Nutraceutical Approach for Preventing Obesity-Related Colorectal and Liver Carcinogenesis
Abstract
:1. Introduction
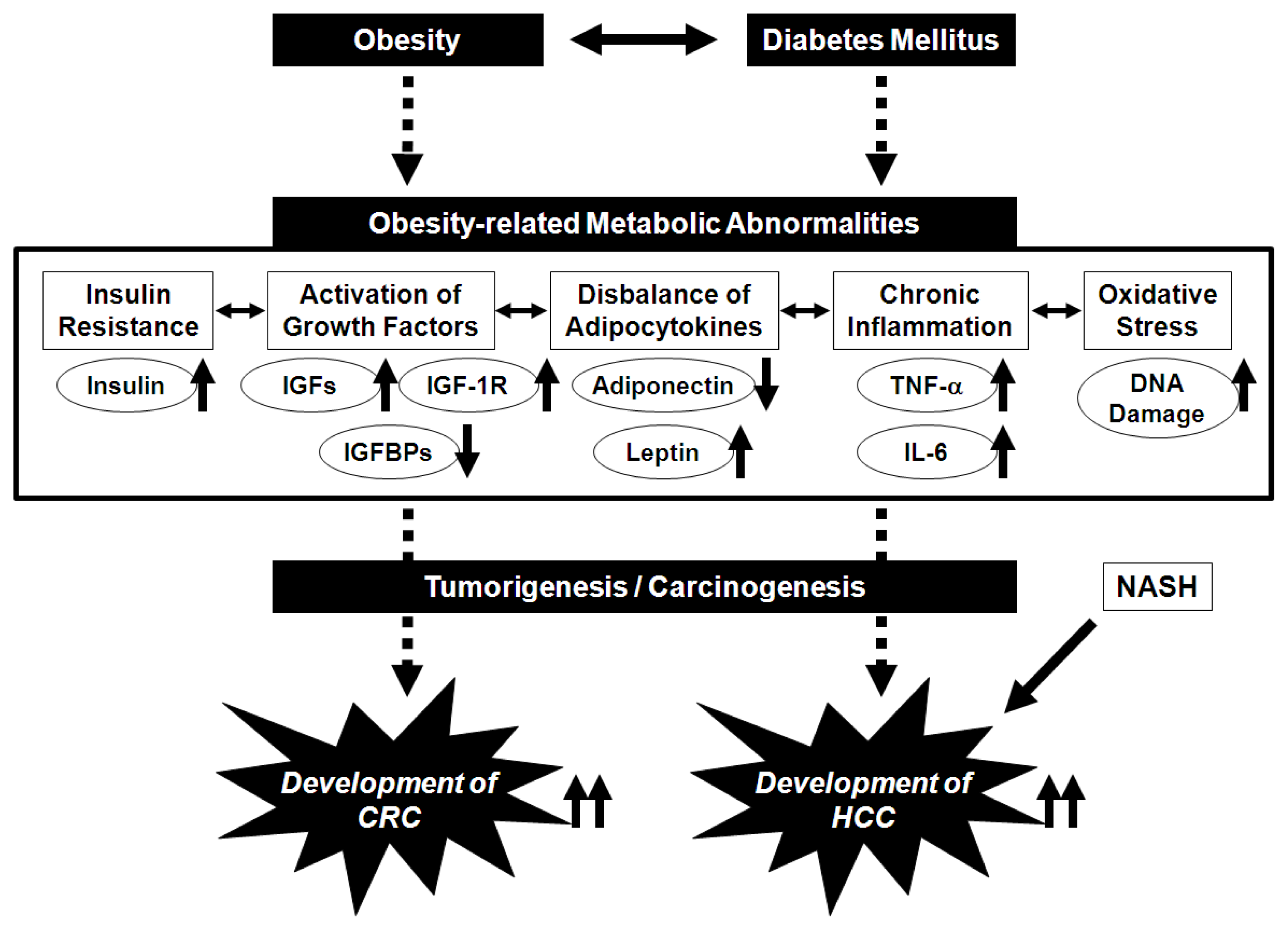
2. Potential Pathophysiological Mechanisms Linking Obesity and the Development of CRC
3. Potential Pathophysiological Mechanisms Linking Obesity, Non-Alcoholic Fatty Liver Disease/Non-Alcoholic Steatohepatitis, and the Development of HCC
4. Preventive Effects of GTCs on the Metabolic Abnormalities and Cancer Development
5. Preventive Effects of BCAA on Metabolic Abnormalities and HCC in Obese, Cirrhotic Patients: Results Form the LOTUS Study
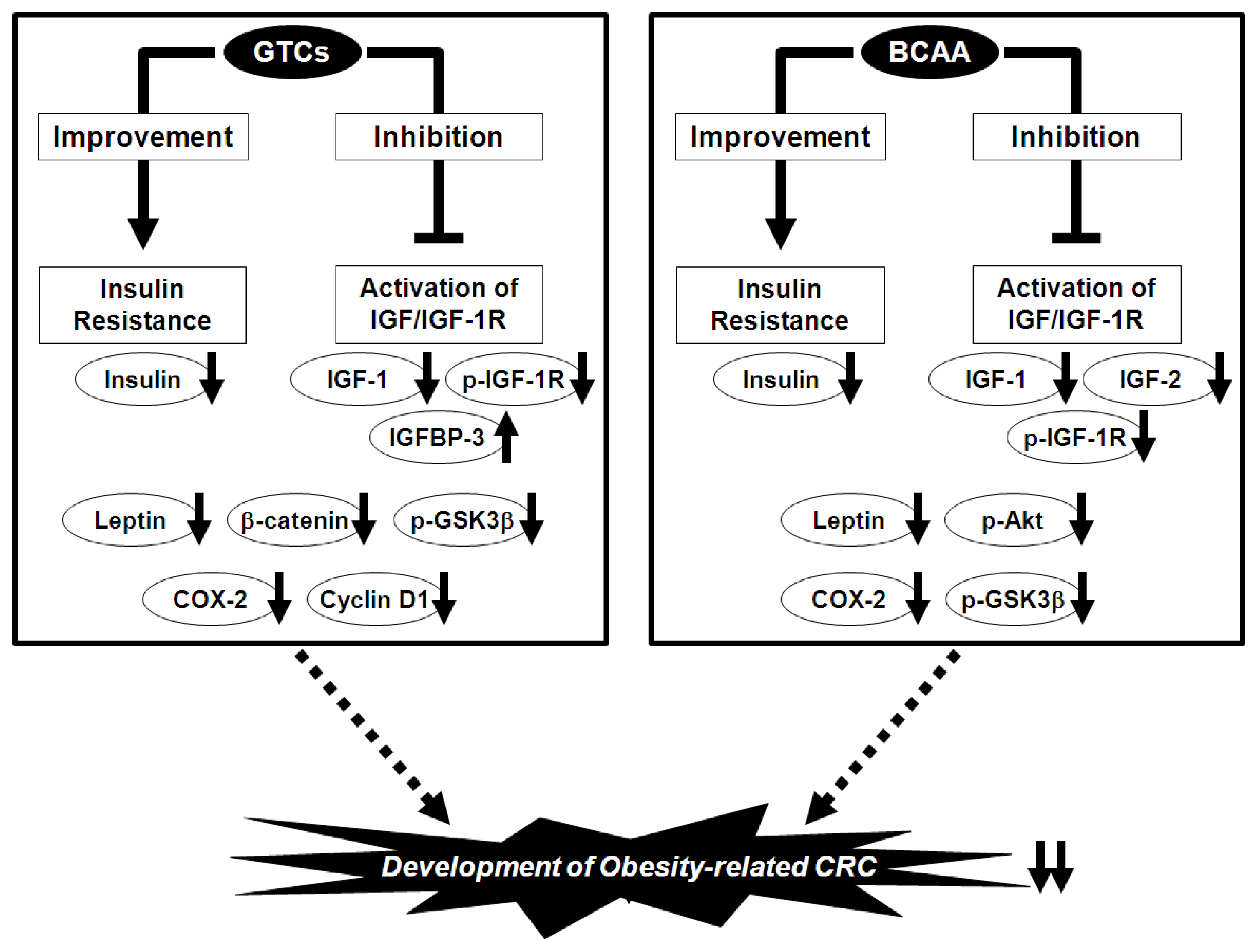
6. Prevention of Obesity-Related CRC via the Nutraceutical Approach—GTCs and BCAA Effectively Prevent Obesity-Related Colorectal Carcinogenesis
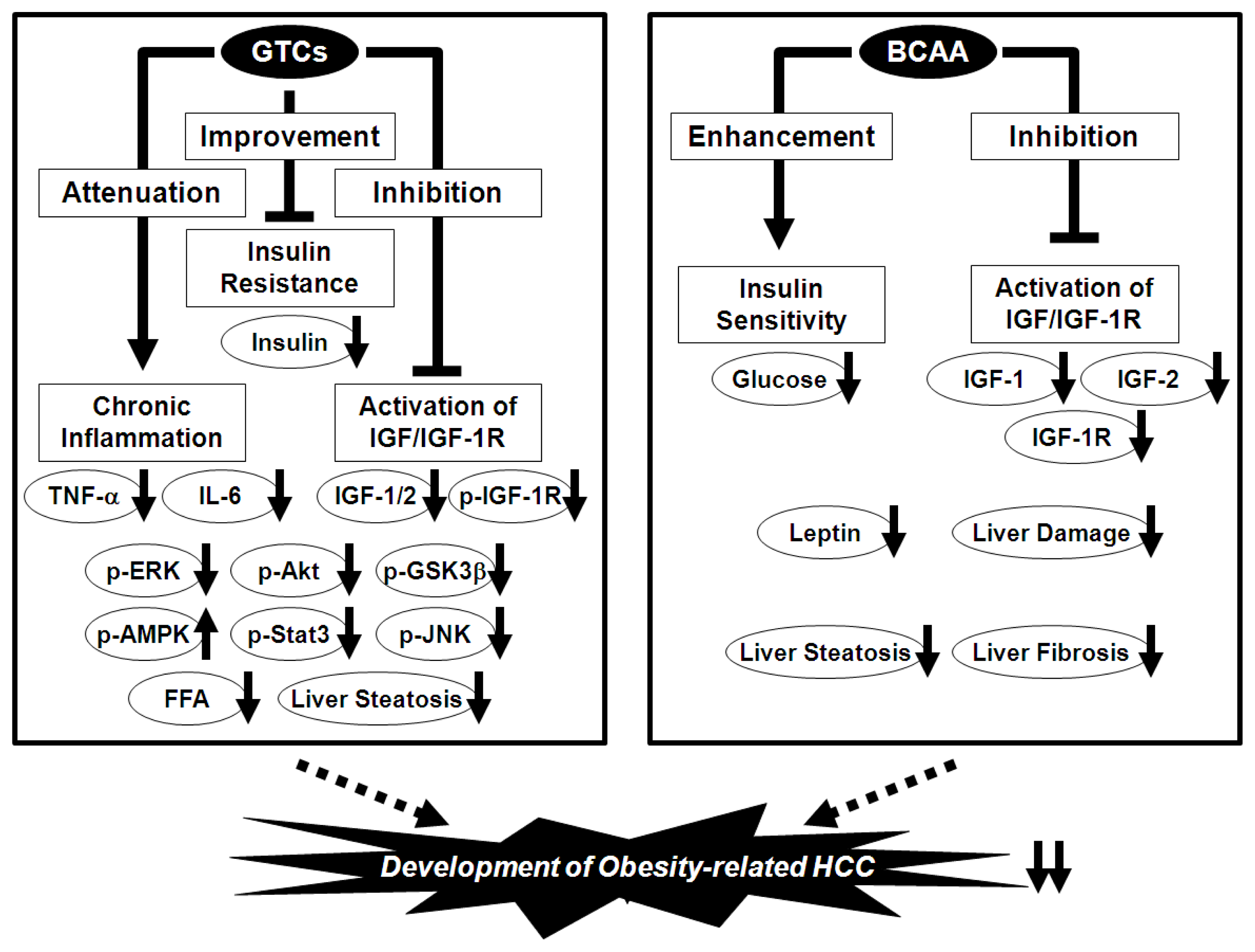
7. Prevention of Obesity-Related HCC via the Nutraceutical Approach—BCAA and GTCs Effectively Prevent Obesity-Related Liver Carcinogenesis
8. Conclusions
Acknowledgments
- Conflict of Interest The authors declare no conflict of interest.
References
- WHO: World Health Organization. Fact Sheet for World Wide Prevalence of Obesity. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/indexhtml (accessed on 27 December 2011).
- Calle, E.E.; Kaaks, R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 2004, 4, 579–591. [Google Scholar]
- Anderson, A.S.; Caswell, S. Obesity management—An opportunity for cancer prevention. Surgeon 2009, 7, 282–285. [Google Scholar]
- Giovannucci, E.; Michaud, D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology 2007, 132, 2208–2225. [Google Scholar]
- Frezza, E.E.; Wachtel, M.S.; Chiriva-Internati, M. Influence of obesity on the risk of developing colon cancer. Gut 2006, 55, 285–291. [Google Scholar]
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar]
- El-Serag, H.B.; Tran, T.; Everhart, J.E. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology 2004, 126, 460–468. [Google Scholar]
- Muto, Y.; Sato, S.; Watanabe, A.; Moriwaki, H.; Suzuki, K.; Kato, A.; Kato, M.; Nakamura, T.; Higuchi, K.; Nishiguchi, S.; et al. Overweight and obesity increase the risk for liver cancer in patients with liver cirrhosis and long-term oral supplementation with branched-chain amino acid granules inhibits liver carcinogenesis in heavier patients with liver cirrhosis. Hepatol. Res 2006, 35, 204–214. [Google Scholar]
- World Cancer Research Fund/American Institute for Cancer Research, Food, Nutrition, Physical activity, and the Prevention of Cancer: A Global Perspective 2007; AICR: Washington, DC, USA, 2007.
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med 2003, 348, 1625–1638. [Google Scholar]
- Yasuda, Y.; Shimizu, M.; Shirakami, Y.; Sakai, H.; Kubota, M.; Hata, K.; Hirose, Y.; Tsurumi, H.; Tanaka, T.; Moriwaki, H. Pitavastatin inhibits azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Cancer Sci 2010, 101, 1701–1707. [Google Scholar]
- Shimizu, M.; Yasuda, Y.; Sakai, H.; Kubota, M.; Terakura, D.; Baba, A.; Ohno, T.; Kochi, T.; Tsurumi, H.; Tanaka, T.; et al. Pitavastatin suppresses diethylnitrosamine-induced liver preneoplasms in male C57BL/KsJ-db/db obese mice. BMC Cancer 2011, 11. [Google Scholar] [CrossRef]
- Kubota, M.; Shimizu, M.; Sakai, H.; Yasuda, Y.; Ohno, T.; Kochi, T.; Tsurumi, H.; Tanaka, T.; Moriwaki, H. Renin-angiotensin system inhibitors suppress azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Biochem. Biophys. Res. Commun 2011, 410, 108–113. [Google Scholar]
- Kao, Y.H.; Chang, H.H.; Lee, M.J.; Chen, C.L. Tea, obesity, and diabetes. Mol. Nutr. Food Res 2006, 50, 188–210. [Google Scholar]
- Yang, C.S.; Wang, X.; Lu, G.; Picinich, S.C. Cancer prevention by tea: Animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer 2009, 9, 429–439. [Google Scholar]
- Shimizu, M.; Shirakami, Y.; Moriwaki, H. Targeting receptor tyrosine kinases for chemoprevention by green tea catechin, EGCG. Int. J. Mol. Sci 2008, 9, 1034–1049. [Google Scholar]
- Shimizu, M.; Adachi, S.; Masuda, M.; Kozawa, O.; Moriwaki, H. Cancer chemoprevention with green tea catechins by targeting receptor tyrosine kinases. Mol. Nutr. Food Res 2011, 55, 832–843. [Google Scholar]
- Shimizu, M.; Weinstein, I.B. Modulation of signal transduction by tea catechins and related phytochemicals. Mutat. Res 2005, 591, 147–160. [Google Scholar]
- Muto, Y.; Sato, S.; Watanabe, A.; Moriwaki, H.; Suzuki, K.; Kato, A.; Kato, M.; Nakamura, T.; Higuchi, K.; Nishiguchi, S.; et al. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin. Gastroenterol. Hepatol 2005, 3, 705–713. [Google Scholar]
- Marchesini, G.; Bianchi, G.; Merli, M.; Amodio, P.; Panella, C.; Loguercio, C.; Fanelli, F.R.; Abbiati, R. Italian BCAA Study Group. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: A double-blind randomized trial. Gastroenterology 2003, 124, 1792–1801. [Google Scholar]
- Kawaguchi, T.; Nagao, Y.; Matsuoka, H.; Ide, T.; Sata, M. Branched-chain amino acid-enriched supplementation improves insulin resistance in patients with chronic liver disease. Int. J. Mol. Med 2008, 22, 105–112. [Google Scholar]
- Chang, C.K.; Ulrich, C.M. Hyperinsulinaemia and hyperglycaemia: Possible risk factors of colorectal cancer among diabetic patients. Diabetologia 2003, 46, 595–607. [Google Scholar]
- Pollak, M. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer 2008, 8, 915–928. [Google Scholar]
- Clayton, P.E.; Banerjee, I.; Murray, P.G.; Renehan, A.G. Growth hormone, the insulin-like growth factor axis, insulin and cancer risk. Nat. Rev. Endocrinol 2011, 7, 11–24. [Google Scholar]
- Bjork, J.; Nilsson, J.; Hultcrantz, R.; Johansson, C. Growth-regulatory effects of sensory neuropeptides, epidermal growth factor, insulin, and somatostatin on the non-transformed intestinal epithelial cell line IEC-6 and the colon cancer cell line HT 29. Scand. J. Gastroenterol 1993, 28, 879–884. [Google Scholar]
- Tran, T.T.; Medline, A.; Bruce, W.R. Insulin promotion of colon tumors in rats. Cancer Epidemiol. Biomark. Prev 1996, 5, 1013–1015. [Google Scholar]
- Singh, P.; Rubin, N. Insulinlike growth factors and binding proteins in colon cancer. Gastroenterology 1993, 105, 1218–1237. [Google Scholar]
- Kajantie, E.; Fall, C.H.; Seppala, M.; Koistinen, R.; Dunkel, L.; Yliharsila, H.; Osmond, C.; Andersson, S.; Barker, D.J.; Forsen, T.; et al. Serum insulin-like growth factor (IGF)-I and IGF-binding protein-1 in elderly people: Relationships with cardiovascular risk factors, body composition, size at birth, and childhood growth. J. Clin. Endocrinol. Metab 2003, 88, 1059–1065. [Google Scholar]
- Esposito, K.; Nappo, F.; Marfella, R.; Giugliano, G.; Giugliano, F.; Ciotola, M.; Quagliaro, L.; Ceriello, A.; Giugliano, D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation 2002, 106, 2067–2072. [Google Scholar]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar]
- Hotamisligil, G.S.; Peraldi, P.; Budavari, A.; Ellis, R.; White, M.F.; Spiegelman, B.M. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 1996, 271, 665–668. [Google Scholar]
- Kern, P.A.; Ranganathan, S.; Li, C.; Wood, L.; Ranganathan, G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab 2001, 280, E745–E751. [Google Scholar]
- Szlosarek, P.; Charles, K.A.; Balkwill, F.R. Tumour necrosis factor-alpha as a tumour promoter. Eur. J. Cancer 2006, 42, 745–750. [Google Scholar]
- Valko, M.; Izakovic, M.; Mazur, M.; Rhodes, C.J.; Telser, J. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell. Biochem 2004, 266, 37–56. [Google Scholar]
- Leslie, N.R. The redox regulation of PI 3-kinase-dependent signaling. Antioxid. Redox Signal 2006, 8, 1765–1774. [Google Scholar]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L.; et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med 1996, 334, 292–295. [Google Scholar]
- Barb, D.; Williams, C.J.; Neuwirth, A.K.; Mantzoros, C.S. Adiponectin in relation to malignancies: A review of existing basic research and clinical evidence. Am. J. Clin. Nutr 2007, 86, s858–s866. [Google Scholar]
- Amemori, S.; Ootani, A.; Aoki, S.; Fujise, T.; Shimoda, R.; Kakimoto, T.; Shiraishi, R.; Sakata, Y.; Tsunada, S.; Iwakiri, R.; et al. Adipocytes and preadipocytes promote the proliferation of colon cancer cells in vitro. Am. J. Physiol. Gastrointest. Liver Physiol 2007, 292, G923–G929. [Google Scholar]
- Stattin, P.; Lukanova, A.; Biessy, C.; Soderberg, S.; Palmqvist, R.; Kaaks, R.; Olsson, T.; Jellum, E. Obesity and colon cancer: Does leptin provide a link? Int. J. Cancer 2004, 109, 149–152. [Google Scholar]
- Faggioni, R.; Feingold, K.R.; Grunfeld, C. Leptin regulation of the immune response and the immunodeficiency of malnutrition. FASEB J 2001, 15, 2565–2571. [Google Scholar]
- Molina, A.; Vendrell, J.; Gutierrez, C.; Simon, I.; Masdevall, C.; Soler, J.; Gomez, J.M. Insulin resistance, leptin and TNF-alpha system in morbidly obese women after gastric bypass. Obes. Surg 2003, 13, 615–621. [Google Scholar]
- Fenton, J.I.; Hursting, S.D.; Perkins, S.N.; Hord, N.G. Interleukin-6 production induced by leptin treatment promotes cell proliferation in an ApcMin/+ colon epithelial cell line. Carcinogenesis 2006, 27, 1507–1515. [Google Scholar]
- Kang, S.; Song, J.; Kang, H.; Kim, S.; Lee, Y.; Park, D. Insulin can block apoptosis by decreasing oxidative stress via phosphatidylinositol 3-kinase- and extracellular signal-regulated protein kinase-dependent signaling pathways in HepG2 cells. Eur. J. Endocrinol 2003, 148, 147–155. [Google Scholar]
- Tornkvist, A.; Parpal, S.; Gustavsson, J.; Stralfors, P. Inhibition of Raf-1 kinase expression abolishes insulin stimulation of DNA synthesis in H4IIE hepatoma cells. J. Biol. Chem 1994, 269, 13919–13921. [Google Scholar]
- Imai, K.; Takai, K.; Nishigaki, Y.; Shimizu, S.; Naiki, T.; Hayashi, H.; Uematsu, T.; Sugihara, J.; Tomita, E.; Shimizu, M.; et al. Insulin resistance raises the risk for recurrence of stage I hepatocellular carcinoma after curative radiofrequency ablation in hepatitis C virus-positive patients: A prospective, case series study. Hepatol. Res 2010, 40, 376–382. [Google Scholar]
- Powell, E.E.; Jonsson, J.R.; Clouston, A.D. Steatosis: Co-factor in other liver diseases. Hepatology 2005, 42, 5–13. [Google Scholar]
- Alexia, C.; Fallot, G.; Lasfer, M.; Schweizer-Groyer, G.; Groyer, A. An evaluation of the role of insulin-like growth factors (IGF) and of type-I IGF receptor signalling in hepatocarcinogenesis and in the resistance of hepatocarcinoma cells against drug-induced apoptosis. Biochem. Pharmacol 2004, 68, 1003–1015. [Google Scholar]
- Shimizu, M.; Shirakami, Y.; Sakai, H.; Tatebe, H.; Nakagawa, T.; Hara, Y.; Weinstein, I.B.; Moriwaki, H. EGCG inhibits activation of the insulin-like growth factor (IGF)/IGF-1 receptor axis in human hepatocellular carcinoma cells. Cancer Lett 2008, 262, 10–18. [Google Scholar]
- Chen, C.; Chang, Y.C.; Liu, C.L.; Liu, T.P.; Chang, K.J.; Guo, I.C. Leptin induces proliferation and anti-apoptosis in human hepatocarcinoma cells by up-regulating cyclin D1 and down-regulating Bax via a Janus kinase 2-linked pathway. Endocr. Relat. Cancer 2007, 14, 513–529. [Google Scholar]
- Watanabe, N.; Takai, K.; Imai, K.; Shimizu, M.; Naiki, T.; Nagaki, M.; Moriwaki, H. Increased levels of serum leptin are a risk factor for the recurrence of stage I/II hepatocellular carcinoma after curative treatment. J. Clin. Biochem. Nutr 2011, 49, 153–158. [Google Scholar]
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med 2002, 346, 1221–1231. [Google Scholar]
- Siegel, A.B.; Zhu, A.X. Metabolic syndrome and hepatocellular carcinoma: Two growing epidemics with a potential link. Cancer 2009, 115, 5651–5661. [Google Scholar]
- Ratziu, V.; Bonyhay, L.; Di Martino, V.; Charlotte, F.; Cavallaro, L.; Sayegh-Tainturier, M.H.; Giral, P.; Grimaldi, A.; Opolon, P.; Poynard, T. Survival, liver failure, and hepatocellular carcinoma in obesity-related cryptogenic cirrhosis. Hepatology 2002, 35, 1485–1493. [Google Scholar]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar]
- Chitturi, S.; Abeygunasekera, S.; Farrell, G.C.; Holmes-Walker, J.; Hui, J.M.; Fung, C.; Karim, R.; Lin, R.; Samarasinghe, D.; Liddle, C.; et al. NASH and insulin resistance: Insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology 2002, 35, 373–379. [Google Scholar]
- Feldstein, A.E.; Werneburg, N.W.; Canbay, A.; Guicciardi, M.E.; Bronk, S.F.; Rydzewski, R.; Burgart, L.J.; Gores, G.J. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology 2004, 40, 185–194. [Google Scholar]
- Crespo, J.; Cayon, A.; Fernandez-Gil, P.; Hernandez-Guerra, M.; Mayorga, M.; Dominguez-Diez, A.; Fernandez-Escalante, J.C.; Pons-Romero, F. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology 2001, 34, 1158–1163. [Google Scholar]
- Chitturi, S.; Farrell, G.; Frost, L.; Kriketos, A.; Lin, R.; Fung, C.; Liddle, C.; Samarasinghe, D.; George, J. Serum leptin in NASH correlates with hepatic steatosis but not fibrosis: A manifestation of lipotoxicity? Hepatology 2002, 36, 403–409. [Google Scholar]
- Wong, V.W.; Wong, G.L.; Tsang, S.W.; Fan, T.; Chu, W.C.; Woo, J.; Chan, A.W.; Choi, P.C.; Chim, A.M.; Lau, J.Y.; et al. High prevalence of colorectal neoplasm in patients with non-alcoholic steatohepatitis. Gut 2011, 60, 829–836. [Google Scholar]
- Hursel, R.; Viechtbauer, W.; Westerterp-Plantenga, M.S. The effects of green tea on weight loss and weight maintenance: A meta-analysis. Int. J. Obes 2009, 33, 956–961. [Google Scholar]
- Rains, T.M.; Agarwal, S.; Maki, K.C. Antiobesity effects of green tea catechins: A mechanistic review. J. Nutr. Biochem 2011, 22, 1–7. [Google Scholar]
- Grove, K.A.; Lambert, J.D. Laboratory, epidemiological, and human intervention studies show that tea (Camellia sinensis) may be useful in the prevention of obesity. J. Nutr 2010, 140, 446–453. [Google Scholar]
- Thielecke, F.; Boschmann, M. The potential role of green tea catechins in the prevention of the metabolic syndrome—A review. Phytochemistry 2009, 70, 11–24. [Google Scholar]
- Ramadan, G.; El-Beih, N.M.; El-Ghffar, E.A.A. Modulatory effects of black v. green tea aqueous extract on hyperglycaemia, hyperlipidaemia and liver dysfunction in diabetic and obese rat models. Br. J. Nutr 2009, 102, 1611–1619. [Google Scholar]
- Murase, T.; Nagasawa, A.; Suzuki, J.; Hase, T.; Tokimitsu, I. Beneficial effects of tea catechins on diet-induced obesity: Stimulation of lipid catabolism in the liver. Int. J. Obes 2002, 26, 1459–1464. [Google Scholar]
- Bose, M.; Lambert, J.D.; Ju, J.; Reuhl, K.R.; Shapses, S.A.; Yang, C.S. The major green tea polyphenol, (−)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J. Nutr 2008, 138, 1677–1683. [Google Scholar]
- Qin, B.; Polansky, M.M.; Harry, D.; Anderson, R.A. Green tea polyphenols improve cardiac muscle mRNA and protein levels of signal pathways related to insulin and lipid metabolism and inflammation in insulin-resistant rats. Mol. Nutr. Food Res 2010, 54, S14–S23. [Google Scholar]
- Bettuzzi, S.; Brausi, M.; Rizzi, F.; Castagnetti, G.; Peracchia, G.; Corti, A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: A preliminary report from a one-year proof-of-principle study. Cancer Res 2006, 66, 1234–1240. [Google Scholar]
- Li, N.; Sun, Z.; Han, C.; Chen, J. The chemopreventive effects of tea on human oral precancerous mucosa lesions. Proc. Soc. Exp. Biol. Med 1999, 220, 218–224. [Google Scholar]
- Shimizu, M.; Fukutomi, Y.; Ninomiya, M.; Nagura, K.; Kato, T.; Araki, H.; Suganuma, M.; Fujiki, H.; Moriwaki, H. Green tea extracts for the prevention of metachronous colorectal adenomas: A pilot study. Cancer Epidemiol. Biomark. Prev 2008, 17, 3020–3025. [Google Scholar]
- Shirakami, Y.; Shimizu, M.; Tsurumi, H.; Hara, Y.; Tanaka, T.; Moriwaki, H. EGCG and Polyphenon E attenuate inflammation-related mouse colon carcinogenesis induced by AOM plus DDS. Mol. Med. Rep 2008, 1, 355–361. [Google Scholar]
- Adachi, S.; Nagao, T.; Ingolfsson, H.I.; Maxfield, F.R.; Andersen, O.S.; Kopelovich, L.; Weinstein, I.B. The inhibitory effect of (−)-epigallocatechin gallate on activation of the epidermal growth factor receptor is associated with altered lipid order in HT29 colon cancer cells. Cancer Res 2007, 67, 6493–6501. [Google Scholar]
- Adachi, S.; Nagao, T.; To, S.; Joe, A.K.; Shimizu, M.; Matsushima-Nishiwaki, R.; Kozawa, O.; Moriwaki, H.; Maxfield, F.R.; Weinstein, I.B. (−)-Epigallocatechin gallate causes internalization of the epidermal growth factor receptor in human colon cancer cells. Carcinogenesis 2008, 29, 1986–1993. [Google Scholar]
- Shimizu, M.; Deguchi, A.; Hara, Y.; Moriwaki, H.; Weinstein, I.B. EGCG inhibits activation of the insulin-like growth factor-1 receptor in human colon cancer cells. Biochem. Biophys. Res. Commun 2005, 334, 947–953. [Google Scholar]
- Yasuda, Y.; Shimizu, M.; Sakai, H.; Iwasa, J.; Kubota, M.; Adachi, S.; Osawa, Y.; Tsurumi, H.; Hara, Y.; Moriwaki, H. (−)-Epigallocatechin gallate prevents carbon tetrachloride-induced rat hepatic fibrosis by inhibiting the expression of the PDGFRbeta and IGF-1R. Chem. Biol. Interact 2009, 182, 159–164. [Google Scholar]
- Moriwaki, H.; Miwa, Y.; Tajika, M.; Kato, M.; Fukushima, H.; Shiraki, M. Branched-chain amino acids as a protein- and energy-source in liver cirrhosis. Biochem. Biophys. Res. Commun 2004, 313, 405–409. [Google Scholar]
- Kawaguchi, T.; Izumi, N.; Charlton, M.R.; Sata, M. Branched-chain amino acids as pharmacological nutrients in chronic liver disease. Hepatology 2011, 54, 1063–1070. [Google Scholar]
- Petrides, A.S.; Vogt, C.; Schulze-Berge, D.; Matthews, D.; Strohmeyer, G. Pathogenesis of glucose intolerance and diabetes mellitus in cirrhosis. Hepatology 1994, 19, 616–627. [Google Scholar]
- Mehta, S.H.; Brancati, F.L.; Sulkowski, M.S.; Strathdee, S.A.; Szklo, M.; Thomas, D.L. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann. Intern. Med 2000, 133, 592–599. [Google Scholar]
- She, P.; Reid, T.M.; Bronson, S.K.; Vary, T.C.; Hajnal, A.; Lynch, C.J.; Hutson, S.M. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab 2007, 6, 181–194. [Google Scholar]
- Zhang, Y.; Guo, K.; LeBlanc, R.E.; Loh, D.; Schwartz, G.J.; Yu, Y.H. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes 2007, 56, 1647–1654. [Google Scholar]
- Ikehara, O.; Kawasaki, N.; Maezono, K.; Komatsu, M.; Konishi, A. Acute and chronic treatment of l-isoleucine ameliorates glucose metabolism in glucose-intolerant and diabetic mice. Biol. Pharm. Bull 2008, 31, 469–472. [Google Scholar]
- Higuchi, N.; Kato, M.; Miyazaki, M.; Tanaka, M.; Kohjima, M.; Ito, T.; Nakamuta, M.; Enjoji, M.; Kotoh, K.; Takayanagi, R. Potential role of branched-chain amino acids in glucose metabolism through the accelerated induction of the glucose-sensing apparatus in the liver. J. Cell. Biochem 2011, 112, 30–38. [Google Scholar]
- Hinault, C.; Mothe-Satney, I.; Gautier, N.; Lawrence, J.C., Jr; van Obberghen, E. Amino acids and leucine allow insulin activation of the PKB/mTOR pathway in normal adipocytes treated with wortmannin and in adipocytes from db/dbmice. FASEB J 2004, 18, 1894–1896. [Google Scholar]
- Nishitani, S.; Takehana, K.; Fujitani, S.; Sonaka, I. Branched-chain amino acids improve glucose metabolism in rats with liver cirrhosis. Am. J. Physiol. Gastrointest. Liver Physiol 2005, 288, G1292–G1300. [Google Scholar]
- Arakawa, M.; Masaki, T.; Nishimura, J.; Seike, M.; Yoshimatsu, H. The effects of branched-chain amino acid granules on the accumulation of tissue triglycerides and uncoupling proteins in diet-induced obese mice. Endocr. J 2011, 58, 161–170. [Google Scholar]
- Nishimura, J.; Masaki, T.; Arakawa, M.; Seike, M.; Yoshimatsu, H. Isoleucine prevents the accumulation of tissue triglycerides and upregulates the expression of PPARalpha and uncoupling protein in diet-induced obese mice. J. Nutr 2010, 140, 496–500. [Google Scholar]
- Sakaida, I.; Tsuchiya, M.; Okamoto, M.; Okita, K. Late evening snack and the change of blood glucose level in patients with liver cirrhosis. Hepatol. Res 2004, 30, 67–72. [Google Scholar]
- Urata, Y.; Okita, K.; Korenaga, K.; Uchida, K.; Yamasaki, T.; Sakaida, I. The effect of supplementation with branched-chain amino acids in patients with liver cirrhosis. Hepatol. Res 2007, 37, 510–516. [Google Scholar]
- Hagiwara, A.; Nishiyama, M.; Ishizaki, S. Branched-chain amino acids prevent insulin-induced hepatic tumor cell proliferation by inducing apoptosis through mTORC1 and mTORC2-dependent mechanisms. J. Cell. Physiol 2011. [Google Scholar] [CrossRef]
- Forte, A.; de Sanctis, R.; Leonetti, G.; Manfredelli, S.; Urbano, V.; Bezzi, M. Dietary chemoprevention of colorectal cancer. Ann. Ital. Chir 2008, 79, 261–267. [Google Scholar]
- Hirose, Y.; Hata, K.; Kuno, T.; Yoshida, K.; Sakata, K.; Yamada, Y.; Tanaka, T.; Reddy, B.S.; Mori, H. Enhancement of development of azoxymethane-induced colonic premalignant lesions in C57BL/KsJ-db/db mice. Carcinogenesis 2004, 25, 821–825. [Google Scholar]
- Lee, G.H.; Proenca, R.; Montez, J.M.; Carroll, K.M.; Darvishzadeh, J.G.; Lee, J.I.; Friedman, J.M. Abnormal splicing of the leptin receptor in diabetic mice. Nature 1996, 379, 632–635. [Google Scholar]
- Shimizu, M.; Shirakami, Y.; Sakai, H.; Adachi, S.; Hata, K.; Hirose, Y.; Tsurumi, H.; Tanaka, T.; Moriwaki, H. (−)-Epigallocatechin gallate suppresses azoxymethane-induced colonic premalignant lesions in male C57BL/KsJ-db/db mice. Cancer Prev. Res 2008, 1, 298–304. [Google Scholar]
- Suzuki, R.; Kohno, H.; Yasui, Y.; Hata, K.; Sugie, S.; Miyamoto, S.; Sugawara, K.; Sumida, T.; Hirose, Y.; Tanaka, T. Diet supplemented with citrus unshiu segment membrane suppresses chemically induced colonic preneoplastic lesions and fatty liver in male db/db mice. Int. J. Cancer 2007, 120, 252–258. [Google Scholar]
- Hayashi, K.; Suzuki, R.; Miyamoto, S.; Shin-Ichiroh, Y.; Kohno, H.; Sugie, S.; Takashima, S.; Tanaka, T. Citrus auraptene suppresses azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/db mice. Nutr. Cancer 2007, 58, 75–84. [Google Scholar]
- Miyamoto, S.; Yasui, Y.; Ohigashi, H.; Tanaka, T.; Murakami, A. Dietary flavonoids suppress azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db mice. Chem. Biol. Interact 2010, 183, 276–283. [Google Scholar]
- Shimizu, M.; Shirakami, Y.; Iwasa, J.; Shiraki, M.; Yasuda, Y.; Hata, K.; Hirose, Y.; Tsurumi, H.; Tanaka, T.; Moriwaki, H. Supplementation with branched-chain amino acids inhibits azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db mice. Clin. Cancer Res 2009, 15, 3068–3075. [Google Scholar]
- Gupta, R.A.; Dubois, R.N. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat. Rev. Cancer 2001, 1, 11–21. [Google Scholar]
- Iwasa, J.; Shimizu, M.; Shiraki, M.; Shirakami, Y.; Sakai, H.; Terakura, Y.; Takai, K.; Tsurumi, H.; Tanaka, T.; Moriwaki, H. Dietary supplementation with branched-chain amino acids suppresses diethylnitrosamine-induced liver tumorigenesis in obese and diabetic C57BL/KsJ-db/db mice. Cancer Sci 2010, 101, 460–467. [Google Scholar]
- Shimizu, M.; Sakai, H.; Shirakami, Y.; Iwasa, J.; Yasuda, Y.; Kubota, M.; Takai, K.; Tsurumi, H.; Tanaka, T.; Moriwaki, H. Acyclic retinoid inhibits diethylnitrosamine-induced liver tumorigenesis in obese and diabetic C57BLKS/J-+Leprdb/+Leprdb mice. Cancer Prev. Res 2011, 4, 128–136. [Google Scholar]
- Shimizu, M.; Sakai, H.; Shirakami, Y.; Yasuda, Y.; Kubota, M.; Terakura, D.; Baba, A.; Ohno, T.; Hara, Y.; Tanaka, T.; et al. Preventive Effects of (−)-Epigallocatechin gallate on diethylnitrosamine-induced liver tumorigenesis in obese and diabetic C57BL/KsJ-db/db mice. Cancer Prev. Res 2011, 4, 396–403. [Google Scholar]
- Yoshiji, H.; Noguchi, R.; Kitade, M.; Kaji, K.; Ikenaka, Y.; Namisaki, T.; Yoshii, J.; Yanase, K.; Yamazaki, M.; Tsujimoto, T.; et al. Branched-chain amino acids suppress insulin-resistance-based hepatocarcinogenesis in obese diabetic rats. J. Gastroenterol 2009, 44, 483–491. [Google Scholar]
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shimizu, M.; Kubota, M.; Tanaka, T.; Moriwaki, H. Nutraceutical Approach for Preventing Obesity-Related Colorectal and Liver Carcinogenesis. Int. J. Mol. Sci. 2012, 13, 579-595. https://doi.org/10.3390/ijms13010579
Shimizu M, Kubota M, Tanaka T, Moriwaki H. Nutraceutical Approach for Preventing Obesity-Related Colorectal and Liver Carcinogenesis. International Journal of Molecular Sciences. 2012; 13(1):579-595. https://doi.org/10.3390/ijms13010579
Chicago/Turabian StyleShimizu, Masahito, Masaya Kubota, Takuji Tanaka, and Hisataka Moriwaki. 2012. "Nutraceutical Approach for Preventing Obesity-Related Colorectal and Liver Carcinogenesis" International Journal of Molecular Sciences 13, no. 1: 579-595. https://doi.org/10.3390/ijms13010579
APA StyleShimizu, M., Kubota, M., Tanaka, T., & Moriwaki, H. (2012). Nutraceutical Approach for Preventing Obesity-Related Colorectal and Liver Carcinogenesis. International Journal of Molecular Sciences, 13(1), 579-595. https://doi.org/10.3390/ijms13010579




