In Vitro and in Vivo Anti-Hyperglycemic Effects of Omija (Schizandra chinensis) Fruit
Abstract
:1. Introduction
2. Results and Discussion
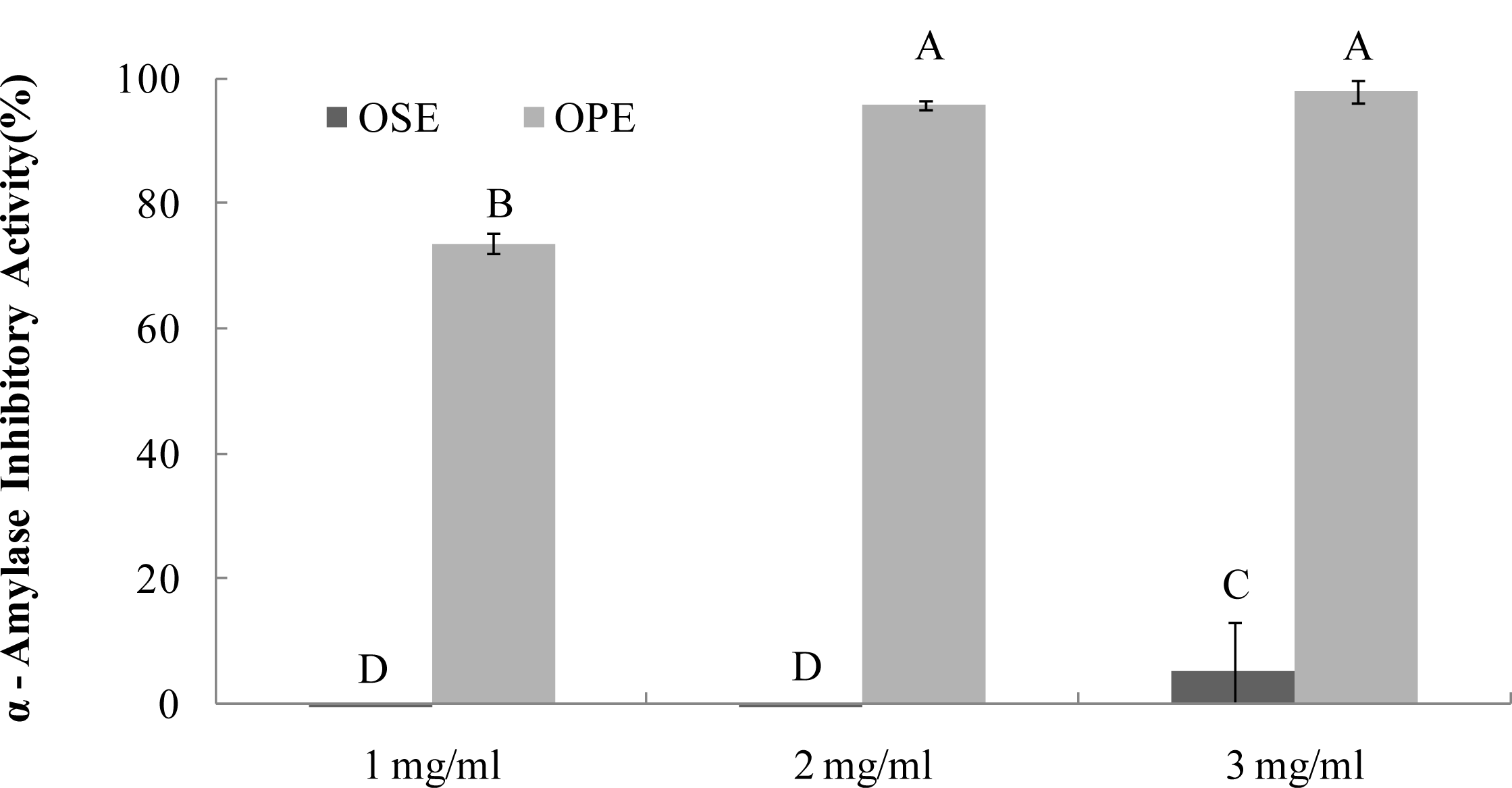
2.1. a-Amylase Inhibition
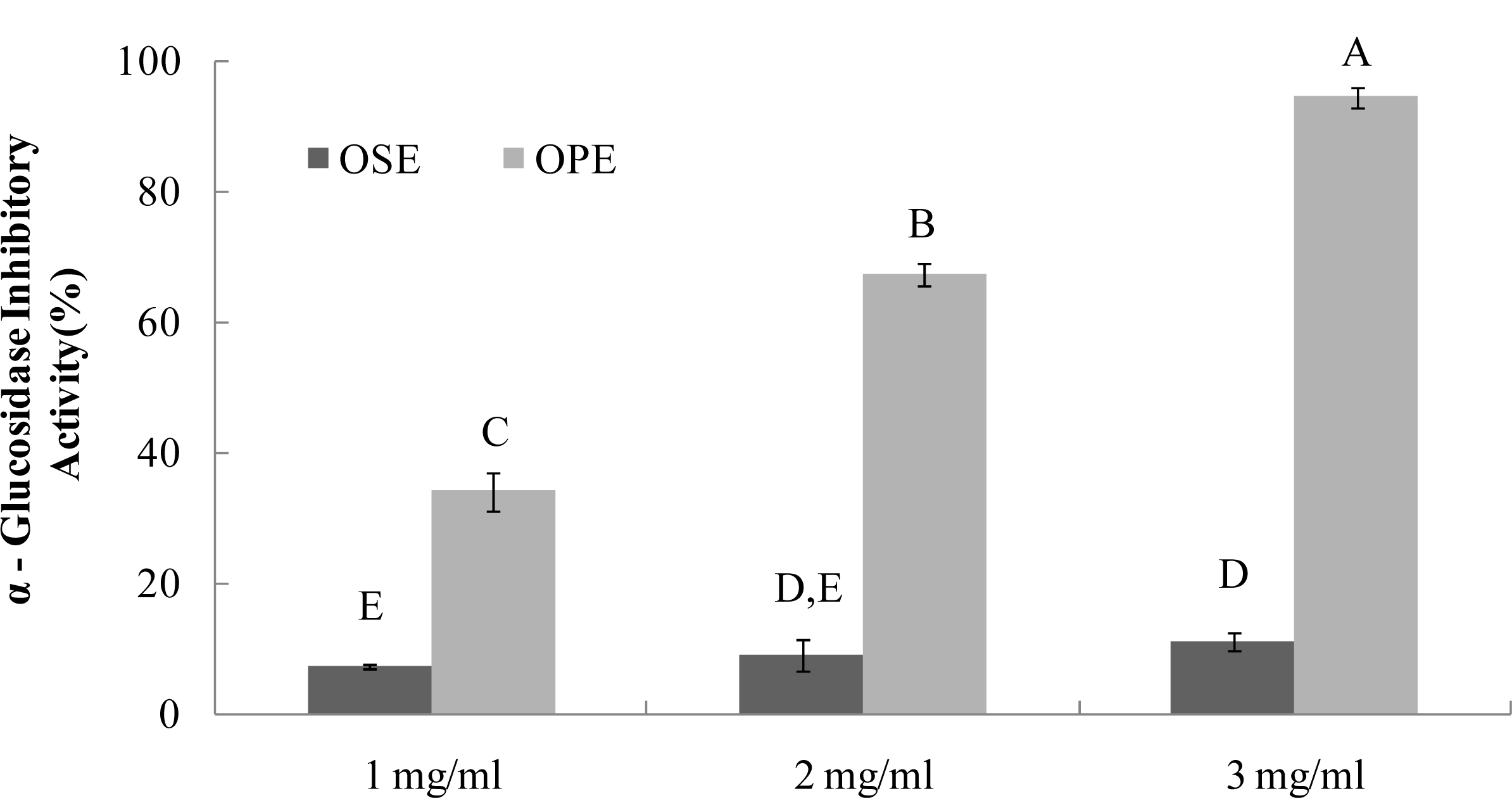
2.2. a-Glucosidase Inhibition
2.3. Antioxidant Activity by ORAC System
2.4. In Vivo Blood Glucose Lowering Effect of Omija Extracts
3. Experimental Section
3.1. Materials
3.2. Preparation of Extracts
3.3. a-Amylase Inhibition Assay
3.4. a-Glucosidase Inhibition Assay
3.5. Oxygen Radical Absorbance Capacity (ORAC) Assay
3.6. Sugar Loading Test
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Wild, S; Roglic, G; Green, A; Sicree, R; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar]
- Deshpande, MC; Venkateswarlu, V; Babu, RK; Trivedi, RK. Design and evaluation of oral bioadhesive controlled release formulations of miglitol, intended for prolonged inhibition of intestinal alpha-glucosidases and enhancement of plasma glycogen like peptide-1 levels. Int. J. Pharm 2009, 380, 16–24. [Google Scholar]
- Hirsh, AJ; Yao, SY; Young, JD; Cheeseman, CI. Inhibition of glucose absorption in the rat jejunum: A novel action of alpha-d-glucosidase inhibitors. Gastroenterology 1997, 113, 205–211. [Google Scholar]
- Brownlee, M. The pathobiology of diabetic complications. Diabetes 2005, 54, 1615–1625. [Google Scholar]
- Kwon, Y-I; Vattem, DA; Shetty, K. Evaluation of clonal herbs of Lamiaceae species for management of diabetes and hypertension. Asia Pac. J. Clin. Nutr 2005, 15, 107–118. [Google Scholar]
- Kaiser, N; Sasson, S; Feener, EP; Boukobza, N; Higashi, S; Moller, DE. Differential regulation of glucose transport and transporters by glucose in vascular endothelial and smooth muscle cells. Diabetes 1993, 42, 80–89. [Google Scholar]
- Kim, S-H; Joo, MH; Yoo, SH. Structural identification and antioxidant properties of major anthocyanin extracted from Omija (Schizandra chinensis) fruit. J. Food Sci 2009, 74, 134–140. [Google Scholar]
- Guo, LY; Hung, TM; Bae, KH; Shin, EM; Zhou, HY; Hong, YN; Kang, SS; Kim, HP; Kim, YS. Anti-inflammatory effects of schisandrin isolated from the fruit of Schisandra chinesis Bail. Eur. J. Pharmacol 2008, 591, 293–299. [Google Scholar]
- Sheng, CH; Kim, KH; Kim, TH; Lee, HJ. Analysis and characterization of aroma-active compounds of Schizandra chinensis (Omija) leaves. J. Sci. Food Agric 2005, 85, 161–166. [Google Scholar]
- Traustadottir, T; Davies, SS; Stock, AA; Heward, CB; Roberts, LJ; Harman, SM. Tart cherry juice decreases oxidative stress in healthy older men and women. J. Nutr 2009, 139, 1896–1900. [Google Scholar]
- Takikawa, M; Inoue, S; Horio, F; Tsuda, T. Dietary anthocyanin-rich bilberry extract ameliorate hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J. Nutr 2010, 140, 527–533. [Google Scholar]
- Kim, D-S; Kwon, HJ; Jang, H-D; Kwon, Y-I. In vitro α-glucosidase inhibitory potential and antioxidant activity of selected Lamiaceae species inhabited in Korean peninsula. Food. Sci. Biotechnol 2009, 18, 239–244. [Google Scholar]
- Lee, SK; Hwang, JY; Kang, MJ; Kim, YM; Jung, SH; Lee, JH; Kim, JI. Hypoglycemic effect of onion skin extract in animal models of diabetes mellitus. Food Sci. Biotechnol 2008, 17, 130–134. [Google Scholar]
- Gao, H; Kawabata, J. Importance of the B ring and its substitution on the α-glucosidase inhibitory activity of Baicalein, 5,6,7-Trihydroxyflavone. Biosci. Biotech. Biochem 2004, 68, 1858–1864. [Google Scholar]
- Gordon, JM; Derek, S. The inhibitory effects of berry polyphenols on digestive enzymes. BioFactors 2005, 23, 189–195. [Google Scholar]
- Yao, Y; Sang, W; Zhou, M; Ren, G. Antioxidant and alpha-glucosidase inhibitory activity of colored grains in China. J. Agric. Food Chem 2010, 27, 770–774. [Google Scholar]
- Cao, G; Sofic, E; Prior, RL. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med 1997, 22, 749–760. [Google Scholar]
- Prior, R; Hoang, H; Gu, L; Wu, X; Bacchiocca, M; Howard, L; Hampsci-Woodill, M; Huang, D; Ou, B; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL)) of plasma and other biological and food samples. J. Agric. Food Chem 2003, 51, 3273–3279. [Google Scholar]
- Wu, X; Beecher, GR; Holden, JM; Haytowitz, DB; Gebhardt, SE; Prior, RL. Lipophilic and hydrophilic capacities of common foods in the United States. J. Agric. Food Chem 2004, 52, 4026–4037. [Google Scholar]
- Kwon, Y-I; Vattem, DA; Shetty, K. Evaluation of clonal herbs of Lamiaceae species for management of diabetes and hypertension. Asia Pac. J. Clin. Nutr 2005, 15, 107–118. [Google Scholar]
- Kwon, Y-I; Apostolidis, E; Kim, Y-C; Shetty, K. Health benefits of traditional corn, beans and pumpkin: In vitro studies for hyperglycemia and hypertension management. J. Med. Foods 2007, 10, 266–275. [Google Scholar]
- Kurihara, H; Fukami, H; Asami, S; Totoda, Y; Nakai, M; Shibata, H; Yao, XS. Effects of oolong tea on plasma antioxidative capacity in mice loaded with restraint stress assessed using the oxygen radical absorbance capacity (ORAC) assay. Biol. Pharm. Bull 2004, 27, 1093–1098. [Google Scholar]
- Hertog, MGL; Kromhout, D; Aravanis, C; Blacburn, H; Buzina, R; Fridanza, F; Giampaoli, S; Jansen, A; Menotti, A; Nedeljkovic, S; et al. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven counties study. Arch. Intern. Med 1995, 155, 381–386. [Google Scholar]
- Paganga, G; Miller, N; Rice-Evans, CA. The polyphenolic contents of fruits and vegetables and their antioxidant activities. What does a serving constitute? Free Radic. Res 1999, 30, 153–162. [Google Scholar]
- Tadera, K; Minami, Y; Takamatsu, K; Matsuoka, T. Inhibition of α-glucosidase and α-amylase by flavonoids. J. Nutr. Sci. Vitaminol 2006, 52, 149–153. [Google Scholar]


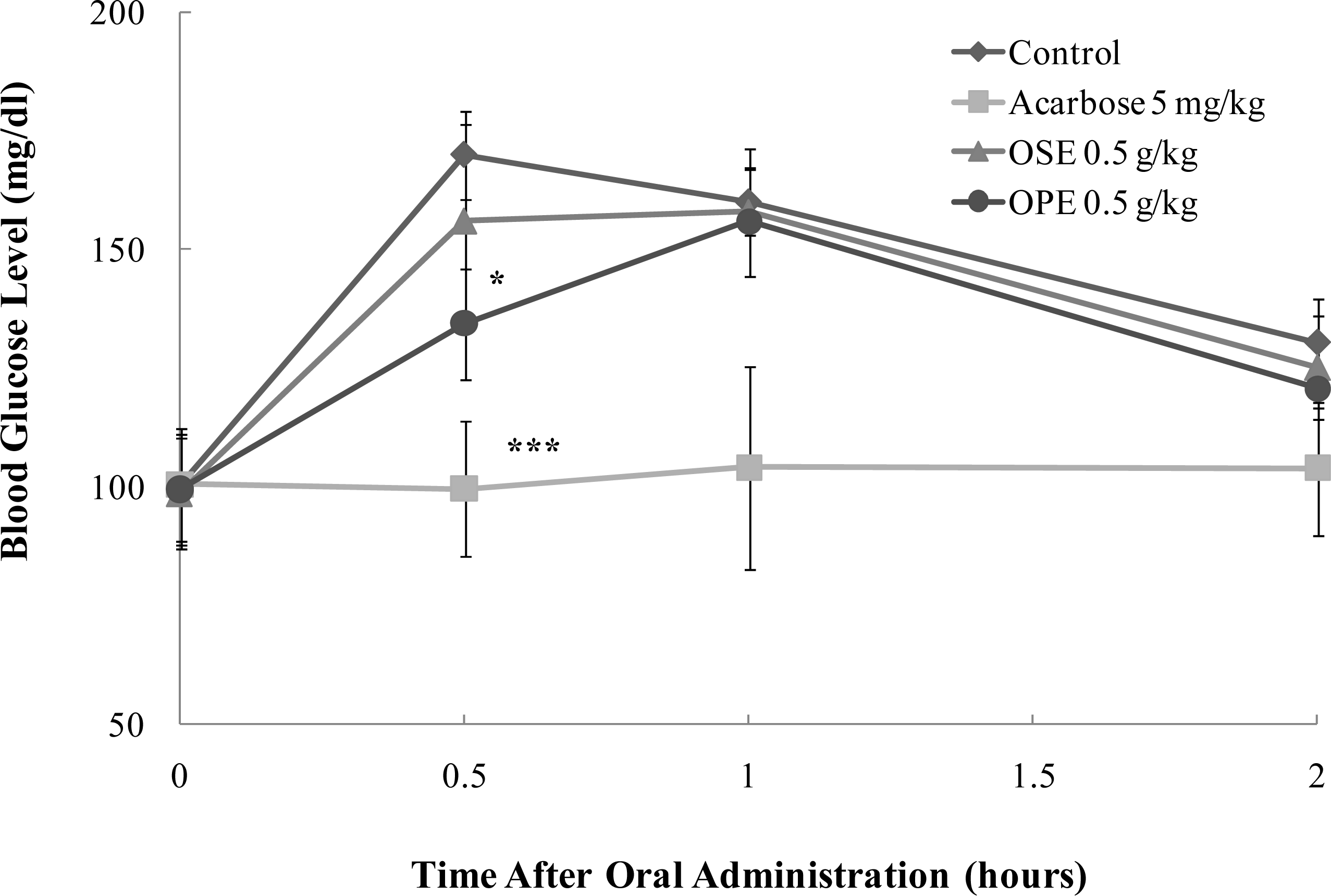
| PK parameters | ||||
|---|---|---|---|---|
| AUClast (mg/dL·h) | Cmax (mg/dL) | Tmax (h) | ||
| Sucrose | Control | 295.3 ± 7.2 | 171.2 ± 7.2 | 0.6 ± 0.2 |
| Acarbose (5 mg/kg) | 205.1 ± 29.4*** | 111.0 ± 15.8*** | 1.2 ± 0.8*** | |
| OSE (0.5 g/kg) | 291.8 ± 22.0 | 173.4 ± 13.5 | 0.8 ± 0.3 | |
| OPE (0.5 g/kg) | 276.6 ± 16.3* | 157.2 ± 10.5* | 1.0 ± 0.0** | |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jo, S.-H.; Ha, K.-S.; Moon, K.-S.; Lee, O.-H.; Jang, H.-D.; Kwon, Y.-I. In Vitro and in Vivo Anti-Hyperglycemic Effects of Omija (Schizandra chinensis) Fruit. Int. J. Mol. Sci. 2011, 12, 1359-1370. https://doi.org/10.3390/ijms12021359
Jo S-H, Ha K-S, Moon K-S, Lee O-H, Jang H-D, Kwon Y-I. In Vitro and in Vivo Anti-Hyperglycemic Effects of Omija (Schizandra chinensis) Fruit. International Journal of Molecular Sciences. 2011; 12(2):1359-1370. https://doi.org/10.3390/ijms12021359
Chicago/Turabian StyleJo, Sung-Hoon, Kyoung-Soo Ha, Kyoung-Sik Moon, Ok-Hwan Lee, Hae-Dong Jang, and Young-In Kwon. 2011. "In Vitro and in Vivo Anti-Hyperglycemic Effects of Omija (Schizandra chinensis) Fruit" International Journal of Molecular Sciences 12, no. 2: 1359-1370. https://doi.org/10.3390/ijms12021359
APA StyleJo, S.-H., Ha, K.-S., Moon, K.-S., Lee, O.-H., Jang, H.-D., & Kwon, Y.-I. (2011). In Vitro and in Vivo Anti-Hyperglycemic Effects of Omija (Schizandra chinensis) Fruit. International Journal of Molecular Sciences, 12(2), 1359-1370. https://doi.org/10.3390/ijms12021359







