The Wnt and BMP Families of Signaling Morphogens at the Vertebrate Neuromuscular Junction
Abstract
:1. Introduction
2. The Neuromuscular Junction
2.1. Presynaptic Differentiation at the Vertebrate NMJ
2.2. Neural Control of Postsynaptic Differentiation at the Vertebrate NMJ
2.3. Aneural Signals Induce Postsynaptic Pre-Patterning at the Vertebrate NMJ
2.4. The Neurotransmitter Acetylcholine Inhibits NMJ Assembly
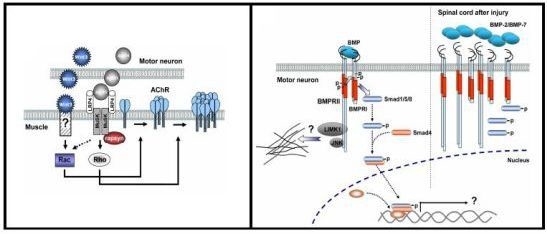
3. Wnt Signaling at the Neuromuscular Synapse
3.1. Wnt Pathways
3.2. Wnts Play Pro- and Anti-Synaptogenic Roles at the Invertebrate NMJ
3.3. Role of Wnt Ligands in Postsynaptic Differentiation of the Vertebrate NMJ
4. BMP Signaling Pathways at the Neuromuscular Synapse
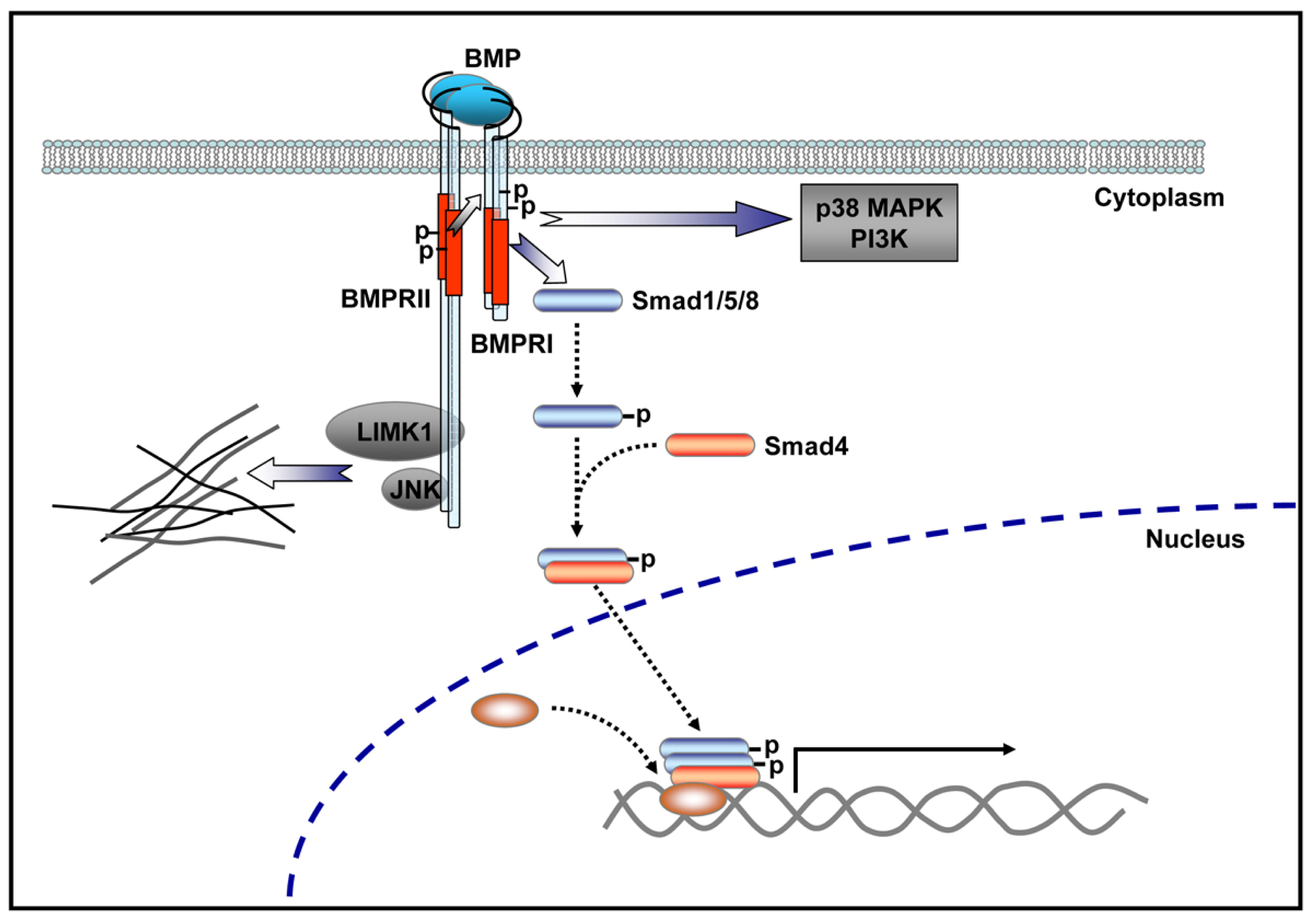
4.1. BMP Signaling Pathways
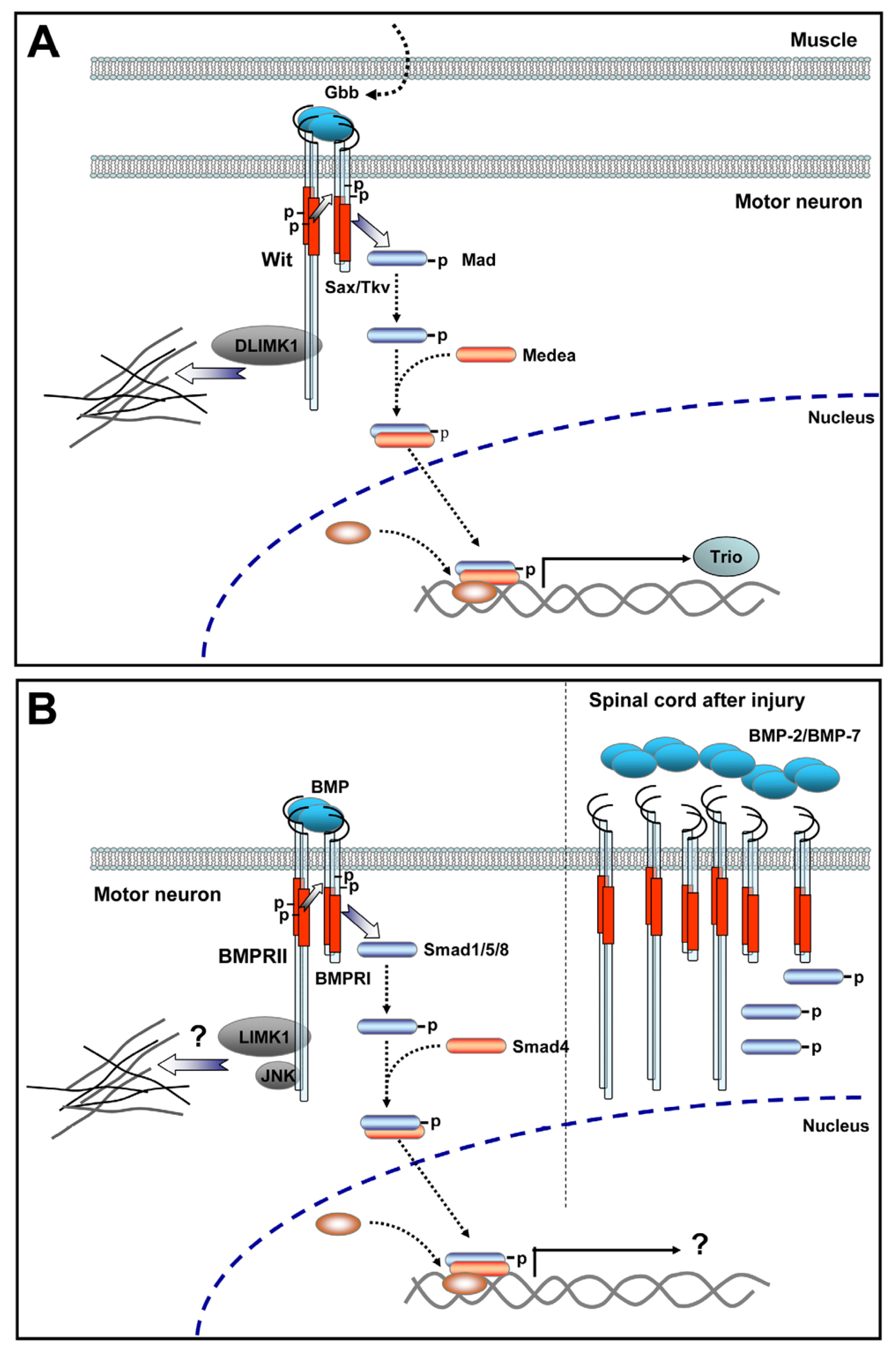
4.2. BMP Signaling at the Invertebrate NMJ
4.3. In Vivo Evidence Supports a Role for BMP Signaling in Vertebrate Motor Neurons
4.4. BMP Signaling Modulation by Proteins Involved in Vertebrate Motor Neuron Dysfunction
5. Conclusions
Acknowledgments
- Conflict of Interest The authors declare no conflict of interest.
References
- Sanes, J.R.; Lichtman, J.W. Development of the vertebrate neuromuscular junction. Annu. Rev. Neurosci 1999, 22, 389–442. [Google Scholar]
- Sanes, J.R.; Lichtman, J.W. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat. Rev. Neurosci 2001, 2, 791–805. [Google Scholar]
- Fox, M.A.; Umemori, H. Seeking long-term relationship: axon and target communicate to organize synaptic differentiation. J. Neurochem 2006, 97, 1215–1231. [Google Scholar]
- Kummer, T.T.; Misgeld, T.; Sanes, J.R. Assembly of the postsynaptic membrane at the neuromuscular junction: paradigm lost. Curr. Opin. Neurobiol 2006, 16, 74–82. [Google Scholar]
- Wu, H.; Xiong, W.C.; Mei, L. To build a synapse: Signaling pathways in neuromuscular junction assembly. Development 2010, 137, 1017–1033. [Google Scholar]
- Feng, G.; Laskowski, M.B.; Feldheim, D.A.; Wang, H.; Lewis, R.; Frisen, J.; Flanagan, J.G.; Sanes, J.R. Roles for ephrins in positionally selective synaptogenesis between motor neurons and muscle fibers. Neuron 2000, 25, 295–306. [Google Scholar]
- Fox, M.A.; Sanes, J.R.; Borza, D.B.; Eswarakumar, V.P.; Fassler, R.; Hudson, B.G.; John, S.W.; Ninomiya, Y.; Pedchenko, V.; Pfaff, S.L.; et al. Distinct target-derived signals organize formation, maturation, and maintenance of motor nerve terminals. Cell 2007, 129, 179–193. [Google Scholar]
- Nishimune, H.; Sanes, J.R.; Carlson, S.S. A synaptic laminin-calcium channel interaction organizes active zones in motor nerve terminals. Nature 2004, 432, 580–587. [Google Scholar]
- Carlson, S.S.; Valdez, G.; Sanes, J.R. Presynaptic calcium channels and alpha3-integrins are complexed with synaptic cleft laminins, cytoskeletal elements and active zone components. J. Neurochem 2010, 115, 654–666. [Google Scholar]
- Gautam, M.; Noakes, P.G.; Moscoso, L.; Rupp, F.; Scheller, R.H.; Merlie, J.P.; Sanes, J.R. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell 1996, 85, 525–535. [Google Scholar]
- Lin, W.; Burgess, R.W.; Dominguez, B.; Pfaff, S.L.; Sanes, J.R.; Lee, K.F. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature 2001, 410, 1057–1064. [Google Scholar]
- Misgeld, T.; Burgess, R.W.; Lewis, R.M.; Cunningham, J.M.; Lichtman, J.W.; Sanes, J.R. Roles of neurotransmitter in synapse formation: Development of neuromuscular junctions lacking choline acetyltransferase. Neuron 2002, 36, 635–648. [Google Scholar]
- Bowe, M.A.; Fallon, J.R. The role of agrin in synapse formation. Annu. Rev. Neurosci 1995, 18, 443–462. [Google Scholar]
- Fallon, J.R.; Gelfman, C.E. Agrin-related molecules are concentrated at acetylcholine receptor clusters in normal and aneural developing muscle. J. Cell Biol 1989, 108, 1527–1535. [Google Scholar]
- Fallon, J.R.; Nitkin, R.M.; Reist, N.E.; Wallace, B.G.; McMahan, U.J. Acetylcholine receptor-aggregating factor is similar to molecules concentrated at neuromuscular junctions. Nature 1985, 315, 571–574. [Google Scholar]
- Nitkin, R.M.; Smith, M.A.; Magill, C.; Fallon, J.R.; Yao, Y.M.; Wallace, B.G.; McMahan, U.J. Identification of agrin, a synaptic organizing protein from Torpedo electric organ. J. Cell Biol 1987, 105, 2471–2478. [Google Scholar]
- Wallace, B.G.; Nitkin, R.M.; Reist, N.E.; Fallon, J.R.; Moayeri, N.N.; McMahan, U.J. Aggregates of acetylcholinesterase induced by acetylcholine receptor-aggregating factor. Nature 1985, 315, 574–577. [Google Scholar]
- Jing, L.; Lefebvre, J.L.; Gordon, L.R.; Granato, M. Wnt signals organize synaptic prepattern and axon guidance through the zebrafish unplugged/MuSK receptor. Neuron 2009, 61, 721–733. [Google Scholar]
- DeChiara, T.M.; Bowen, D.C.; Valenzuela, D.M.; Simmons, M.V.; Poueymirou, W.T.; Thomas, S.; Kinetz, E.; Compton, D.L.; Rojas, E.; Park, J.S.; et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell 1996, 85, 501–512. [Google Scholar]
- Glass, D.J.; Bowen, D.C.; Stitt, T.N.; Radziejewski, C.; Bruno, J.; Ryan, T.E.; Gies, D.R.; Shah, S.; Mattsson, K.; Burden, S.J.; et al. Agrin acts via a MuSK receptor complex. Cell 1996, 85, 513–523. [Google Scholar]
- Valenzuela, D.M.; Stitt, T.N.; DiStefano, P.S.; Rojas, E.; Mattsson, K.; Compton, D.L.; Nunez, L.; Park, J.S.; Stark, J.L.; Gies, D.R.; et al. Receptor tyrosine kinase specific for the skeletal muscle lineage: expression in embryonic muscle, at the neuromuscular junction, and after injury. Neuron 1995, 15, 573–584. [Google Scholar]
- Noakes, P.G.; Phillips, W.D.; Hanley, T.A.; Sanes, J.R.; Merlie, J.P. 43K protein and acetylcholine receptors colocalize during the initial stages of neuromuscular synapse formation in vivo. Dev. Biol 1993, 155, 275–280. [Google Scholar]
- Inoue, A.; Setoguchi, K.; Matsubara, Y.; Okada, K.; Sato, N.; Iwakura, Y.; Higuchi, O.; Yamanashi, Y. Dok-7 activates the muscle receptor kinase MuSK and shapes synapse formation. Sci. Signal 2009, 2, ra7. [Google Scholar]
- Linnoila, J.; Wang, Y.; Yao, Y.; Wang, Z.Z. A mammalian homolog of Drosophila tumorous imaginal discs, Tid1, mediates agrin signaling at the neuromuscular junction. Neuron 2008, 60, 625–641. [Google Scholar]
- Okada, K.; Inoue, A.; Okada, M.; Murata, Y.; Kakuta, S.; Jigami, T.; Kubo, S.; Shiraishi, H.; Eguchi, K.; Motomura, M.; et al. The muscle protein Dok-7 is essential for neuromuscular synaptogenesis. Science 2006, 312, 1802–1805. [Google Scholar]
- Flanagan-Steet, H.; Fox, M.A.; Meyer, D.; Sanes, J.R. Neuromuscular synapses can form in vivo by incorporation of initially aneural postsynaptic specializations. Development 2005, 132, 4471–4481. [Google Scholar]
- Lin, S.; Landmann, L.; Ruegg, M.A.; Brenner, H.R. The role of nerve- versus muscle-derived factors in mammalian neuromuscular junction formation. J. Neurosci 2008, 28, 3333–3340. [Google Scholar]
- Pun, S.; Sigrist, M.; Santos, A.F.; Ruegg, M.A.; Sanes, J.R.; Jessell, T.M.; Arber, S.; Caroni, P. An intrinsic distinction in neuromuscular junction assembly and maintenance in different skeletal muscles. Neuron 2002, 34, 357–370. [Google Scholar]
- Yang, X.; Arber, S.; William, C.; Li, L.; Tanabe, Y.; Jessell, T.M.; Birchmeier, C.; Burden, S.J. Patterning of muscle acetylcholine receptor gene expression in the absence of motor innervation. Neuron 2001, 30, 399–410. [Google Scholar]
- Ponomareva, O.N.; Ma, H.; Vock, V.M.; Ellerton, E.L.; Moody, S.E.; Dakour, R.; Chodosh, L.A.; Rimer, M. Defective neuromuscular synaptogenesis in mice expressing constitutively active ErbB2 in skeletal muscle fibers. Mol. Cell. Neurosci 2006, 31, 334–345. [Google Scholar]
- Vock, V.M.; Ponomareva, O.N.; Rimer, M. Evidence for muscle-dependent neuromuscular synaptic site determination in mammals. J. Neurosci 2008, 28, 3123–3130. [Google Scholar]
- Panzer, J.A.; Gibbs, S.M.; Dosch, R.; Wagner, D.; Mullins, M.C.; Granato, M.; Balice-Gordon, R.J. Neuromuscular synaptogenesis in wild-type and mutant zebrafish. Dev. Biol 2005, 285, 340–357. [Google Scholar]
- Lin, W.; Dominguez, B.; Yang, J.; Aryal, P.; Brandon, E.P.; Gage, F.H.; Lee, K.F. Neurotransmitter acetylcholine negatively regulates neuromuscular synapse formation by a Cdk5-dependent mechanism. Neuron 2005, 46, 569–579. [Google Scholar]
- Misgeld, T.; Kummer, T.T.; Lichtman, J.W.; Sanes, J.R. Agrin promotes synaptic differentiation by counteracting an inhibitory effect of neurotransmitter. Proc. Natl. Acad. Sci. USA 2005, 102, 11088–11093. [Google Scholar]
- An, M.C.; Lin, W.; Yang, J.; Dominguez, B.; Padgett, D.; Sugiura, Y.; Aryal, P.; Gould, T.W.; Oppenheim, R.W.; Hester, M.E.; et al. Acetylcholine negatively regulates development of the neuromuscular junction through distinct cellular mechanisms. Proc. Natl. Acad. Sci. USA 2010, 107, 10702–10707. [Google Scholar]
- Logan, C.Y.; Nusse, R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol 2004, 20, 781–810. [Google Scholar]
- Farias, G.G.; Godoy, J.A.; Cerpa, W.; Varela-Nallar, L.; Inestrosa, N.C. Wnt signaling modulates pre- and postsynaptic maturation: therapeutic considerations. Dev. Dyn 2010, 239, 94–101. [Google Scholar]
- Salinas, P.C.; Zou, Y. Wnt signaling in neural circuit assembly. Annu. Rev. Neurosci 2008, 31, 339–358. [Google Scholar]
- Gordon, M.D.; Nusse, R. Wnt signaling: Multiple pathways, multiple receptors, and multiple transcription factors. J. Biol. Chem 2006, 281, 22429–22433. [Google Scholar]
- Sheldahl, L.C.; Slusarski, D.C.; Pandur, P.; Miller, J.R.; Kuhl, M.; Moon, R.T. Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. J. Cell Biol 2003, 161, 769–777. [Google Scholar]
- Wharton, K.A., Jr. Runnin’ with the Dvl: Proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev. Biol. 2003, 253, 1–17. [Google Scholar]
- Kim, N.G.; Xu, C.; Gumbiner, B.M. Identification of targets of the Wnt pathway destruction complex in addition to beta-catenin. Proc. Natl. Acad. Sci. USA 2009, 106, 5165–5170. [Google Scholar]
- Macdonald, B.T.; Semenov, M.V.; He, X. SnapShot: Wnt/beta-catenin signaling. Cell 2007, 131, 1204. [Google Scholar]
- Kuhl, M.; Sheldahl, L.C.; Park, M.; Miller, J.R.; Moon, R.T. The Wnt/Ca2+ pathway: A new vertebrate Wnt signaling pathway takes shape. Trends Genet 2000, 16, 279–283. [Google Scholar]
- McEwen, D.G.; Peifer, M. Wnt signaling: Moving in a new direction. Curr. Biol 2000, 10, R562–R564. [Google Scholar]
- Semenov, M.V.; Habas, R.; Macdonald, B.T.; He, X. SnapShot: Noncanonical Wnt Signaling Pathways. Cell 2007, 131, 1378. [Google Scholar]
- Cadigan, K.M.; Liu, Y.I. Wnt signaling: Complexity at the surface. J. Cell Sci 2006, 119, 395–402. [Google Scholar]
- He, X.; Semenov, M.; Tamai, K.; Zeng, X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development 2004, 131, 1663–1677. [Google Scholar]
- Matthews, H.K.; Marchant, L.; Carmona-Fontaine, C.; Kuriyama, S.; Larrain, J.; Holt, M.R.; Parsons, M.; Mayor, R. Directional migration of neural crest cells in vivo is regulated by Syndecan-4/Rac1 and non-canonical Wnt signaling/RhoA. Development 2008, 135, 1771–1780. [Google Scholar]
- Kawano, Y.; Kypta, R. Secreted antagonists of the Wnt signalling pathway. J. Cell Sci 2003, 116, 2627–2634. [Google Scholar]
- Packard, M.; Koo, E.S.; Gorczyca, M.; Sharpe, J.; Cumberledge, S.; Budnik, V. The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell 2002, 111, 319–330. [Google Scholar]
- Ataman, B.; Ashley, J.; Gorczyca, D.; Gorczyca, M.; Mathew, D.; Wichmann, C.; Sigrist, S.J.; Budnik, V. Nuclear trafficking of Drosophila Frizzled-2 during synapse development requires the PDZ protein dGRIP. Proc. Natl. Acad. Sci. USA 2006, 103, 7841–7846. [Google Scholar]
- Mathew, D.; Ataman, B.; Chen, J.; Zhang, Y.; Cumberledge, S.; Budnik, V. Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2. Science 2005, 310, 1344–1347. [Google Scholar]
- Franco, B.; Bogdanik, L.; Bobinnec, Y.; Debec, A.; Bockaert, J.; Parmentier, M.L.; Grau, Y. Shaggy, the homolog of glycogen synthase kinase 3, controls neuromuscular junction growth in Drosophila. J. Neurosci 2004, 24, 6573–6577. [Google Scholar]
- Miech, C.; Pauer, H.U.; He, X.; Schwarz, T.L. Presynaptic local signaling by a canonical wingless pathway regulates development of the Drosophila neuromuscular junction. J. Neurosci 2008, 28, 10875–10884. [Google Scholar]
- Klassen, M.P.; Shen, K. Wnt signaling positions neuromuscular connectivity by inhibiting synapse formation in C. elegans. Cell 2007, 130, 704–716. [Google Scholar]
- Henriquez, J.P.; Webb, A.; Bence, M.; Bildsoe, H.; Sahores, M.; Hughes, S.M.; Salinas, P.C. Wnt signaling promotes AChR aggregation at the neuromuscular synapse in collaboration with agrin. Proc. Natl. Acad. Sci. USA 2008, 105, 18812–18817. [Google Scholar]
- Luo, Z.G.; Wang, Q.; Zhou, J.Z.; Wang, J.; Luo, Z.; Liu, M.; He, X.; Wynshaw-Boris, A.; Xiong, W.C.; Lu, B.; et al. Regulation of AChR clustering by Dishevelled interacting with MuSK and PAK1. Neuron 2002, 35, 489–505. [Google Scholar]
- Krylova, O.; Herreros, J.; Cleverley, K.E.; Ehler, E.; Henriquez, J.P.; Hughes, S.M.; Salinas, P.C. WNT-3, expressed by motoneurons, regulates terminal arborization of neurotrophin-3-responsive spinal sensory neurons. Neuron 2002, 35, 1043–1056. [Google Scholar]
- Kim, M.; Lee, H.C.; Tsedensodnom, O.; Hartley, R.; Lim, Y.S.; Yu, E.; Merle, P.; Wands, J.R. Functional interaction between Wnt3 and Frizzled-7 leads to activation of the Wnt/beta-catenin signaling pathway in hepatocellular carcinoma cells. J. Hepatol 2008, 48, 780–791. [Google Scholar]
- Kobune, M.; Chiba, H.; Kato, J.; Kato, K.; Nakamura, K.; Kawano, Y.; Takada, K.; Takimoto, R.; Takayama, T.; Hamada, H.; et al. Wnt3/RhoA/ROCK signaling pathway is involved in adhesion-mediated drug resistance of multiple myeloma in an autocrine mechanism. Mol. Cancer Ther 2007, 6, 1774–1784. [Google Scholar]
- Henriquez, J.P.; Salinas, P.C. Dual roles for Wnt signalling during the formation of the vertebrate neuromuscular junction. Acta Physiol. (Oxf.) 2011. [Google Scholar] [CrossRef]
- Weston, C.; Gordon, C.; Teressa, G.; Hod, E.; Ren, X.D.; Prives, J. Cooperative regulation by Rac and Rho of agrin-induced acetylcholine receptor clustering in muscle cells. J. Biol. Chem 2003, 278, 6450–6455. [Google Scholar]
- Weston, C.; Yee, B.; Hod, E.; Prives, J. Agrin-induced acetylcholine receptor clustering is mediated by the small guanosine triphosphatases Rac and Cdc42. J. Cell Biol 2000, 150, 205–212. [Google Scholar]
- Wang, J.; Ruan, N.J.; Qian, L.; Lei, W.L.; Chen, F.; Luo, Z.G. Wnt/beta-catenin signaling suppresses Rapsyn expression and inhibits acetylcholine receptor clustering at the neuromuscular junction. J. Biol. Chem 2008, 283, 21668–21675. [Google Scholar]
- Li, X.M.; Dong, X.P.; Luo, S.W.; Zhang, B.; Lee, D.H.; Ting, A.K.; Neiswender, H.; Kim, C.H.; Carpenter-Hyland, E.; Gao, T.M.; et al. Retrograde regulation of motoneuron differentiation by muscle beta-catenin. Nat. Neurosci 2008, 11, 262–268. [Google Scholar]
- Lefebvre, J.L.; Jing, L.; Becaficco, S.; Franzini-Armstrong, C.; Granato, M. Differential requirement for MuSK and dystroglycan in generating patterns of neuromuscular innervation. Proc. Natl. Acad. Sci. USA 2007, 104, 2483–2488. [Google Scholar]
- Zhang, J.; Lefebvre, J.L.; Zhao, S.; Granato, M. Zebrafish unplugged reveals a role for muscle-specific kinase homologs in axonal pathway choice. Nat. Neurosci 2004, 7, 1303–1309. [Google Scholar]
- Ahmad-Annuar, A.; Ciani, L.; Simeonidis, I.; Herreros, J.; Fredj, N.B.; Rosso, S.B.; Hall, A.; Brickley, S.; Salinas, P.C. Signaling across the synapse: A role for Wnt and Dishevelled in presynaptic assembly and neurotransmitter release. J. Cell Biol 2006, 174, 127–139. [Google Scholar]
- Avila, M.E.; Sepulveda, F.J.; Burgos, C.F.; Moraga-Cid, G.; Parodi, J.; Moon, R.T.; Aguayo, L.G.; Opazo, C.; De Ferrari, G.V. Canonical Wnt3a modulates intracellular calcium and enhances excitatory neurotransmission in hippocampal neurons. J. Biol. Chem 2010, 285, 18939–18947. [Google Scholar]
- Varela-Nallar, L.; Grabowski, C.P.; Alfaro, I.E.; Alvarez, A.R.; Inestrosa, N.C. Role of the Wnt receptor Frizzled-1 in presynaptic differentiation and function. Neural. Dev 2009, 4, 41. [Google Scholar]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar]
- Wozney, J.M.; Rosen, V.; Celeste, A.J.; Mitsock, L.M.; Whitters, M.J.; Kriz, R.W.; Hewick, R.M.; Wang, E.A. Novel regulators of bone formation: Molecular clones and activities. Science 1988, 242, 1528–1534. [Google Scholar]
- Kishigami, S.; Mishina, Y. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev 2005, 16, 265–278. [Google Scholar]
- De Robertis, E.M.; Kuroda, H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu. Rev. Cell Dev. Biol 2004, 20, 285–308. [Google Scholar]
- Sieber, C.; Kopf, J.; Hiepen, C.; Knaus, P. Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev 2009, 20, 343–355. [Google Scholar]
- Zhao, G.Q. Consequences of knocking out BMP signaling in the mouse. Genesis 2003, 35, 43–56. [Google Scholar]
- Anitha, M.; Shahnavaz, N.; Qayed, E.; Joseph, I.; Gossrau, G.; Mwangi, S.; Sitaraman, S.V.; Greene, J.G.; Srinivasan, S. BMP2 promotes differentiation of nitrergic and catecholaminergic enteric neurons through a Smad1-dependent pathway. Am. J. Physiol. Gastrointest. Liver Physiol 2010, 298, G375–G383. [Google Scholar]
- Gratacos, E.; Checa, N.; Alberch, J. Bone morphogenetic protein-2, but not bone morphogenetic protein-7, promotes dendritic growth and calbindin phenotype in cultured rat striatal neurons. Neuroscience 2001, 104, 783–790. [Google Scholar]
- Horbinski, C.; Stachowiak, E.K.; Chandrasekaran, V.; Miuzukoshi, E.; Higgins, D.; Stachowiak, M.K. Bone morphogenetic protein-7 stimulates initial dendritic growth in sympathetic neurons through an intracellular fibroblast growth factor signaling pathway. J. Neurochem 2002, 80, 54–63. [Google Scholar]
- Iwasaki, S.; Iguchi, M.; Watanabe, K.; Hoshino, R.; Tsujimoto, M.; Kohno, M. Specific activation of the p38 mitogen-activated protein kinase signaling pathway and induction of neurite outgrowth in PC12 cells by bone morphogenetic protein-2. J. Biol. Chem 1999, 274, 26503–26510. [Google Scholar]
- Lee-Hoeflich, S.T.; Causing, C.G.; Podkowa, M.; Zhao, X.; Wrana, J.L.; Attisano, L. Activation of LIMK1 by binding to the BMP receptor, BMPRII, regulates BMP-dependent dendritogenesis. EMBO J 2004, 23, 4792–4801. [Google Scholar]
- Matsuura, I.; Endo, M.; Hata, K.; Kubo, T.; Yamaguchi, A.; Saeki, N.; Yamashita, T. BMP inhibits neurite growth by a mechanism dependent on LIM-kinase. Biochem. Biophys. Res. Commun 2007, 360, 868–873. [Google Scholar]
- Yabe, T.; Samuels, I.; Schwartz, J.P. Bone morphogenetic proteins BMP-6 and BMP-7 have differential effects on survival and neurite outgrowth of cerebellar granule cell neurons. J. Neurosci. Res 2002, 68, 161–168. [Google Scholar]
- Gilboa, L.; Nohe, A.; Geissendorfer, T.; Sebald, W.; Henis, Y.I.; Knaus, P. Bone morphogenetic protein receptor complexes on the surface of live cells: A new oligomerization mode for serine/threonine kinase receptors. Mol. Biol. Cell 2000, 11, 1023–1035. [Google Scholar]
- Nohe, A.; Hassel, S.; Ehrlich, M.; Neubauer, F.; Sebald, W.; Henis, Y.I.; Knaus, P. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J. Biol. Chem 2002, 277, 5330–5338. [Google Scholar]
- Miyazono, K.; Maeda, S.; Imamura, T. BMP receptor signaling: Transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev 2005, 16, 251–263. [Google Scholar]
- Nohe, A.; Keating, E.; Knaus, P.; Petersen, N.O. Signal transduction of bone morphogenetic protein receptors. Cell. Signal 2004, 16, 291–299. [Google Scholar]
- Shi, Y.; Massague, J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar]
- Bragdon, B.; Moseychuk, O.; Saldanha, S.; King, D.; Julian, J.; Nohe, A. Bone morphogenetic proteins: A critical review. Cell. Signal 2011, 23, 609–620. [Google Scholar]
- Aberle, H.; Haghighi, A.P.; Fetter, R.D.; McCabe, B.D.; Magalhaes, T.R.; Goodman, C.S. wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron 2002, 33, 545–558. [Google Scholar]
- Estevez, M.; Attisano, L.; Wrana, J.L.; Albert, P.S.; Massague, J.; Riddle, D.L. The daf-4 gene encodes a bone morphogenetic protein receptor controlling C. elegans dauer larva development. Nature 1993, 365, 644–649. [Google Scholar]
- Ishikawa, T.; Yoshioka, H.; Ohuchi, H.; Noji, S.; Nohno, T. Truncated type II receptor for BMP-4 induces secondary axial structures in Xenopus embryos. Biochem. Biophys. Res. Commun 1995, 216, 26–33. [Google Scholar]
- Foletta, V.C.; Lim, M.A.; Soosairajah, J.; Kelly, A.P.; Stanley, E.G.; Shannon, M.; He, W.; Das, S.; Massague, J.; Bernard, O.; et al. Direct signaling by the BMP type II receptor via the cytoskeletal regulator LIMK1. J. Cell Biol 2003, 162, 1089–1098. [Google Scholar]
- Podkowa, M.; Zhao, X.; Chow, C.W.; Coffey, E.T.; Davis, R.J.; Attisano, L. Microtubule stabilization by bone morphogenetic protein receptor-mediated scaffolding of c-Jun N-terminal kinase promotes dendrite formation. Mol. Cell Biol 2010, 30, 2241–2250. [Google Scholar]
- Machado, R.D.; Rudarakanchana, N.; Atkinson, C.; Flanagan, J.A.; Harrison, R.; Morrell, N.W.; Trembath, R.C. Functional interaction between BMPR-II and Tctex-1, a light chain of Dynein, is isoform-specific and disrupted by mutations underlying primary pulmonary hypertension. Hum. Mol. Genet 2003, 12, 3277–3286. [Google Scholar]
- Ball, R.W.; Warren-Paquin, M.; Tsurudome, K.; Liao, E.H.; Elazzouzi, F.; Cavanagh, C.; An, B.S.; Wang, T.T.; White, J.H.; Haghighi, A.P. Retrograde BMP signaling controls synaptic growth at the NMJ by regulating trio expression in motor neurons. Neuron 2010, 66, 536–549. [Google Scholar]
- Eaton, B.A.; Davis, G.W. LIM Kinase1 controls synaptic stability downstream of the type II BMP receptor. Neuron 2005, 47, 695–708. [Google Scholar]
- Marques, G.; Bao, H.; Haerry, T.E.; Shimell, M.J.; Duchek, P.; Zhang, B.; O’Connor, M.B. The Drosophila BMP type II receptor Wishful Thinking regulates neuromuscular synapse morphology and function. Neuron 2002, 33, 529–543. [Google Scholar]
- Marques, G.; Zhang, B. Retrograde signaling that regulates synaptic development and function at the Drosophila neuromuscular junction. Int. Rev. Neurobiol 2006, 75, 267–285. [Google Scholar]
- James, R.E.; Broihier, H.T. Crimpy inhibits the BMP homolog Gbb in motoneurons to enable proper growth control at the Drosophila neuromuscular junction. Development 2011, 138, 3273–3286. [Google Scholar]
- McCabe, B.D.; Marques, G.; Haghighi, A.P.; Fetter, R.D.; Crotty, M.L.; Haerry, T.E.; Goodman, C.S.; O’Connor, M.B. The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron 2003, 39, 241–254. [Google Scholar]
- McCabe, B.D.; Hom, S.; Aberle, H.; Fetter, R.D.; Marques, G.; Haerry, T.E.; Wan, H.; O’Connor, M.B.; Goodman, C.S.; Haghighi, A.P. Highwire regulates presynaptic BMP signaling essential for synaptic growth. Neuron 2004, 41, 891–905. [Google Scholar]
- Rawson, J.M.; Lee, M.; Kennedy, E.L.; Selleck, S.B. Drosophila neuromuscular synapse assembly and function require the TGF-beta type I receptor saxophone and the transcription factor Mad. J. Neurobiol 2003, 55, 134–150. [Google Scholar]
- Salinas, S.; Proukakis, C.; Crosby, A.; Warner, T.T. Hereditary spastic paraplegia: Clinical features and pathogenetic mechanisms. Lancet Neurol 2008, 7, 1127–1138. [Google Scholar]
- Wang, X.; Shaw, W.R.; Tsang, H.T.; Reid, E.; O’Kane, C.J. Drosophila spichthyin inhibits BMP signaling and regulates synaptic growth and axonal microtubules. Nat. Neurosci 2007, 10, 177–185. [Google Scholar]
- Hirth, F. Drosophila melanogaster in the study of human neurodegeneration. CNS Neurol. Disord. Drug Targets 2010, 9, 504–523. [Google Scholar]
- Bayat, V.; Jaiswal, M.; Bellen, H.J. The BMP signaling pathway at the Drosophila neuromuscular junction and its links to neurodegenerative diseases. Curr. Opin. Neurobiol 2011, 21, 182–188. [Google Scholar]
- Matsuura, I.; Taniguchi, J.; Hata, K.; Saeki, N.; Yamashita, T. BMP inhibition enhances axonal growth and functional recovery after spinal cord injury. J. Neurochem 2008, 105, 1471–1479. [Google Scholar]
- Setoguchi, T.; Yone, K.; Matsuoka, E.; Takenouchi, H.; Nakashima, K.; Sakou, T.; Komiya, S.; Izumo, S. Traumatic injury-induced BMP7 expression in the adult rat spinal cord. Brain Res 2001, 921, 219–225. [Google Scholar]
- Setoguchi, T.; Nakashima, K.; Takizawa, T.; Yanagisawa, M.; Ochiai, W.; Okabe, M.; Yone, K.; Komiya, S.; Taga, T. Treatment of spinal cord injury by transplantation of fetal neural precursor cells engineered to express BMP inhibitor. Exp. Neurol 2004, 189, 33–44. [Google Scholar]
- Enzmann, G.U.; Benton, R.L.; Woock, J.P.; Howard, R.M.; Tsoulfas, P.; Whittemore, S.R. Consequences of noggin expression by neural stem, glial, and neuronal precursor cells engrafted into the injured spinal cord. Exp. Neurol 2005, 195, 293–304. [Google Scholar]
- Xiao, Q.; Du, Y.; Wu, W.; Yip, H.K. Bone morphogenetic proteins mediate cellular response and, together with Noggin, regulate astrocyte differentiation after spinal cord injury. Exp. Neurol 2010, 221, 353–366. [Google Scholar]
- Silver, J.; Miller, J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci 2004, 5, 146–156. [Google Scholar]
- Sahni, V.; Mukhopadhyay, A.; Tysseling, V.; Hebert, A.; Birch, D.; McGuire, T.L.; Stupp, S.I.; Kessler, J.A. BMPR1a and BMPR1b signaling exert opposing effects on gliosis after spinal cord injury. J. Neurosci 2010, 30, 1839–1855. [Google Scholar]
- Okuyama, N.; Kiryu-Seo, S.; Kiyama, H. Altered expression of Smad family members in injured motor neurons of rat. Brain Res 2007, 1132, 36–41. [Google Scholar]
- Parikh, P.; Hao, Y.; Hosseinkhani, M.; Patil, S.B.; Huntley, G.W.; Tessier-Lavigne, M.; Zou, H. Regeneration of axons in injured spinal cord by activation of bone morphogenetic protein/Smad1 signaling pathway in adult neurons. Proc. Natl. Acad. Sci. USA 2011, 108, E99–E107. [Google Scholar]
- Dion, P.A.; Daoud, H.; Rouleau, G.A. Genetics of motor neuron disorders: new insights into pathogenic mechanisms. Nat. Rev. Genet 2009, 10, 769–782. [Google Scholar]
- Tsang, H.T.; Edwards, T.L.; Wang, X.; Connell, J.W.; Davies, R.J.; Durrington, H.J.; O’Kane, C.J.; Luzio, J.P.; Reid, E. The hereditary spastic paraplegia proteins NIPA1, spastin and spartin are inhibitors of mammalian BMP signalling. Hum. Mol. Genet 2009, 18, 3805–3821. [Google Scholar]
- Fassier, C.; Hutt, J.A.; Scholpp, S.; Lumsden, A.; Giros, B.; Nothias, F.; Schneider-Maunoury, S.; Houart, C.; Hazan, J. Zebrafish atlastin controls motility and spinal motor axon architecture via inhibition of the BMP pathway. Nat. Neurosci 2010, 13, 1380–1387. [Google Scholar]
- Fuentealba, L.C.; Eivers, E.; Ikeda, A.; Hurtado, C.; Kuroda, H.; Pera, E.M.; De Robertis, E.M. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell 2007, 131, 980–993. [Google Scholar]
- Packard, M.; Mathew, D.; Budnik, V. Wnts and TGF beta in synaptogenesis: Old friends signalling at new places. Nat. Rev. Neurosci 2003, 4, 113–120. [Google Scholar]

© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Henríquez, J.P.; Krull, C.E.; Osses, N. The Wnt and BMP Families of Signaling Morphogens at the Vertebrate Neuromuscular Junction. Int. J. Mol. Sci. 2011, 12, 8924-8946. https://doi.org/10.3390/ijms12128924
Henríquez JP, Krull CE, Osses N. The Wnt and BMP Families of Signaling Morphogens at the Vertebrate Neuromuscular Junction. International Journal of Molecular Sciences. 2011; 12(12):8924-8946. https://doi.org/10.3390/ijms12128924
Chicago/Turabian StyleHenríquez, Juan P., Catherine E. Krull, and Nelson Osses. 2011. "The Wnt and BMP Families of Signaling Morphogens at the Vertebrate Neuromuscular Junction" International Journal of Molecular Sciences 12, no. 12: 8924-8946. https://doi.org/10.3390/ijms12128924
APA StyleHenríquez, J. P., Krull, C. E., & Osses, N. (2011). The Wnt and BMP Families of Signaling Morphogens at the Vertebrate Neuromuscular Junction. International Journal of Molecular Sciences, 12(12), 8924-8946. https://doi.org/10.3390/ijms12128924