Principal Component Analysis of HPLC Retention Data and Molecular Modeling Structural Parameters of Cardiovascular System Drugs in View of Their Pharmacological Activity
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Drugs
3.2. Chromatographic Conditions
3.3. Structural Parameters
3.4. Statistical Analysis
4. Conclusions
References
- Hardman, JG; Limbird, LE; Gilman, AG. Goodman and Gilman’s the Pharmacological Basis of Therapeutics; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Feely, J. New Drugs, 3rd ed; BMJ: London, UK, 1994. [Google Scholar]
- DiPiro, JT; Talbert, RL; Matzke, GR; Wells, BG; Posey, LM. Pharmacotherapy A Pathophysiologic Approach; McGraw-Hill: New York, NY, USA, 1999. [Google Scholar]
- Pugsley, MK. Antiarrhythmic drug development: historical review and future perspective. Drug Dev. Res 2002, 55, 3–16. [Google Scholar]
- Singh, BN; Vaughan Williams, EM. The effect of amiodarone, a new anti-anginal drug, on cardiac muscle. Br. J. Pharmac 1970, 39, 657–667. [Google Scholar]
- Rosenbaum, MB; Chiale, PA; Halpern, MS; Nau, GJ; Przybylski, J; Levi, RJ; Lázzari, JO; Elizari, MV. Clinical efficacy of amiodarone as an antiarrhythmic agent. Am. J. Cardiol 1976, 38, 934–944. [Google Scholar]
- McMurray, JJV. Clinical practice. Systolic heart failure. N. Engl. J. Med 2010, 362, 228–238. [Google Scholar]
- Tronvik, E; Stovner, LJ; Helde, G; Sand, T; Bovim, G. Prophylactic treatment of migraine with an angiotensin II receptor blocker: a randomized controlled trial. JAMA 2003, 1, 65–69. [Google Scholar]
- Triggle, DJ. 1,4-dihydropyridine calcium channel ligands: Selectivity of action. The roles of pharmacokinetics, state-dependent interactions, channel isoforms, and other factors. Drug Dev. Res 2003, 58, 5–17. [Google Scholar]
- Katz, AM; Leach, NM. Differential effects of 1,4-dihydropyridine calcium channel blockers: Therapeutic implications. J. Clin. Pharmacol 1987, 27, 825–834. [Google Scholar]
- Janis, RA; Triggle, DJ. 1,4-Dihydropyridine Ca2+ channel antagonists and activators: A comparison of binding characteristics with pharmacology. Drug Dev. Res 1984, 4, 257–274. [Google Scholar]
- Grossman, E; Messerli, FH. Calcium antagonists. Prog. Cardiovasc. Dis 2004, 47, 34–57. [Google Scholar]
- Frampton, JE; Brodgen, RN. Pentoxifylline (oxpentifylline). A review of its therapeutic efficacy in the management of peripheral vascular and cerebrovascular disorders. Drugs Aging 1995, 7, 480–503. [Google Scholar]
- Jull, A; Waters, J; Arroll, B. Pentoxifylline for treatment of venous leg ulcers: a systematic review. Lancet 2002, 359, 1550–1554. [Google Scholar]
- Ward, A; Clissold, SP. Pentoxifylline. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic efficacy. Drugs 1987, 34, 50–97. [Google Scholar]
- Velicer, WF; Jackson, DN. Component analysis versus common factor analysis: Some issues in selecting an appropriate procedure. Multivar. Behav. Res 1990, 25, 1–28. [Google Scholar]
- Abraham, MH; Chadha, HS; Leitao, RAE; Mitchell, RC; Lambert, WJ; Kaliszan, R; Nasal, A; Haber, P. Determination of solute lipophilicity, as log P(octanol) and log P(alkane) using poly(styrene-divinylbenzene) and immobilised artificial membrane stationary phases in reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1997, 766, 35–47. [Google Scholar]
- Gami-Yilinkou, R; Nasal, A; Kaliszan, R. Application of chemometrically processed chromatographic data for pharmacologically relevant classification of antihistamine drugs. J. Chromatogr 1993, 633, 57–63. [Google Scholar]
- Petrusewicz, J; Gami-Yilinkou, R; Kaliszan, R; Pilarski, B; Foks, H. Pyrazine CH-and NH-acids. Antithrombotic activity and chromatographic behaviour. Gen. Pharmacol 1993, 24, 17–22. [Google Scholar]
- Nasal, A; Buciński, A; Bober, L; Kaliszan, R. Prediction of pharmacological classification by means of chromatographic parameters processed by principal component analysis. Int. J. Pharm 1997, 153, 43–55. [Google Scholar]
- Nasal, A; Wojdełko, A; Bączek, T; Kaliszan, R; Cybulski, M; Chilmończyk, Z. Relationship between chromatographic behavior and affinity to 5–HT1A serotonin receptors of new buspirone analogues. J. Sep. Sci 2002, 25, 273–279. [Google Scholar]
- Koba, M; Stasiak, J; Bober, L; Bączek, T. Evaluation of molecular descriptors and HPLC retention data of analgesic and anti-inflamatory drugs by factor analysis in retention to their pharmacological activity. J Mol Model 2010, in press.. [Google Scholar]
- HyperChem® Computational Chemistry, Practical Guide – Theory and Method, HC 70-00-04-00. Hypercube Inc: Gainesville, FL, USA, 2002.
- Mulliken, RS. A new electroaffinity scale; together with data on valence states and on valence ionization potentials and electron affinities. J. Chem. Phys 1934, 2, 782–793. [Google Scholar]
- Mulliken, RS. Electronic structures of molecules. XI. Electroaffinity, molecular orbitals and dipole moments. J. Chem. Phys 1935, 3, 573–585. [Google Scholar]
- Parr, RG; Pearson, RG. Absolute hardness: companion parameter to absolute electronegativity. J. Am. Chem. Soc 1983, 105, 7512. [Google Scholar]
- Robles, J; Bartolotti, LJ. Electronegativities, electron affinities, ionization potentials, and hardnesses of elements within spin polarized density functional theory. J. Am. Chem. Soc 1984, 106, 3723–3727. [Google Scholar]
- Leonard, RG; Talbert, RL. Calcium-channel blocking agents. Clin. Pharm 1982, 1, 17–33. [Google Scholar]
- Antman, EM; Stone, PH; Muller, JE; Braunwald, E. Calcium channel blocking agents in the treatment of cardiovascular disorders. Part I: Basic and clinical electrophysiologic effects. Ann. Intern. Med 1980, 93, 875–885. [Google Scholar]
- Stone, PH; Antman, EM; Muller, JE; Braunwald, E. Calcium channel blocking agents in the treatment of cardiovascular disorders. Part II: Hemodynamic effects and clinical applications. Ann. Intern. Med 1980, 93, 886–904. [Google Scholar]
- Henry, PD. Comparative pharmacology of calcium antagonists: nifedipine, verapamil and diltiazem. Am. J. Cardiol 1980, 46, 1047–1058. [Google Scholar]
- Kenakin, TP; Beek, D. The activity of nifedipine, diltiazem, verapamil, and lidoflazine in isolated tissues: An approach to the determination of calcium channel blocking activity. Drug Dev. Res 1985, 5, 347–358. [Google Scholar]
- Boatto, G; Nieddu, M; Faedda, MV; De Caprariis, P. Enantiomeric separation by HPLC of 1,4-dihydropyridines with vancomycin as chiral selector. Chirality 2003, 15, 494–497. [Google Scholar]
- Triggle, DJ. 1,4-Dihydropyridines as calcium channel ligands and privileged structures. Cell Mol. Neurobiol 2003, 23, 293–303. [Google Scholar]
- Fantin, M; Quintieri, L; Kúsz, E; Kis, E; Glavinas, H; Floreani, M; Padrini, R; Duda, E; Vizler, C. Pentoxifylline and its major oxidative metabolites exhibit different pharmacological properties. Eur. J. Pharmacol 2006, 535, 301–309. [Google Scholar]
- Beerman, B; Ings, R; Mansby, J; Chamberlain, J; McDonald, A. Kinetics of intravenous and oral pentoxifylline in healthy subjects. Clin. Pharmacol. Ther 1985, 37, 25–28. [Google Scholar]
- Crouch, SP; Fletcher, J. Effect of ingested pentoxifylline on neutrophil superoxide anion production. Infect. Immun 1992, 60, 4504–4509. [Google Scholar]
- Dominguez-Jimenez, C; Sancho, D; Nieto, M; Montova, MC; Barreiro, O; Sanchez-Madrid, F; Gonzales-Amaro, R. Effect of pentoxifylline on polarization and migration of human leukocytes. J. Leukoc. Biol 2002, 71, 588–596. [Google Scholar]
- Nicklasson, M; Bjorkman, S; Roth, B; Jonsson, M; Hoglund, P. Stereoselective metabolism of pentoxifylline in vitro and in vivo in humans. Chirality 2002, 14, 643–652. [Google Scholar]
- Zabel, P; Schade, FU; Schlaak, M. Inhibition of endogenous TNF formation by pentoxifylline. Immunobiology 1993, 187, 447–463. [Google Scholar]
- Vukanić, ZS; Colić, M; Dimitrijević, M. Effect of pentoxifylline on differentiation and maturation of human monocyte-derived dendritic cells in vitro. Int. Immunopharmacol 2007, 7, 167–174. [Google Scholar]
- Ishchenko, MM; Korol'kov, AS. The effect of pentoxifylline on the systemic and cerebral hemodynamics in patients with circulatory encephalopathy and an atherosclerotic lesion of the precerebral arteries. Lik Sprava 1994, 5–6, 144–146. [Google Scholar]
- Stojic Vukanic, Z; Dimitrijevic, M; Colic, M; Popovic, P; Jandric, D. Modulation of human peripheral blood mononuclear cell activation by the combination of leflunomide and pentoxifylline. Transplant. Proc 2001, 33, 2137–2138. [Google Scholar]
- Bahl, VK; Jadhav, UM; Thacker, HP. Management of hypertension with the fixed combination of perindopril and amlodipine in daily clinical practice: results from the strong prospective, observational, multicenter study. Am. J. Cardiovasc. Drugs 2009, 9, 135–142. [Google Scholar]
- Claas, SA; Glasser, SP. Long-acting diltiazem HCl for the chronotherapeutic treatment of hypertension and chronic stable angina pectoris. Expert Opin Pharmacother 2005, 5–6, 765–776. [Google Scholar]
- Basile, J. The role of existing and newer calcium channel blockers in the treatment of hypertension. J. Clin. Hypertens 2004, 6, 621–629. [Google Scholar]



| Compound | Antiarrhythmic activity a | Lack of anti-arrhythmic activity b | Blood vassels activity c | Anti hypertension activity d | |||||
|---|---|---|---|---|---|---|---|---|---|
| Receptor | Chanel blockers | ||||||||
| M2 muscarinic antagonist | α-receptors | Na+ class I | K+ class III | Ca2+ class IV | Ca2+ | ||||
| Ia | Ic | ||||||||
| amiodarone | − | + | − | − | + | − | − | − | + |
| amlodipine | − | − | − | − | − | − | + | − | + |
| diltiazem | − | − | − | − | − | + | − | − | + |
| disopyramide | + | − | + | − | − | − | − | − | + |
| nifedipine | − | − | − | − | − | − | + | − | + |
| nimodipine | − | − | − | − | − | − | + | − | + |
| nisoldipine | − | − | − | − | − | − | + | − | + |
| nitrendipine | − | − | − | − | − | − | + | − | + |
| pentoxyphylline | − | − | − | − | − | − | − | + | − |
| propaphenone | − | − | − | + | − | − | − | − | + |
| verapamil | − | + | − | − | − | + | − | − | + |
| Compound | HPLC retention data | Molecular descriptors | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nucleosil C18 2.5 | Nucleosil C18 7.0 | IAM 2.5 | IAM 7.0 | Nucleosil OH 2.5 | Nucleosil OH 7.0 | TE | BE | AIE | EE | ECC | HF | EHOMO | ELUMO | EN | HARD | MAX_NEG | MAX_POS | DELTA | TDM | SA | V | HE | LOG_P | R | P | |
| 1 | 2.51 | 4.32 | −0.19 | 1.82 | −0.41 | 0.99 | −125943 | −6134 | −119809 | −1014808 | 888865 | −8.1 | −9.2 | −0.81 | −5.0 | −4.2 | −0.30 | 0.34 | 0.64 | 2.61 | 792 | 1385 | −1.4 | 6.57 | 144 | 56 |
| 2 | 1.75 | 2.31 | −0.49 | 1.23 | −0.44 | 0.77 | −122106 | −5447 | −116660 | −986416 | 864310 | −173.6 | −8.8 | −0.38 | −4.6 | −4.2 | −0.40 | 0.36 | 0.76 | 4.99 | 635 | 1123 | −8.6 | −0.01 | 109 | 42 |
| 3 | 1.37 | 2.25 | −0.58 | 0.40 | −0.44 | 0.67 | −116762 | −5719 | −111043 | −975452 | 858691 | −73.8 | −8.6 | −0.40 | −4.5 | −4.1 | −0.37 | 0.30 | 0.67 | 1.15 | 660 | 1155 | −4.7 | 2.4 | 114 | 45 |
| 4 | 0.60 | 1.59 | 0.17 | 0.61 | −0.44 | 0.65 | −92905 | −5482 | −87423 | −793186 | 700281 | 16.3 | −8.9 | −0.10 | −4.5 | −4.4 | −0.43 | 0.31 | 0.74 | 2.31 | 586 | 1041 | −3.6 | 3.99 | 102 | 41 |
| 5 | 2.14 | 2.22 | 0.07 | 0.03 | 0.22 | 0.26 | −109704 | −4536 | −105168 | −816705 | 707002 | −109.6 | −9.0 | −0.61 | −4.8 | −4.2 | −0.35 | 0.36 | 0.71 | 9.67 | 545 | 939 | −6.7 | −3.48 | 91 | 34 |
| 6 | 2.62 | 2.69 | 0.14 | 0.09 | 0.22 | 0.31 | −131466 | −5762 | −125704 | −1089747 | 958282 | −175.4 | −9.1 | −0.88 | −5.0 | −4.1 | −0.39 | 0.57 | 0.95 | 7.11 | 652 | 1154 | −7.7 | −2.89 | 112 | 42 |
| 7 | 2.77 | 2.86 | 0.23 | 0.17 | 0.23 | 0.32 | −120482 | −5379 | −115103 | −991599 | 871116 | −127.8 | −9.0 | −0.69 | −4.9 | −4.2 | −0.38 | 0.57 | 0.95 | 8.38 | 600 | 1062 | −3.9 | −2.27 | 105 | 40 |
| 8 | 2.40 | 2.51 | 0.17 | 0.12 | 0.23 | 0.31 | −113306 | −4827 | −108480 | −856446 | 743139 | −125.3 | −9.1 | −0.82 | −5.0 | −4.1 | −0.38 | 0.57 | 0.95 | 7.18 | 570 | 981 | −7.0 | −3.14 | 96 | 36 |
| 9 | 0.56 | 0.57 | −0.13 | −0.15 | 0.22 | 0.24 | −85329 | −3839 | −81490 | −550132 | 464803 | −49.2 | −9.0 | −0.36 | −4.7 | −4.3 | −0.36 | 0.41 | 0.77 | 4.44 | 515 | 837 | −1.2 | −0.14 | 74 | 28 |
| 10 | 1.68 | 2.17 | −0.53 | 1.12 | −0.43 | 0.74 | −97523 | −5382 | −92141 | −768876 | 671353 | −95.0 | −9.3 | −0.36 | −4.8 | −4.5 | −0.31 | 0.28 | 0.59 | 4.72 | 589 | 1050 | −5.4 | 3.4 | 100 | 39 |
| 11 | 1.79 | 2.15 | −0.58 | 0.61 | −0.43 | 0.68 | −130850 | −7144 | −123706 | −1123270 | 992420 | −85.9 | −8.5 | 0.12 | −4.2 | −4.2 | −0.26 | 0.15 | 0.41 | 4.69 | 798 | 1394 | −7.6 | 5.05 | 133 | 58 |
| No. of factor | Structural parametersa | HPLC retention datab | All datac | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Eigen-value | Variance explained (%) | Total variance explained (%) | Eigen-value | Variance explained (%) | Total variance explained (%) | Eigen-value | Variance explained (%) | Total variance explained (%) | |
| 1 | 9.24 | 46.18 | 46.18 | 3.42 | 56.92 | 56.92 | 11.34 | 43.60 | 43.60 |
| 2 | 5.82 | 29.10 | 75.27 | 1.98 | 33.08 | 90.00 | 7.59 | 29.21 | 72.81 |
| 3 | 2.41 | 12.07 | 87.34 | 0.46 | 7.61 | 97.61 | 3.20 | 12.30 | 85.11 |
| 4 | 1.16 | 5.81 | 93.15 | 0.10 | 1.67 | 99.28 | 1.34 | 5.16 | 90.27 |
| 5 | 0.74 | 3.68 | 96.83 | - | - | - | 1.16 | 4.46 | 94.73 |
| 6 | - | - | - | - | - | - | 0.74 | 2.86 | 97.59 |
| Structural parameters | Factor 1 | Factor 2 |
|---|---|---|
| TE | 0.7651 | −0.6392 |
| BE | 0.9569 | 0.0165 |
| AIE | 0.7404 | −0.6655 |
| EE | 0.8450 | −0.4976 |
| ECC | −0.8492 | 0.4809 |
| HF | −0.0313 | −0.7792 |
| EHOMO | −0.4810 | −0.2329 |
| ELUMO | −0.2627 | −0.7643 |
| EN | −0.3883 | −0.5919 |
| HARD | −0.3389 | 0.7326 |
| MAX_NEG | −0.5966 | −0.2413 |
| MAX_POS | 0.4951 | 0.7760 |
| DELTA | 0.5749 | 0.7005 |
| TDM | 0.3436 | 0.6671 |
| SA | −0.9666 | −0.0155 |
| V | −0.9749 | 0.0133 |
| HE | 0.2474 | −0.4908 |
| LOG_P | −0.5978 | −0.7178 |
| R | −0.9384 | 0.0650 |
| P | −0.9750 | −0.0713 |
| HPLC retention data | Factor 1 | Factor 2 |
|---|---|---|
| Nucleosil C18 2.5 | −0.0666 | −0.9490 |
| Nucleosil C18 7.0 | 0.4324 | −0.8885 |
| IAM 2.5 | −0.7218 | −0.3973 |
| IAM 7.0 | 0.9270 | −0.2296 |
| Nucleosil OH 2.5 | −0.9296 | −0.2897 |
| Nucleosil OH 7.0 | 0.9896 | −0.0226 |
| All data | Factor 1 | Factor 2 |
|---|---|---|
| Nucleosil C18 2.5 | −0.2015 | −0.9114 |
| Nucleosil C18 7.0 | −0.5561 | −0.6065 |
| IAM 2.5 | 0.6107 | −0.4332 |
| IAM 7.0 | −0.7057 | 0.1657 |
| Nucleosil OH 2.5 | 0.7475 | −0.5362 |
| Nucleosil OH 7.0 | −0.8065 | 0.3277 |
| TE | 0.6488 | 0.7362 |
| BE | 0.9308 | 0.1287 |
| AIE | 0.6210 | 0.7579 |
| EE | 0.7461 | 0.6032 |
| ECC | −0.7525 | −0.5869 |
| HF | −0.1472 | 0.6855 |
| EHOMO | −0.4196 | 0.2353 |
| ELUMO | −0.3112 | 0.7591 |
| EN | −0.3914 | 0.5898 |
| HARD | −0.1857 | −0.7573 |
| MAX_NEG | −0.6159 | 0.0915 |
| MAX_POS | 0.5959 | −0.7068 |
| DELTA | 0.6618 | −0.5966 |
| TDM | 0.4756 | −0.6349 |
| SA | −0.9554 | −0.1519 |
| V | −0.9681 | −0.1769 |
| HE | 0.1498 | 0.4308 |
| LOG_P | −0.7316 | 0.5915 |
| R | −0.9413 | −0.2332 |
| P | −0.9753 | −0.0994 |
| Compound | Structural parametersa | HPLC retention datab | All datac | ||||
|---|---|---|---|---|---|---|---|
| No. | Name | Factor 1 | Factor 2 | Factor 1 | Factor 2 | Factor 1 | Factor 2 |
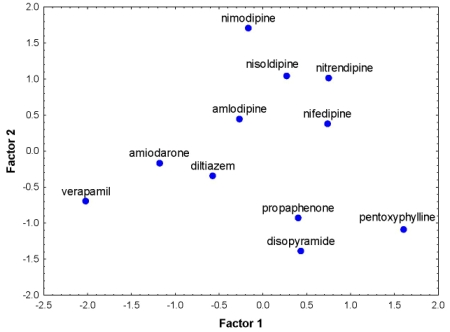
| 1 | amiodarone | −1.1793 | −0.1644 | 1.5754 | −1.5335 | −1.4266 | −0.2749 |
| 2 | amlodipine | −0.2667 | 0.4491 | 1.0088 | 0.2542 | −0.4023 | −0.1355 |
| 3 | diltiazem | −0.5703 | −0.3440 | 0.5879 | 0.7398 | −0.5999 | 0.4120 |
| 4 | disopyramide | 0.4344 | −1.3892 | 0.1143 | 1.0613 | 0.2651 | 1.4139 |
| 5 | nifedipine | 0.7363 | 0.3826 | −0.9964 | −0.3150 | 0.8611 | −0.3602 |
| 6 | nimodipine | −0.1657 | 1.7114 | −0.9049 | −0.9047 | 0.1784 | −1.6478 |
| 7 | nisoldipine | 0.2700 | 1.0449 | −0.8930 | −1.1573 | 0.4737 | −1.1303 |
| 8 | nitrendipine | 0.7518 | 1.0173 | −0.9329 | −0.7018 | 0.8768 | −0.9184 |
| 9 | pentoxyphylline | 1.6061 | −1.0869 | −1.1599 | 1.6600 | 1.5499 | 1.3053 |
| 10 | propaphenone | 0.4022 | −0.9245 | 0.9337 | 0.4110 | 0.0136 | 0.9640 |
| 11 | verapamil | −2.0187 | −0.6963 | 0.6671 | 0.4860 | −1.7899 | 0.3719 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Stasiak, J.; Koba, M.; Bober, L.; Bączek, T. Principal Component Analysis of HPLC Retention Data and Molecular Modeling Structural Parameters of Cardiovascular System Drugs in View of Their Pharmacological Activity. Int. J. Mol. Sci. 2010, 11, 2681-2698. https://doi.org/10.3390/ijms11072681
Stasiak J, Koba M, Bober L, Bączek T. Principal Component Analysis of HPLC Retention Data and Molecular Modeling Structural Parameters of Cardiovascular System Drugs in View of Their Pharmacological Activity. International Journal of Molecular Sciences. 2010; 11(7):2681-2698. https://doi.org/10.3390/ijms11072681
Chicago/Turabian StyleStasiak, Jolanta, Marcin Koba, Leszek Bober, and Tomasz Bączek. 2010. "Principal Component Analysis of HPLC Retention Data and Molecular Modeling Structural Parameters of Cardiovascular System Drugs in View of Their Pharmacological Activity" International Journal of Molecular Sciences 11, no. 7: 2681-2698. https://doi.org/10.3390/ijms11072681
APA StyleStasiak, J., Koba, M., Bober, L., & Bączek, T. (2010). Principal Component Analysis of HPLC Retention Data and Molecular Modeling Structural Parameters of Cardiovascular System Drugs in View of Their Pharmacological Activity. International Journal of Molecular Sciences, 11(7), 2681-2698. https://doi.org/10.3390/ijms11072681