat-(epi)cat | 15.87 | 6731 | 4786 | 2630 | n.d. | n.d. | n.d. | n.d. |
| (epi)cat-(epi)cat-(epi)cat | 16.45 | n.d. | n.d. | 1685 | n.d. | n.d. | n.d. | n.d. |
| (epi)cat-(epi)cat | 17.58 | 12219 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Cat | 19.37 | 28346 | 9966 | 3225 | n.d. | n.d. | - | - |
| (epi)cat-(epi)cat-(epi)cat | 19.73 | n.d. | n.d. | n.d. | n.d. | n.d. | 50524 | 8592 |
| (epi)cat-(epi)cat-(epi)cat | 20.98 | n.d. | n.d. | n.d. | n.d. | n.d. | 82616 | 14036 |
| (epi)cat-(epi)cat | 22.30 | 45400 | n.d. | n.d. | n.d. | n.d. | 422618 | 64600 |
| Epicat | 28.93 | 48261 | 1285 | 413 | n.d. | n.d. | 463423 | 112225 |
| (epi)cat-(epi)cat-(epi)cat | 29.37 | n.d. | n.d. | n.d. | n.d. | n.d. | 523159 | n.d. |
| (epi)cat-(epi)cat-(epi)cat | 31.22 | n.d. | n.d. | n.d. | n.d. | n.d. | 129118 | 23137 |
| (epi)cat-(epi)cat-(epi)cat | 34.17 | n.d. | n.d. | n.d. | n.d. | n.d. | 41978 | 4828 |
| (epi)cat-(epi)cat-(epi)cat | 37.22 | n.d. | n.d. | n.d. | n.d. | n.d. | 83613 | n.d. |
| (epi)cat-(epi)cat-(epi)cat | 85.17 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 1627 |
| ∑FVL | n.d. | 11232 | 967 | 12401 | n.d. | 106166 | 2696 | |
| Que-pent-deoxyhex-hex | 75.53 | n.d. | 1045 | 165 | n.d. | n.d. | n.d. | n.d. |
| Kam-deoxyhex-hex | 76.18 | n.d. | n.d. | n.d. | 5605 | n.d. | n.d | n.d. |
| Que-hex | 82.28 | n.d. | n.d. | n.d. | n.d. | n.d. | 23930 | n.d. |
| Que-deoxyhex-hex | 83.73 | n.d. | 1521 | 344 | n.d. | n.d. | 6678 | 1788 |
| Kam-pent-deoxyhex-hex | 84.37 | n.d. | 2016 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Kam-pent-deoxyhex-hex | 87.00 | n.d. | 1771 | 56 | n.d. | n.d. | n.d. | n.d. |
| Coelution of iso-pent-deoxyhex-hex and kam-deoxyhex-hex | 92.42 | n.d. | 1207 | 123a | n.d. | n.d. | n.d. | n.d. |
| Que-deoxyhex | 96.18 | n.d. | n.d. | n.d. | n.d. | n.d. | 31258 | 908 |
| Kam-hex | 97.28 | n.d. | n.d. | n.d. | n.d. | n.d. | 22859 | n.d. |
| Kam-deoxyhex-hex | 99.78 | n.d. | 1877 | n.d. | n.d. | n.d. | n.d. | n.d. |
| ∑HCA | n.d. | 111242 | 4323 | 1140885 | 565630 | 117394 | n.d. | |
| CQA | 16.35 | n.d. | 18512 | n.d. | 110605 | 40783 | n.d | n.d. |
| p-CoQA | 21.03 | n.d. | 8420 | n.d. | n.d. | n.d. | n.d. | n.d. |
| p-CoQA | 21.32 | n.d. | 29335 | 1318 | 11566 | n.d. | n.d | n.d. |
| CQA | 24.13 | n.d. | 4568 | n.d. | 350237 | 239158 | 91738 | n.d. |
| CQA | 26.77 | n.d. | 12856 | n.d. | 112527 | 61954 | n.d | n.d. |
| p-CoQA | 30.53 | n.d. | 8222 | 389 | n.d. | n.d. | n.d. | n.d. |
| p-CoQA | 38.12 | n.d. | 17518 | 727 | n.d. | n.d. | n.d | n.d. |
| diCQA | 82.88 | n.d. | n.d. | n.d. | 221270 | 94436 | n.d | n.d. |
| diCQA | 84.60 | n.d. | n.d. | n.d. | 180674 | 49423 | n.d | n.d. |
| diCQA | 98.50 | n.d. | n.d. | n.d. | 110713 | 79875 | n.d | n.d. |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lizcano, L.J.; Viloria-Bernal, M.; Vicente, F.; Berrueta, L.A.; Gallo, B.; Martínez-Cañamero, M.; Ruiz-Larrea, M.B.; Ruiz-Sanz, J.I. Lipid Oxidation Inhibitory Effects and Phenolic Composition of Aqueous Extracts from Medicinal Plants of Colombian Amazonia. Int. J. Mol. Sci. 2012, 13, 5454-5467. https://doi.org/10.3390/ijms13055454
Lizcano LJ, Viloria-Bernal M, Vicente F, Berrueta LA, Gallo B, Martínez-Cañamero M, Ruiz-Larrea MB, Ruiz-Sanz JI. Lipid Oxidation Inhibitory Effects and Phenolic Composition of Aqueous Extracts from Medicinal Plants of Colombian Amazonia. International Journal of Molecular Sciences. 2012; 13(5):5454-5467. https://doi.org/10.3390/ijms13055454
Chicago/Turabian StyleLizcano, Leandro J., María Viloria-Bernal, Francisca Vicente, Luis Angel Berrueta, Blanca Gallo, Magdalena Martínez-Cañamero, Maria Begoña Ruiz-Larrea, and José Ignacio Ruiz-Sanz. 2012. "Lipid Oxidation Inhibitory Effects and Phenolic Composition of Aqueous Extracts from Medicinal Plants of Colombian Amazonia" International Journal of Molecular Sciences 13, no. 5: 5454-5467. https://doi.org/10.3390/ijms13055454
APA StyleLizcano, L. J., Viloria-Bernal, M., Vicente, F., Berrueta, L. A., Gallo, B., Martínez-Cañamero, M., Ruiz-Larrea, M. B., & Ruiz-Sanz, J. I. (2012). Lipid Oxidation Inhibitory Effects and Phenolic Composition of Aqueous Extracts from Medicinal Plants of Colombian Amazonia. International Journal of Molecular Sciences, 13(5), 5454-5467. https://doi.org/10.3390/ijms13055454





