Pharmacological Evaluation and Preliminary Pharmacokinetics Studies of a New Diclofenac Prodrug without Gastric Ulceration Effect
Abstract
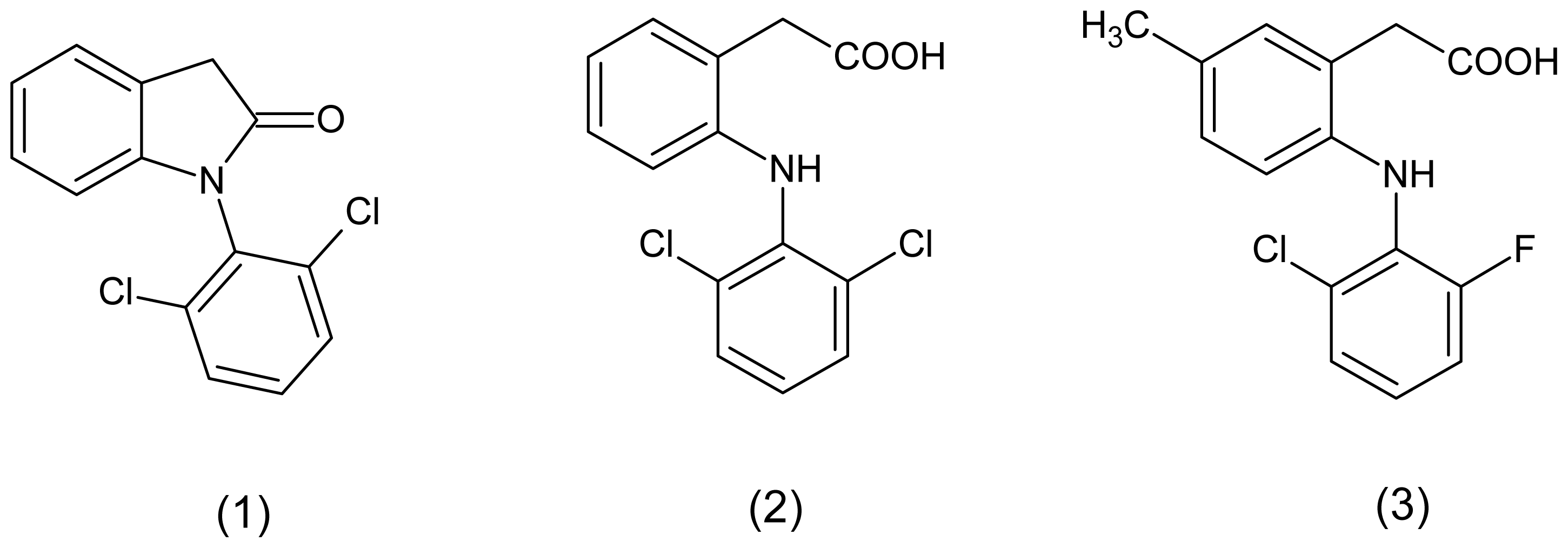
:1. Introduction
2. Results and Discussion
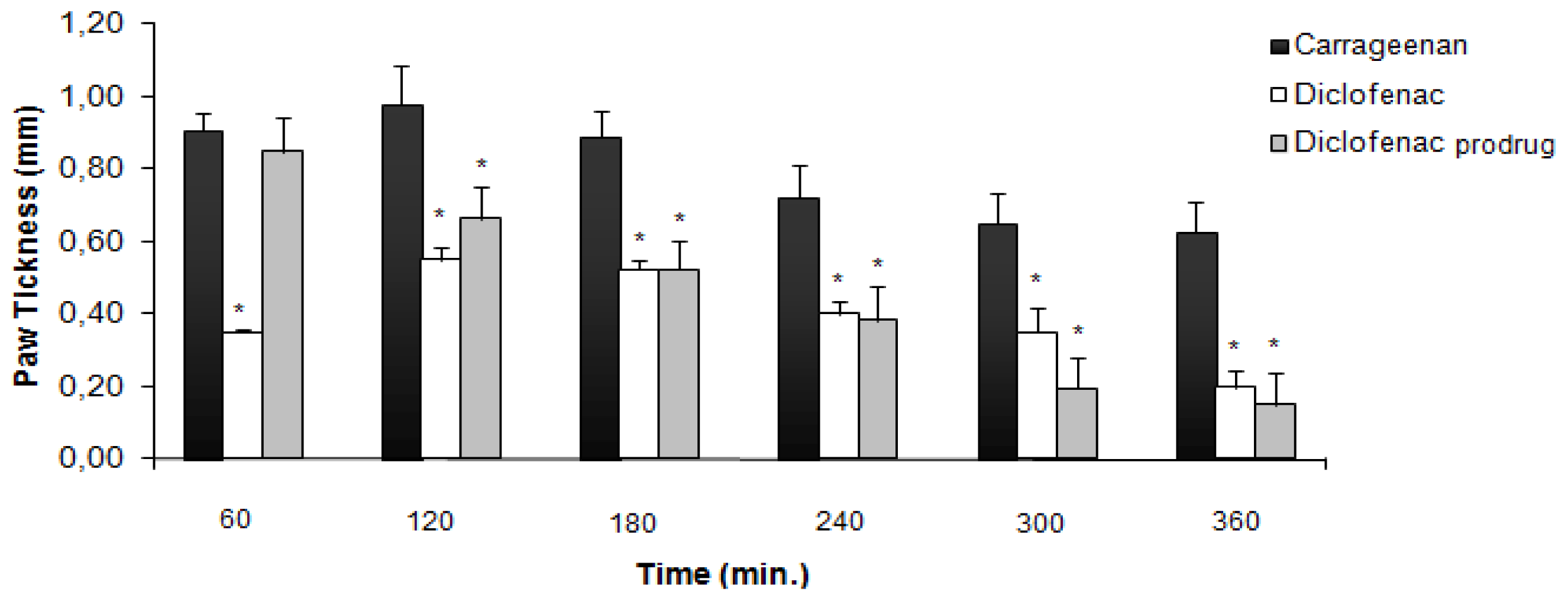
2.1. Carrageenan-Induced Paw Edema
2.2. Ulcerogenicity
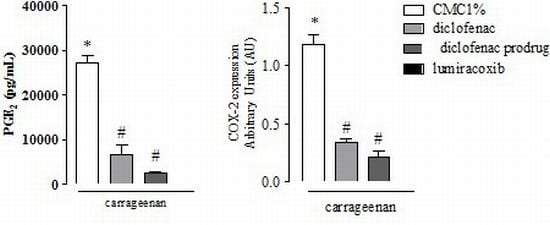
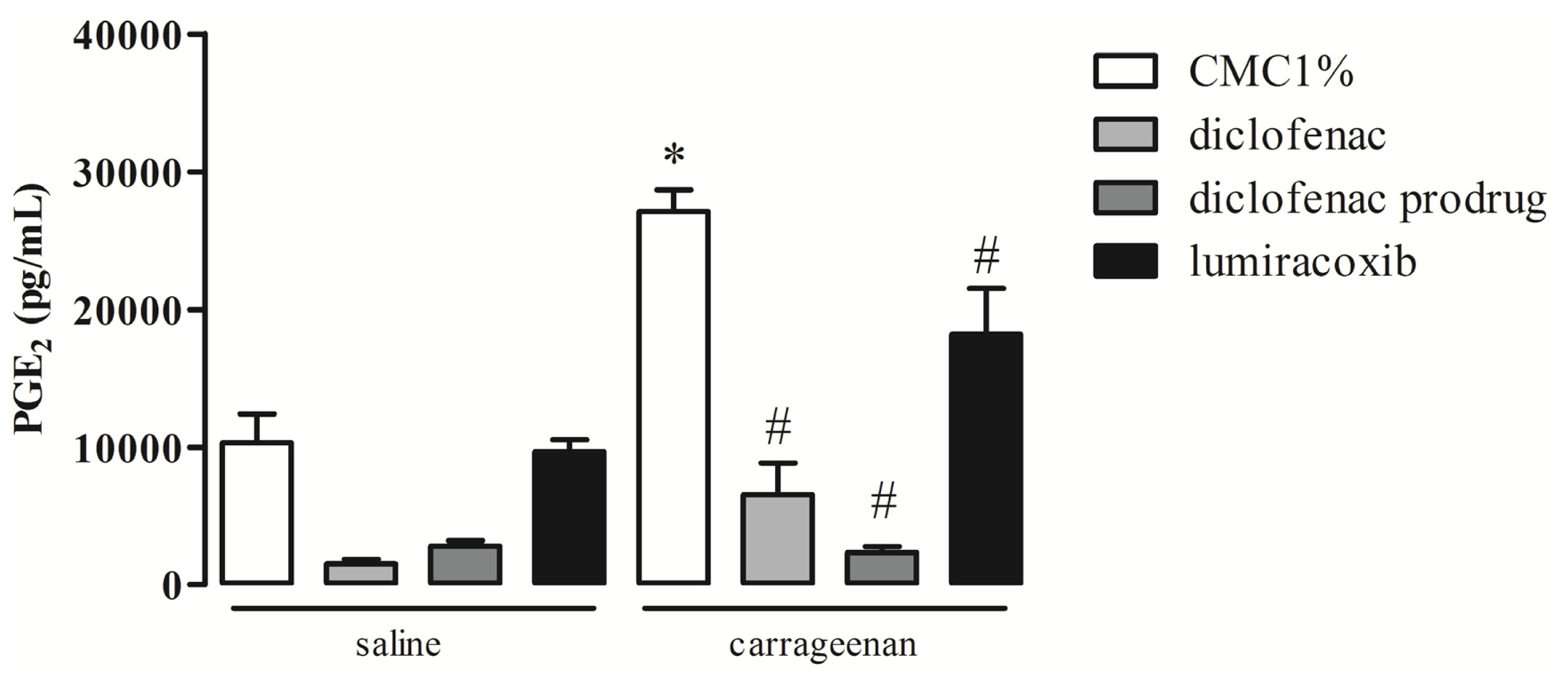
2.3. Effect of Diclofenac Prodrug Treatment on Carrageenan-Induced Release of PGE2
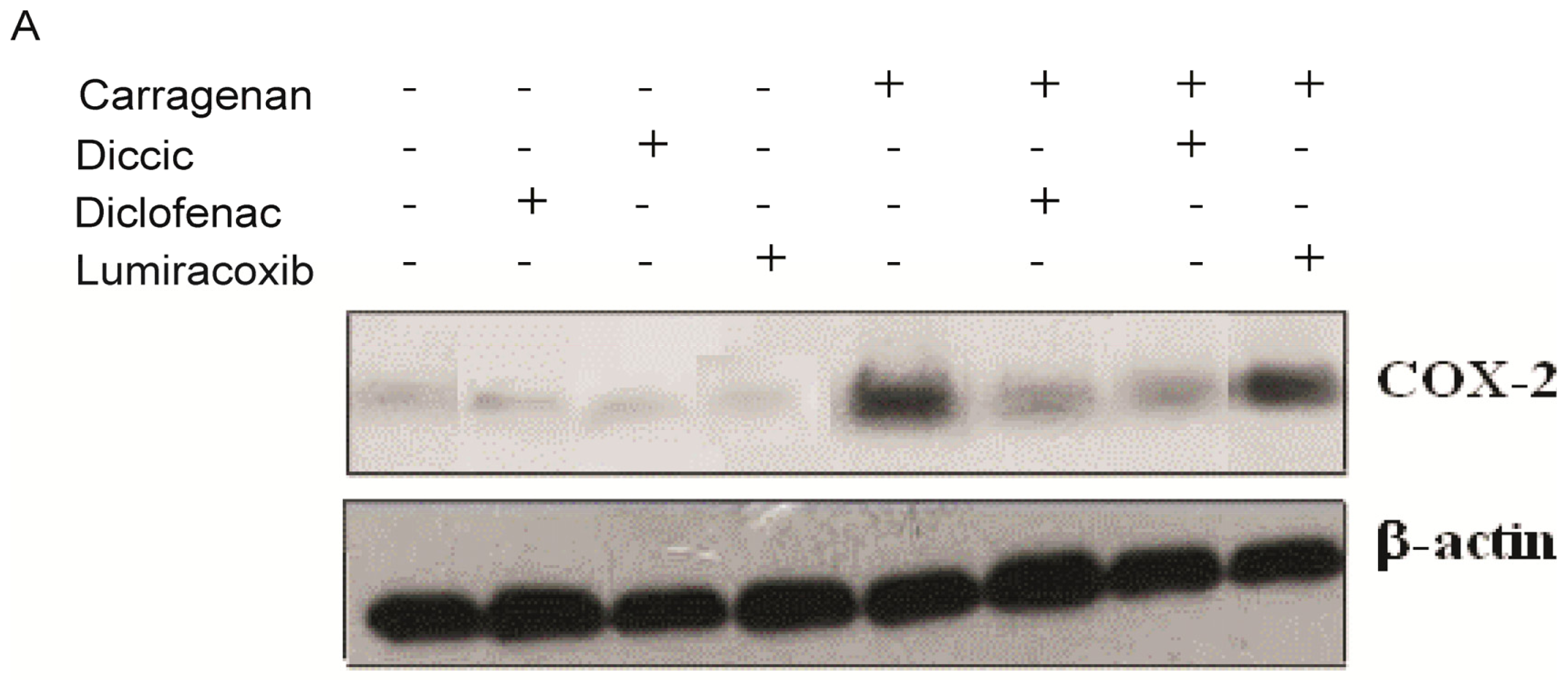
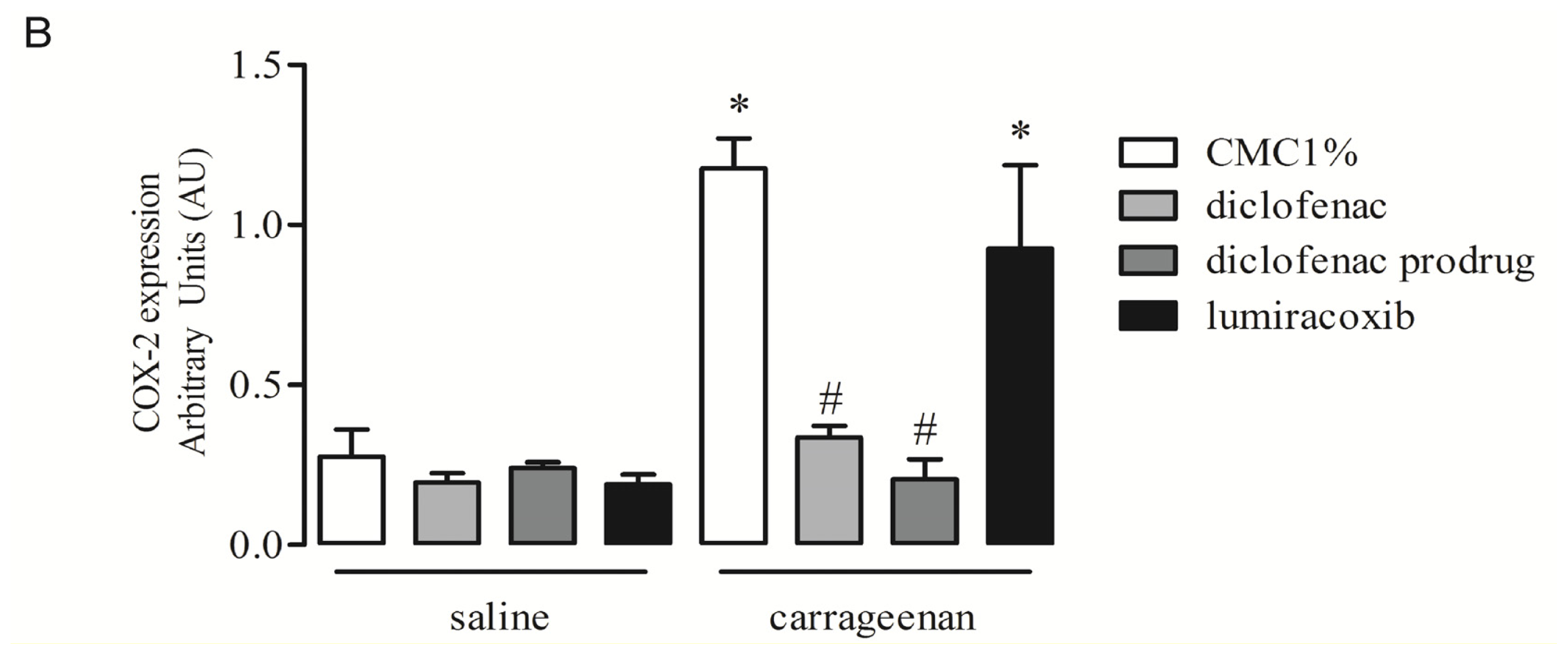
2.4. Effect of Diclofenac Prodrug Treatment on Carrageenan-Induced COX-2 Protein Expression by Peritoneal Leukocytes
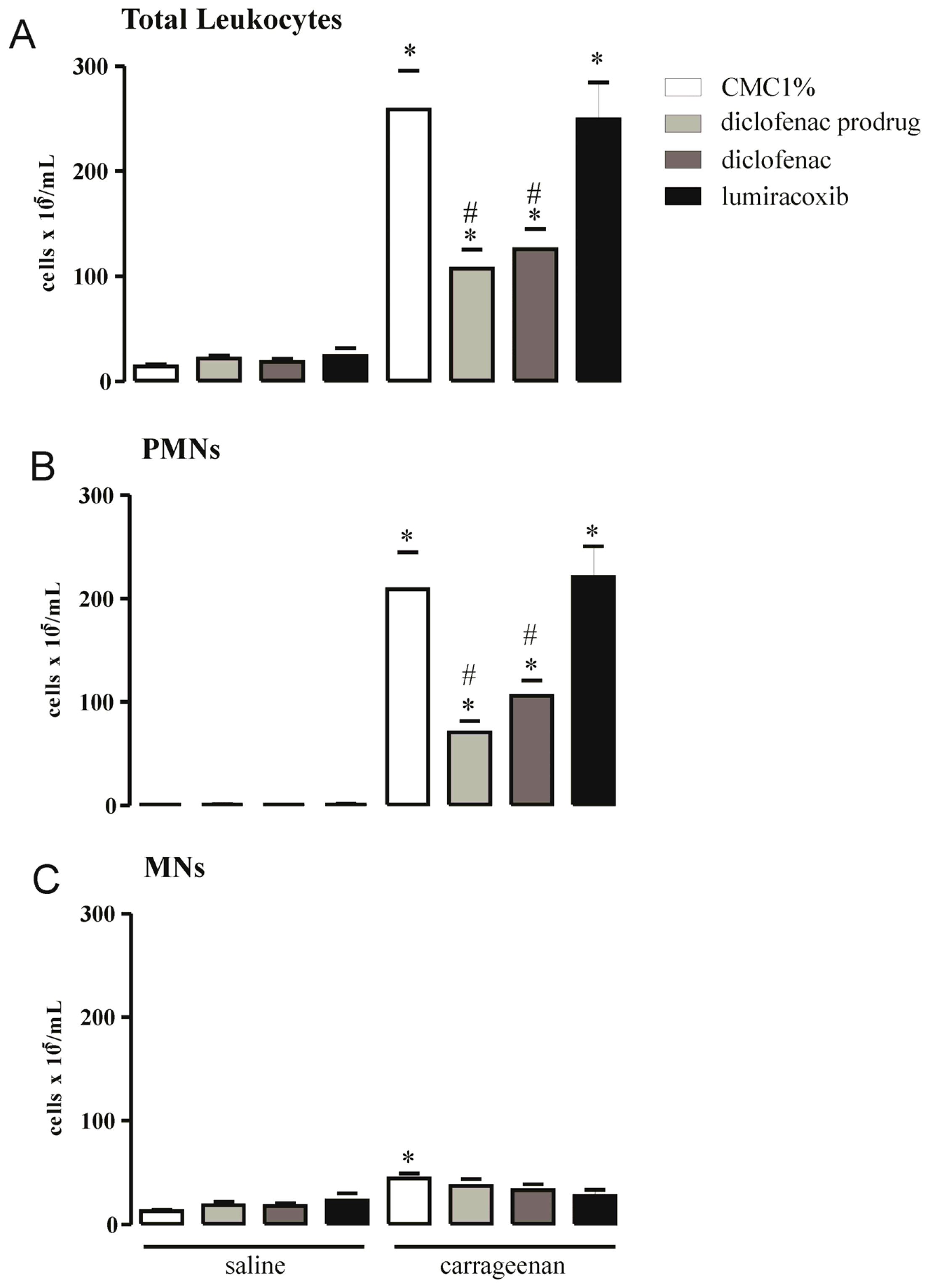
2.5. Effect of Diclofenac Prodrug Treatment on Carrageenan-Induced Cellular Influx into Peritoneal Cavity
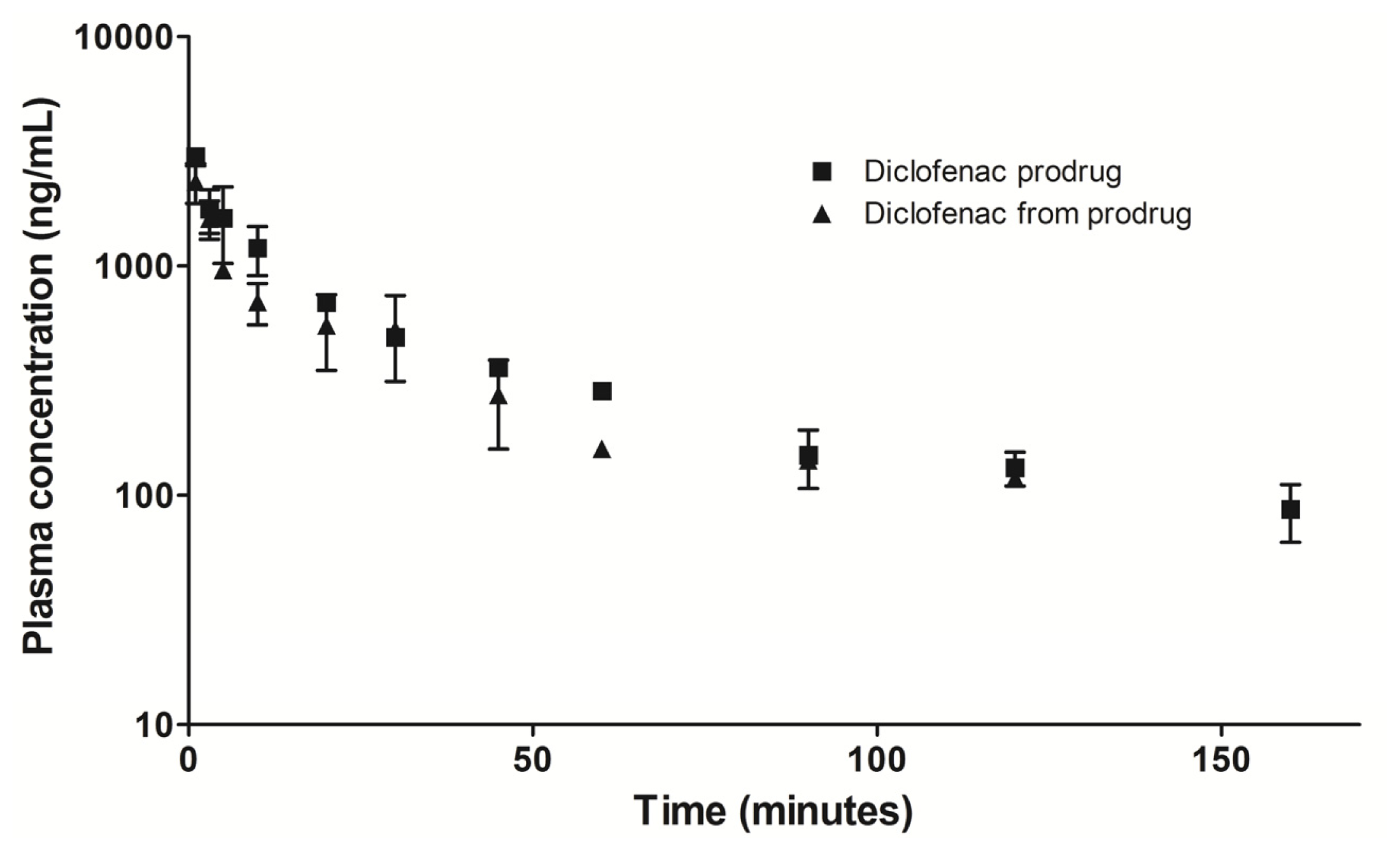
2.6. Preliminary Plasma Concentration-Time Profile
3. Experimental Section
3.1. Material
3.2. Animals
3.3. Carrageenan-Induced Paw Edema
3.4. Ulcerogenicity
3.5. Treatments with Diclofenac Prodrug and NSAIDs
3.6. Carrageenan-Induced Inflammation in Peritoneum of Mice
3.7. Leukocyte Harvesting and Counting
3.8. Western Blotting
3.9. PGE2 Quantification
3.10. Data Analysis
3.11. Preliminary Plasma Concentration-Time Profile
4. Conclusions
Acknowledgments
References
- Cena, C.; Lolli, M.L.; Lazzarato, L.; Guaita, E.; Morini, G.; Coruzzi, G.; McElroy, S.P.; Megson, I.L.; Fruttero, R.; Gasco, A. Antiinflammatory, gastrosparing and antiplatelet properties of new NO-donor esters of aspirin. J. Med. Chem 2003, 46, 747–754. [Google Scholar]
- Sardi, F.; Fassina, L.; Venturini, L.; Inguscio, M.; Guerriero, F.; Rolfo, E.; Ricevuti, G. Alzheimer’s disease, autoimmunety and inflammation. The good, the bad and the ugly. Autoimmun. Rev 2011, 11, 149–153. [Google Scholar]
- Charakida, M.; O’Neil, F.; Masi, S.; Papageorgiou, N.; Tousoulis, D. Inflammatory disorders and atherosclerosis: New therapeutic approaches. Curr. Pharm. Des 2011, 17, 4111–4120. [Google Scholar]
- Cha, Y.I.; DuBois, R.N. NSAIDs and cancer prevention: Targets downstream of COX-2. Annu. Rev. Med 2007, 58, 239–252. [Google Scholar]
- Becker, R.C. Aspirin and the prevention of venous thromboembolism. N. Engl. J. Med 2012, 366, 2028–2030. [Google Scholar]
- Rouzer, C.A.; Marnett, L.J. Cyclooxygenases: Structural and functional insights. J. Lipid. Res 2009, 50, S29–S34. [Google Scholar]
- Go, M.F. Drug injury in the upper gastrointestinal tract: Nonsteroidal anti-inflammatory drugs. Gastrointest. Endosc. Clin. N. Am 2006, 16, 83–97. [Google Scholar]
- Laine, L. Approaches to nonsteroidal anti-inflammatory drug use in the high-risk patient. Gastroenterology 2001, 120, 594–606. [Google Scholar]
- Silverstein, F.E.; Graham, D.Y.; Senior, J.R.; Davies, H.W.; Struthers, B.J.; Bittman, R.M.; Geis, G.S. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med 1995, 123, 241–249. [Google Scholar]
- Mukherjee, D.; Nissen, S.E.; Topol, E.J. Risk of cardiovascular events associated with selective COX-2 inhibitors. J. Am. Med. Assoc 2001, 286, 954–959. [Google Scholar]
- Wermuth, C.G. Selective optimization of side activities: Another way for drug discovery. J. Med. Chem 2004, 47, 1303–1314. [Google Scholar]
- Bosquesi, P.L.; Melo, T.R.F.; Vizioli, E.O.; Santos, J.L.; Chung, M.C. Anti-inflammatory drug design using a molecular hybridization approach. Pharmaceuticals 2011, 4, 1450–1474. [Google Scholar]
- Halen, P.K.; Murumkar, P.R.; Giridhar, R.; Yadav, M.R. Prodrug designing of NSAIDs. Mini Rev. Med. Chem 2009, 9, 124–139. [Google Scholar]
- Khan, M.S.; Akhter, M. Glyceride derivatives as potential prodrugs: Synthesis, biological activity and kinetic studies of glyceride derivatives of mefenamic acid. Pharmazie 2005, 60, 110–114. [Google Scholar]
- Halen, P.K.; Chagti, K.K.; Giridhar, R.; Yadav, M.R. Synthesis and pharmacological evaluation of some dual-acting amino-alcohol ester derivatives of flurbiprofen and 2-[1,1′-biphenyl-4-yl]acetic acid: A potential approach to reduce local gastrointestinal toxicity. Chem. Biodivers 2006, 3, 1238–1248. [Google Scholar]
- Chung, M.C.; Santos, J.L.; Oliveira, E.V.; Blau, L.; Menegon, R.F.; Peccinini, R.G. Synthesis, ex vivo and in vitro hydrolysis study of an indoline derivative designed as an anti-inflammatory with reduced gastric ulceration properties. Molecules 2009, 14, 3187–3197. [Google Scholar]
- Bandarage, U.K.; Chen, L.; Fang, X.; Garvey, D.S.; Glavin, A.; Janero, D.R.; Letts, L.G.; Mercer, G.J.; Saha, J.K.; Schroeder, J.D.; et al. Nitrosothiol esters of diclofenac: Synthesis and pharmacological characterization as gastrointestinal-sparing prodrugs. J. Med. Chem 2000, 43, 4005–4016. [Google Scholar]
- Kim, H.; Jeon, H.; Kong, H.; Yang, Y.; Choi, B.; Kim, Y.M.; Neckers, L.; Jung, Y. A molecular mechanism for the anti-inflammatory effect of taurine-conjugated 5-amino-salicylic acid in inflamed colon. Mol. Pharmacol 2006, 69, 1405–1412. [Google Scholar]
- Pathan, A.R.; Karwa, M.; Pamidiboina, V.; Deshattiwar, J.J.; Deshmukh, N.J.; Gaikwad, P.P.; Mali, S.V.; Desai, D.C.; Dhiman, M.; Thanga Mariappan, T.; et al. Oral bioavailability, efficacy and gastric tolerability of P2026, a novel nitric oxide-releasing diclofenac in rat. Inflammopharmacology 2010, 18, 157–168. [Google Scholar]
- Agrawal, N.; Chandrasekar, M.J.; Sara, U.V.; Rohini, A. Synthesis, characterization, and in vitro drug release study of methacrylate diclofenac conjugate as macromolecular prodrug. PDA J. Pharm. Sci. Technol 2010, 64, 348–355. [Google Scholar]
- Tammara, V.K.; Narurkar, M.M.; Crider, A.M.; Khan, M.A. Morpholinoalkyl ester prodrugs of diclofenac: Synthesis, in vitro and in vivo evaluation. J. Pharm. Sci 1994, 83, 644–648. [Google Scholar]
- Bandgar, B.P.; Sarangdhar, R.J.; Ahamed, F.A.; Viswakarma, S. Synthesis, characterization, and biological evaluation of novel diclofenac prodrugs. J. Med. Chem 2011, 54, 1202–1210. [Google Scholar]
- Dos Santos, J.L.; Chelucci, R.; Chiquetto, R.; Chung, M.C.; Campos, M.L.; Peccinini, R.G. Synthesis, characterization and pharmacological evaluation of 1-(2-Chloro-6-Fluorophenyl)-5- Methylindolin-2-One: A new anti-inflammatory compound with reduced gastric ulceration properties. Molecules 2010, 15, 8039–8047. [Google Scholar]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-induced edema in hind paw of rat as an assay for antiinflammatory drugs. Proc. Soc. Exp. Biol. Med 1962, 111, 544–547. [Google Scholar]
- Cioli, V.; Putzolu, S.; Rossi, V.; Corza, B.P.; Corradino, C. The role of direct tissue contact in the production of gastrointestinal ulcers by anti-inflammatory drugs in rats. Toxicol. Appl. Pharmacol 1979, 50, 283–289. [Google Scholar]
- Portanova, J.P.; Zhang, Y.; Anderson, G.D.; Hauser, S.D.; Masferrer, J.L.; Seibert, K.; Gregory, S.A.; Isakson, P.C. Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J. Exp. Med 1996, 184, 883–889. [Google Scholar]
- Nakamura, M.; Ferreira, S.H. A peripheral sympathetic component in inflammatory hyperalgesia. Eur. J. Pharmacol 1987, 135, 145–153. [Google Scholar]
- Poole, S.; Lorenzetti, B.B.; Cunha, J.M.; Cunha, F.Q.; Ferreira, S.H. Bradykinin B1 and B2 receptors, tumour necrosis factor alpha and inflammatory hyperalgesia. Br. J. Pharmacol 1999, 126, 649–656. [Google Scholar]
- Posadas, I.; Bucci, M.; Roviezzo, F.; Rossi, A.; Parente, L.; Sautebin, L.; Cirino, G. Carrageenan-induced mouse paw oedema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. Br. J. Pharmacol 2004, 142, 331–338. [Google Scholar]
- Elder, D.J.; Paraskeva, C. NSAIDs to prevent colorectal cancer: A question of sensitivity. Gastroenterology 1997, 113, 1999–2003. [Google Scholar]
- Tegeder, I.; Niederberger, E.; Israr, E.; Gühring, H.; Brune, K.; Euchenhofer, C.; Grösch, S.; Geisslinger, G. Inhibition of NF-kappaB and AP-1 activation by R- and S-flurbiprofen. FASEB J 2001, 15, 2–4. [Google Scholar]
- Grösch, S.; Tegeder, I.; Niederberger, E.; Bräutigam, L.; Geisslinger, G. COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. FASEB J 2001, 15, 2742–2744. [Google Scholar]
- Maier, T.J.; Janssen, A.; Schmidt, R.; Geisslinger, G.; Grösch, S. Targeting the beta-catenin/APC pathway: A novel mechanism to explain the cyclooxygenase-2-independent anticarcinogenic effects of celecoxib in human colon carcinoma cells. FASEB J 2005, 19, 1353–1355. [Google Scholar]
- Bonaterra, G.A.; Heinrichc, E.U.; Kelberc, O.; Weiserc, D.; Metzd, J.; Kinscherfa, R. Anti-inflammatory effects of the willow bark extract STW 33-I (Proaktiv®) in LPS-activated human monocytes and differentiated macrophages. Phytomedicine 2010, 17, 1106–1113. [Google Scholar]
- Adamson, D.J.A.; Frew, D.; Tatoud, R.C.; Wolf, R.; Palmer, C.N.A. Diclofenac antagonizes peroxisome proliferator-activated receptor-signaling. Mol. Pharmacol 2001, 61, 7–12. [Google Scholar]
- Cuzzocrea, S.; Mazzon, E.; Di Paola, R.; Peli, A.; Bonato, A.; Britti, D.; Genovese, T.; Muia, C.; Crisafulli, C.; Caputi, A.P. The role of the peroxisome proliferator-activated receptor-(PPAR-) in the regulation of acute inflammation. J. Leuk. Biol 2006, 79, 999–1010. [Google Scholar]
- Kaur, J.; Sanyal, S.N. Modulation of inflammatory changes in early stages of colon cancer through activation of PPAR gamma by diclofenac. Eur. J. Cancer Prev 2010, 19, 319–327. [Google Scholar]
- Kaur, J.; Sanyal, S.N. Diclofenac, a selective COX-2 inhibitor, inhibits DMH-induced colon tumorigenesis through suppression of MCP-1, MIP-1α and VEGF. Mol. Carcinog 2011, 50, 707–718. [Google Scholar]
- Niederberger, E.; Manderscheid, C.; Geisslinger, G. Different COX-independent effects of the COX-2 inhibitors etoricoxib and lumiracoxib. Biochem. Biophys. Res. Commun 2006, 342, 940–948. [Google Scholar]
- Malech, H.L.; Gallin, J.I. Current concepts: Immunology. Neutrophils in human diseases. N. Engl. J. Med 1987, 317, 687–694. [Google Scholar]
- Utsunomiya, I.; Nagai, S.; Oh-ishi, S. Sequential appearance of IL-1 and IL-6 activities in rat carrageenin-induced pleurisy. J. Immunol 1991, 147, 1803–1809. [Google Scholar]
- Saleh, T.S.; Calixto, J.B.; Medeiros, Y.S. Effects of anti-inflammatory drugs upon nitrate and myeloperoxidase levels in the mouse pleurisy induced by carrageenan. Peptides 1999, 20, 949–956. [Google Scholar]
- Cuzzocrea, S.; Sautebin, L.; De Sarro, G.; Costantino, G.; Rombola, L.; Mazzon, E.; Ialenti, A.; De Sarro, A.; Ciliberto, G.; Di Rosa, M.; et al. Role of IL-6 in the pleurisy and lung injury caused by carrageenan. J. Immunol 1999, 163, 5094–5104. [Google Scholar]
- Scheja, A.; Forsgren, A.; Marsal, L.; Wollheim, F. Inhibition of in vivo leucocyte migration by NSAIDs. Clin. Exp. Rheumatol 1985, 3, 53–58. [Google Scholar]
- Hartmann, P.; Szabó, A.; Eros, G.; Gurabi, D.; Horváth, G.; Németh, I.; Ghyczy, M.; Boros, M. Anti-inflammatory effects of phosphatidylcholine in neutrophil leukocyte-dependent acute arthritis in rats. Eur. J. Pharmacol 2009, 622, 58–64. [Google Scholar]
- Cardoso, M.L.; Xavier, C.A.C.; Bezerra, M.E.B.; Paiva, A.A.O.; Carvalho, M.; Goretti, F.; Benevides, N.M.B.; Rocha, F.A.C.; Leite, E.L. Assessment of zymosan-induced leukocyte influx in a rat model using sulfated polysaccharides. Planta Med 2010, 76, 113–119. [Google Scholar]
- Laemmli, U.K. Cleavange of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar]
- Pradelles, P.; Grassi, J.; Mac Louf, J. Enzyme immunoassays of eicosanoids using acetylcholine esterase as label: An alternative to radioimmunoassay. Anal. Chem 1985, 57, 1170–1173. [Google Scholar]
| Compounds | Number of ulcers | <1 mm | 1–2 mm | >2 mm |
|---|---|---|---|---|
| Lumiracoxib | 19 ± 5.1 * | 17 ± 4.2 (89%) | 2 ± 0.5 (11%) | 0 |
| Diclofenac | 94 ± 12.8 | 66 ± 8.2 (70%) | 20 ± 2.8 (29.9%) | 8 ± 3.2 (0.1%) |
| Diclofenac prodrug | 3 ± 1.1 * | 3 ± 1.1 (100%) | 0 | 0 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Santos, J.L.d.; Moreira, V.; Campos, M.L.; Chelucci, R.C.; Barbieri, K.P.; Souto, P.C.M.d.C.; Matsubara, M.H.; Teixeira, C.; Bosquesi, P.L.; Peccinini, R.G.; et al. Pharmacological Evaluation and Preliminary Pharmacokinetics Studies of a New Diclofenac Prodrug without Gastric Ulceration Effect. Int. J. Mol. Sci. 2012, 13, 15305-15320. https://doi.org/10.3390/ijms131115305
Santos JLd, Moreira V, Campos ML, Chelucci RC, Barbieri KP, Souto PCMdC, Matsubara MH, Teixeira C, Bosquesi PL, Peccinini RG, et al. Pharmacological Evaluation and Preliminary Pharmacokinetics Studies of a New Diclofenac Prodrug without Gastric Ulceration Effect. International Journal of Molecular Sciences. 2012; 13(11):15305-15320. https://doi.org/10.3390/ijms131115305
Chicago/Turabian StyleSantos, Jean Leandro dos, Vanessa Moreira, Michel Leandro Campos, Rafael Consolin Chelucci, Karina Pereira Barbieri, Pollyana Cristina Maggio de Castro Souto, Márcio Hideki Matsubara, Catarina Teixeira, Priscila Longhin Bosquesi, Rosângela Gonçalves Peccinini, and et al. 2012. "Pharmacological Evaluation and Preliminary Pharmacokinetics Studies of a New Diclofenac Prodrug without Gastric Ulceration Effect" International Journal of Molecular Sciences 13, no. 11: 15305-15320. https://doi.org/10.3390/ijms131115305
APA StyleSantos, J. L. d., Moreira, V., Campos, M. L., Chelucci, R. C., Barbieri, K. P., Souto, P. C. M. d. C., Matsubara, M. H., Teixeira, C., Bosquesi, P. L., Peccinini, R. G., & Chin, C. M. (2012). Pharmacological Evaluation and Preliminary Pharmacokinetics Studies of a New Diclofenac Prodrug without Gastric Ulceration Effect. International Journal of Molecular Sciences, 13(11), 15305-15320. https://doi.org/10.3390/ijms131115305