One-Dimensional Hydrogen-Bonded Infinite Chain from Nickel(II) Tetraaza Macrocyclic Complex and 1,2-Cyclopentanedicarboxylate Ligand
Abstract
:1. Introduction
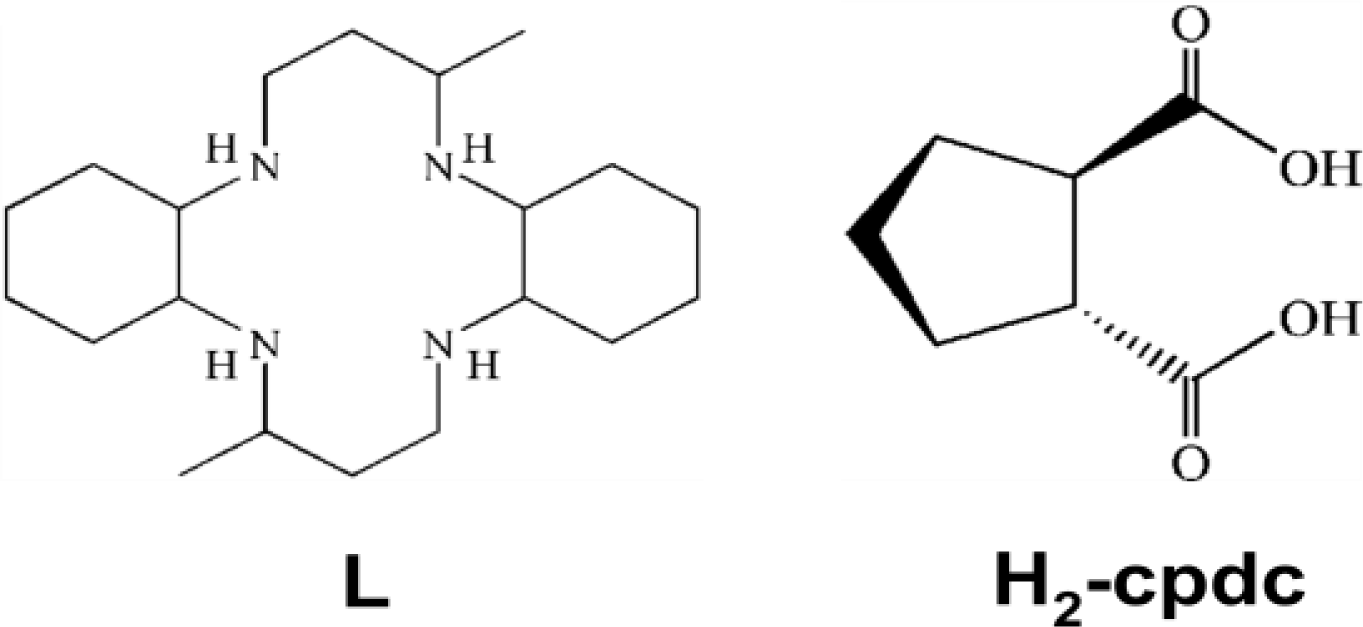
2. Results and Discussion
2.1. Structural Description
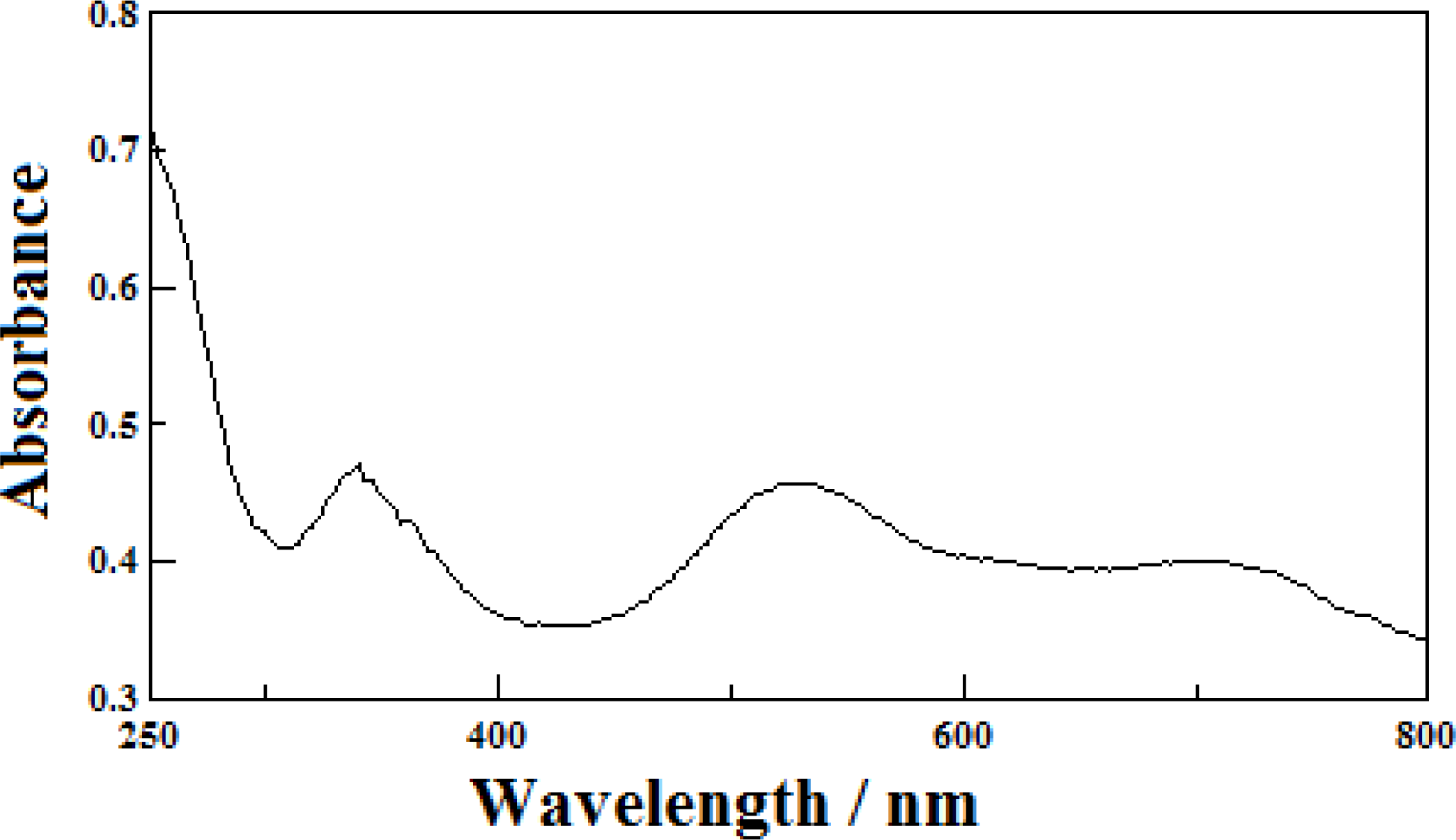
2.2. Chemical Properties
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis of [Ni(L)(H-cpdc–)2] (1)
3.3. X-ray Crystallography
4. Conclusions
Acknowledgments
References
- Piguet, C; Bernardinelli, G; Hopfgartner, G. Helicates as versatile supramolecular complexes. Chem. Rev 1997, 97, 2005–2062. [Google Scholar]
- Munakata, M; Ning, GL; Kuroda-Sowa, T; Maekawa, M; Suenaga, Y; Harino, T. Construction of copper (I) coordination with zigzag sheet and porous frameworks. Inorg. Chem 1998, 37, 5651–5656. [Google Scholar]
- Evans, OR; Xiong, RG. Crystal engineering of acentric diamonded metal-organic coordination networks. Angew. Chem. Int. Ed 1999, 38, 536–538. [Google Scholar]
- Li, H; Eddaoudi, M. Design and synthesis of an exceptionally stable and highly porous meta-organic framework. Nature 1999, 402, 276–279. [Google Scholar]
- Liu, L; Huang, S-P; Yang, G-D; Zhang, H; Wang, X-L; Fu, Z-Y; Dai, J-C. Zn [Htma][ddm]: An interesting three-dimensional chiral nonlinear optical-active zinc-trimestate framework. Cryst. Growth Des 2010, 10, 930–936. [Google Scholar]
- Choi, HJ; Lee, TS; Suh, MP. Self-assembly of a molecular floral lace with one-dimensional channels and inclusion of glucose. Angew. Chem. Int. Ed 1999, 38, 1405–1408. [Google Scholar]
- Moulton, B; Zaworotko, MJ. From molecules to crystal engineering: Supramolecular isomerism and polymorphism in network solids. Chem. Rev 2001, 101, 1629–1658. [Google Scholar]
- Suh, MP; Cheon, YE; Lee, EY. Syntheses and functions of porous metallosupramolecular networks. Coord. Chem. Rev 2008, 252, 1007–1026. [Google Scholar]
- Eryazici, I; Moorefield, CN; Newkome, GR. Square planar Pd(II), Pt(II), and Au(III) terpyridine complexes; Their syntheses, physical properties, supramolecular constructs, and biomedical Activities. Chem. Rev 2008, 108, 1834–1895. [Google Scholar]
- Tominaga, M; Masu, H; Azumaya, I. Hydrogen-bonding networks of adamantane-based bisphenol molecules: Toward the preparation of molecular crystals with channels. Cryst. Growth Des 2011, 11, 542–546. [Google Scholar]
- Sharma, CVK; Rogers, RD. “Molecular chinese blinds”: Self-organization of tetranitrato lanthanide complexes into open, chiral hydrogen bond network. Chem. Commun 1999, 1, 83–84. [Google Scholar]
- Ye, B-H; Tong, M-J; Chen, X-M. Metal-organic molecular architectures with 2,2’-bipyridine-like and carboxylate ligands. Coord. Chem. Rev 2005, 249, 545–546. [Google Scholar]
- Zhao, X; He, H; Dai, F; Sun, D; Ke, Y. Supramolecular isomerism in honeycomb metal-organic frameworks driven by CH…π interactions: Homochiral crystallization from an achiral ligand through chiral inducement. Inorg. Chem 2010, 49, 8650–8652. [Google Scholar]
- Lehn, JM; Mascal, M; MeCian, A; Fisher, J. Molecular ribbons from molecular recognition directed self-assembly of self-complementary molecular components. J. Chem. Soc. Perkin Trans. 1992, 461–467. [Google Scholar]
- Choi, K-Y; Chun, K-M; Suh, I-H. Synthesis and characterization of one-dimensional nickel(II) macrocyclic complexes with bridging organic ligands. Polyhedron 2001, 20, 57–65. [Google Scholar]
- Choi, K-Y; Kim, K-J. Self-assembly of 1D coordination polymers from nickel (II) tetraaza macrocycle and carboxylate ligands. Polyhedron 2008, 27, 1311. [Google Scholar]
- Kim, JC; Cho, J; Lough, AJ. Syntheses, isolation, and structures of nickel (II) and copper (II) coordination polymers with tetraaza macrocyclic ligands. Inorg. Chim. Acta 2001, 317, 252–258. [Google Scholar]
- Choi, K-Y. Self-assembly of one-dimensional coordination polymer from nickel (II) macrocyclic complex and malonate ligand. J. Chem. Crystallogr 2010, 40, 477–481. [Google Scholar]
- Choi, K-Y. One-dimensional hydrogen-bonded polymer from hexaazamacrotetracyclic nickel(II) complex and trans-1,4-cyclohexanedicarboxylate ligand. J. Inorg. Organomet. Poly 2010, 20, 867–872. [Google Scholar]
- Choi, K-Y; Kim, K-J. Self-assembly of metal(II) tetraaza macrocyclic complexes with cyclobutane-1-carboxylic acid-1-carboxylate ligand linked by hydrogen bond. Struct. Chem 2008, 19, 741–747. [Google Scholar]
- Farrugia, LJ. ORTEP-3 for windows-a version of ORTEP-III with a graphical user interface (GUI). J. Appl. Cryst 1997, 30, 568. [Google Scholar]
- Choi, K-Y; Kim, JC; Jensen, WP; Suh, I-H; Choi, S-S. Copper (II) and nickel (II) complex with 3,14-dimethyl-2,6,13,17-tetraazatricyclo[14,4,01.18,07.12]docosane [M(C20H40N4)]Cl2·2H2O (M = CuII and NiII). Acta Cryst 1996, C52, 2166–2168. [Google Scholar]
- Bakalbassis, EG; Tsipis, CA; Bozopoulos, AP; Dreissing, DW; Hartl, H; Mrozinski, J. Strong ferromagnetism between copper(II) ions separated by 6.7. ANG. In a new phthalato-bridged copper(II) binuclear complex. Inorg. Chem 1991, 30, 2801–2806. [Google Scholar]
- Cao, R; Shi, Q; Sun, D; Hong, M; Bi, W; Zhao, Y. Syntheses and characterization of copper(II) polymeric complexes constructed from 1,2,4,5-benzenetetracarboxylic acid. Inorg. Chem 2002, 41, 6161–6168. [Google Scholar]
- Donlevy, TM; Gahan, LR; Hambley, TW; Hanson, GR; McMahon, KL; Stranger, R. Structure and spectroscopy of the copper(II) complex of the unsymmetric encapsulating ligand 1-methyl-8-ammonio-3,13-diothia-6,10,16,19-tetraazabicyclo[6.6.6]icosane (AMN4S2sar). Inorg. Chem 1994, 33, 5131–5137. [Google Scholar]
- Mochizuki, K; Kondo, T. Isolation and Vis-absorption spectrum of trans-[Ni(OH2)2(cyclam)] Cl2·4H2O. Inorg. Chem 1995, 34, 6241–6243. [Google Scholar]
- Kim, JC; Cho, J; Kim, H; Lough, AJ. Isolation and characterization of the first stable bicarbonato complex in a nickel (II) system: Identification of unusual monodentate coordination. Chem. Commun 2004, 16, 1796–1797. [Google Scholar]
- Kang, S-G; Kweon, JK; Jung, S-K. Synthesis of new tetraaza macrocyclic ligands with cyclohexane rings and their Ni(II) and Cu(II) complexes. Bull. Korean Chem. Soc 1991, 12, 483–487. [Google Scholar]
- North, ACT; Phillips, DC; Mathews, FS. A Semi-empirical method of absorption correction. Acta Cryst 1968, A24, 351–359. [Google Scholar]
- Sheldrick, GM. SHELXS 97, Program for Crystal Structure Solution; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, GM. SHELXL 97, Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
Supplementary Material

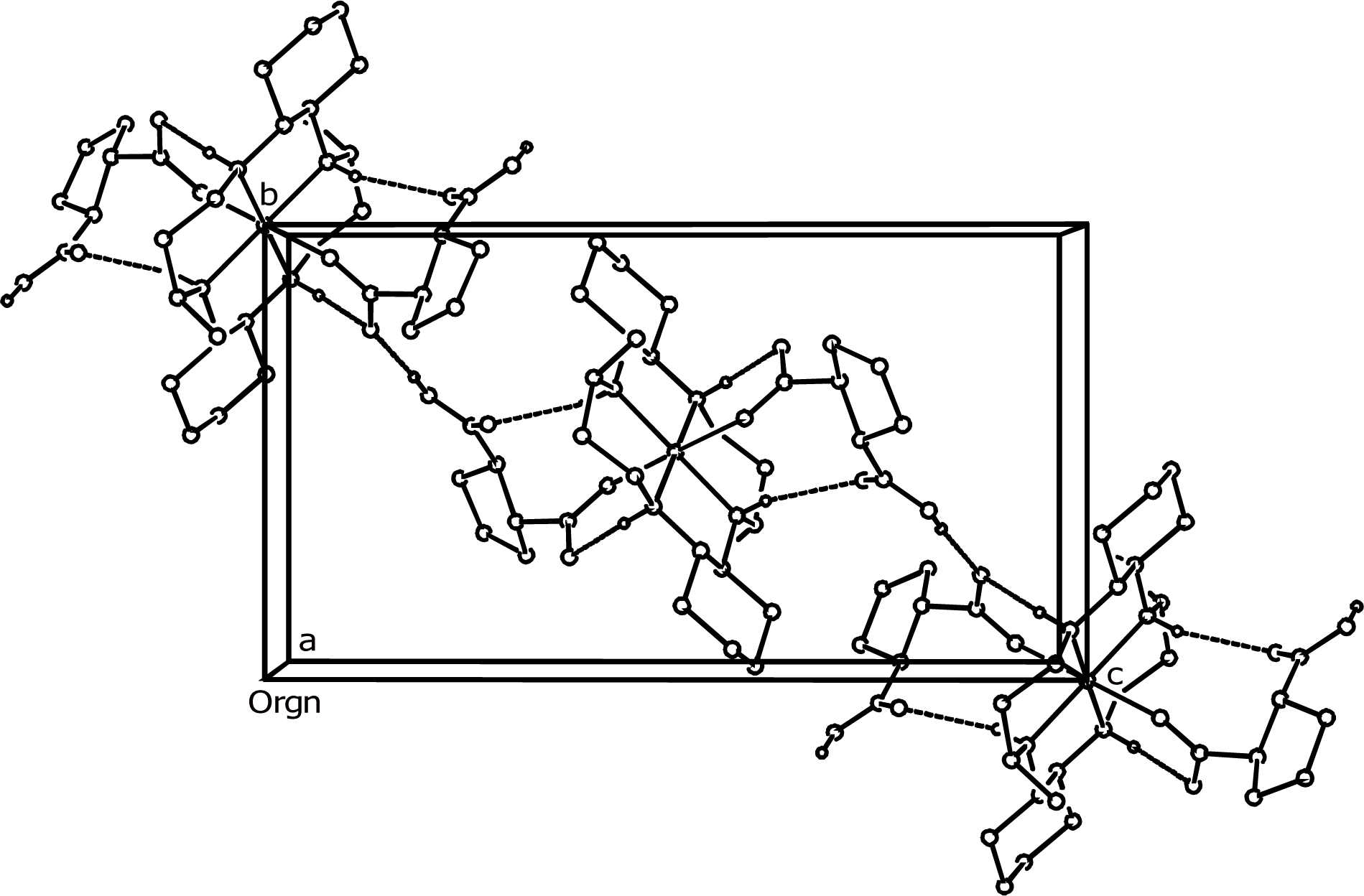

| Bond Lengths | |||
| Ni-N(1) | 2.043(5) | Ni-N(2) | 2.082(5) |
| Ni-O(1) | 2.176(4) | O(1)-C(11) | 1.259(7) |
| O(2)-C(11) | 1.253(7) | O(3)-C(17) | 1.203(7) |
| O(4)-C(17) | 1.290(8) | Ni…Ni#1 | 8.743(2) |
| Bond Angles | |||
| N(1)-Ni-N(2) | 83.8(2) | N(1)-Ni-N(2)#2 | 96.2(2) |
| N(1)-Ni-O(1) | 93.2(2) | N(1)#2-Ni-O(1) | 86.8(2) |
| N(2)-Ni-O(1) | 97.0(2) | N(2)#2-Ni-O(1) | 83.0(2) |
| Ni-O(1)-C(11) | 130.1(4) | O(1)-C(11)-O(2) | 122.9(6) |
| O(3)-C(4)-O(4) | 122.0(6) |
| D-H…A | D-H (Å) | H…A (Å) | D…A (Å) | ∠D-H…A (°) |
|---|---|---|---|---|
| N(1)-H(17)…O(2)#3 | 0.85(6) | 1.97(6) | 2.797(6) | 163(6) |
| N(2)-H(18)…O(3)#2 | 0.82(6) | 2.31(6) | 3.085(7) | 158(6) |
| O(4)-H(19)…O(2)#4 | 0.79(9) | 1.75(9) | 2.522(7) | 166(10) |
| Complex | State | λmax/nm (ɛ/M−1 cm−1) |
|---|---|---|
| [Ni(L)](ClO4)2·2H2O b | MeCN | 465(66) |
| H2O | 459(70) | |
| [Ni(L)(H-cpdc−)2] (1) | Solid | 340, 530, 694 |
| H2O | 260(2.5 × 102), 458(67) |
| Potentials (V) versus Ag/AgCl | ||
|---|---|---|
| Complex | Ni(II)/Ni(III) | Ni(II)/Ni(I) |
| [Ni(L)](ClO4)2 b | +0.73 | −1.63 |
| [Ni(L)(H-cpdc−)2] (1) | +0.66 (i) c | −1.23 |
| Empirical Formula | C34H58N4NiO8 |
|---|---|
| Formula weight | 709.55 |
| Temperature (K) | 293(2) |
| Crystal system | Monoclinic |
| Space group | P21/c |
| a (Å) | 8.7429(17) |
| b (Å) | 10.488(2) |
| c (Å) | 18.929(4) |
| β (°) | 91.82(2) |
| V (Å3) | 1734.8(6) |
| Z | 2 |
| Dcalc (Mg m−3) | 1.358 |
| Absorption coefficient (mm−1) | 0.615 |
| F(000) | 764 |
| Crystal size (mm) | 0.30 × 0.20 × 0.10 |
| θ range (°) | 2.15 to 24.99 |
| Limiting indices | −10 ≤ h ≤ 10, −1 ≤ k ≤ 12, −1 ≤ l ≤ 22 |
| Reflection collected | 3476 |
| Reflection unique | 3055 |
| Absorption correction | φ-scan |
| Max./min. transmission | 0.9403 and 0.8192 |
| Parameters | 223 |
| Goodness of fit on F2 | 1.136 |
| Final R indices [I > 2σ(I)] | R1a = 0.0628, wR2b = 0.1617 |
| R indices (all data) | R1 = 0.1573, wR2 = 0.1855 |
| Largest difference peak and hole (eÅ−3) | 0.366 and −0.338 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lim, I.-T.; Choi, K.-Y. One-Dimensional Hydrogen-Bonded Infinite Chain from Nickel(II) Tetraaza Macrocyclic Complex and 1,2-Cyclopentanedicarboxylate Ligand. Int. J. Mol. Sci. 2011, 12, 2232-2241. https://doi.org/10.3390/ijms12042232
Lim I-T, Choi K-Y. One-Dimensional Hydrogen-Bonded Infinite Chain from Nickel(II) Tetraaza Macrocyclic Complex and 1,2-Cyclopentanedicarboxylate Ligand. International Journal of Molecular Sciences. 2011; 12(4):2232-2241. https://doi.org/10.3390/ijms12042232
Chicago/Turabian StyleLim, In-Taek, and Ki-Young Choi. 2011. "One-Dimensional Hydrogen-Bonded Infinite Chain from Nickel(II) Tetraaza Macrocyclic Complex and 1,2-Cyclopentanedicarboxylate Ligand" International Journal of Molecular Sciences 12, no. 4: 2232-2241. https://doi.org/10.3390/ijms12042232
APA StyleLim, I.-T., & Choi, K.-Y. (2011). One-Dimensional Hydrogen-Bonded Infinite Chain from Nickel(II) Tetraaza Macrocyclic Complex and 1,2-Cyclopentanedicarboxylate Ligand. International Journal of Molecular Sciences, 12(4), 2232-2241. https://doi.org/10.3390/ijms12042232




