Application of the Principles of Systems Biology and Wiener's Cybernetics for Analysis of Regulation of Energy Fluxes in Muscle Cells in Vivo
Abstract
:1. Introduction
2. Controversial State of the Art of Muscle Bioenergetics
Tissue Specificity of Energy Metabolism
3. Experimental Evidence for the Mechanism of Respiration Regulation in Muscle Cells in Situ: Human m. vastus lateralis and Adult Rat Heart Cells
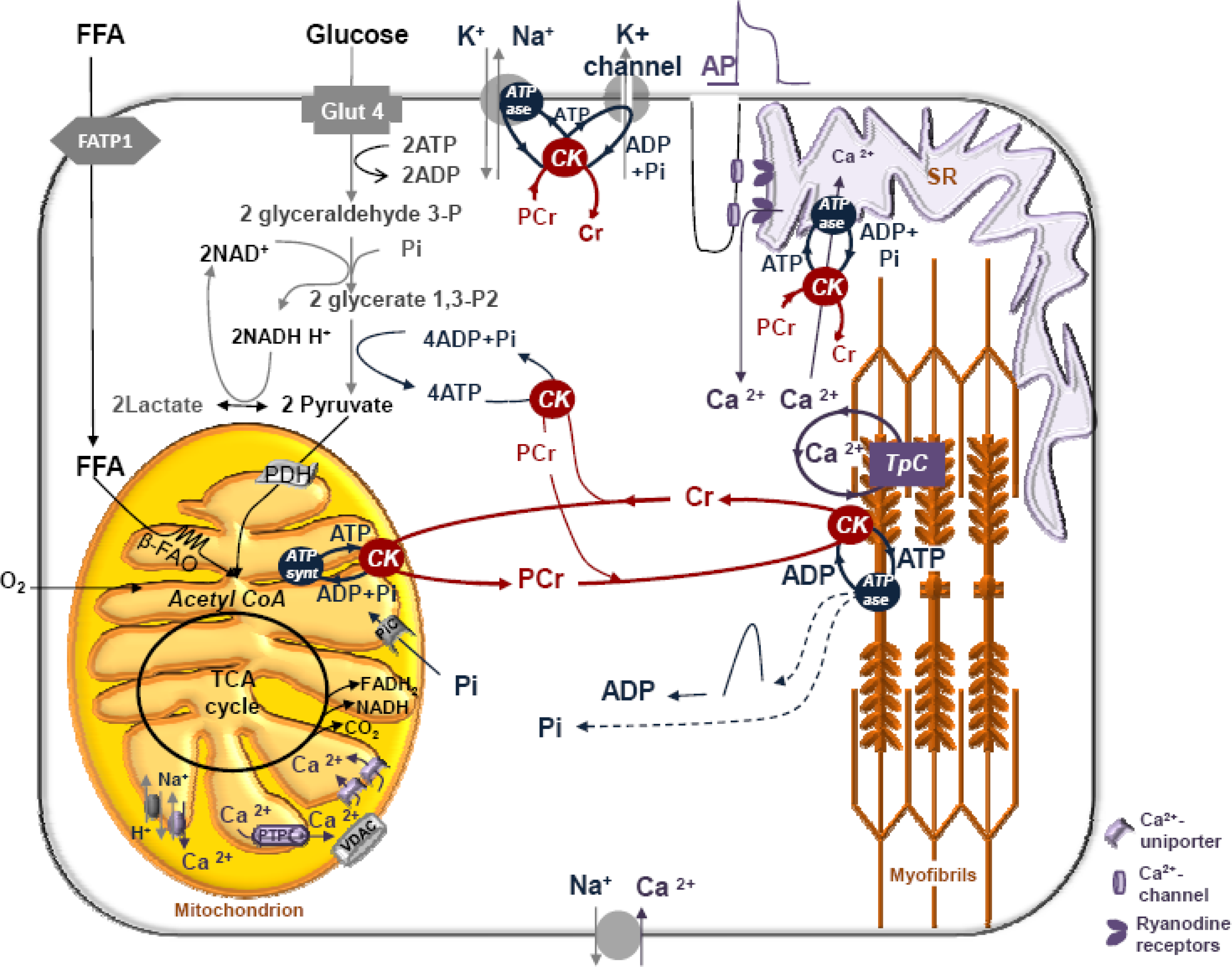
4. Non-equilibrium Steady State of Phosphotransfer Reactions in Muscle Cells
Origin of Controversies
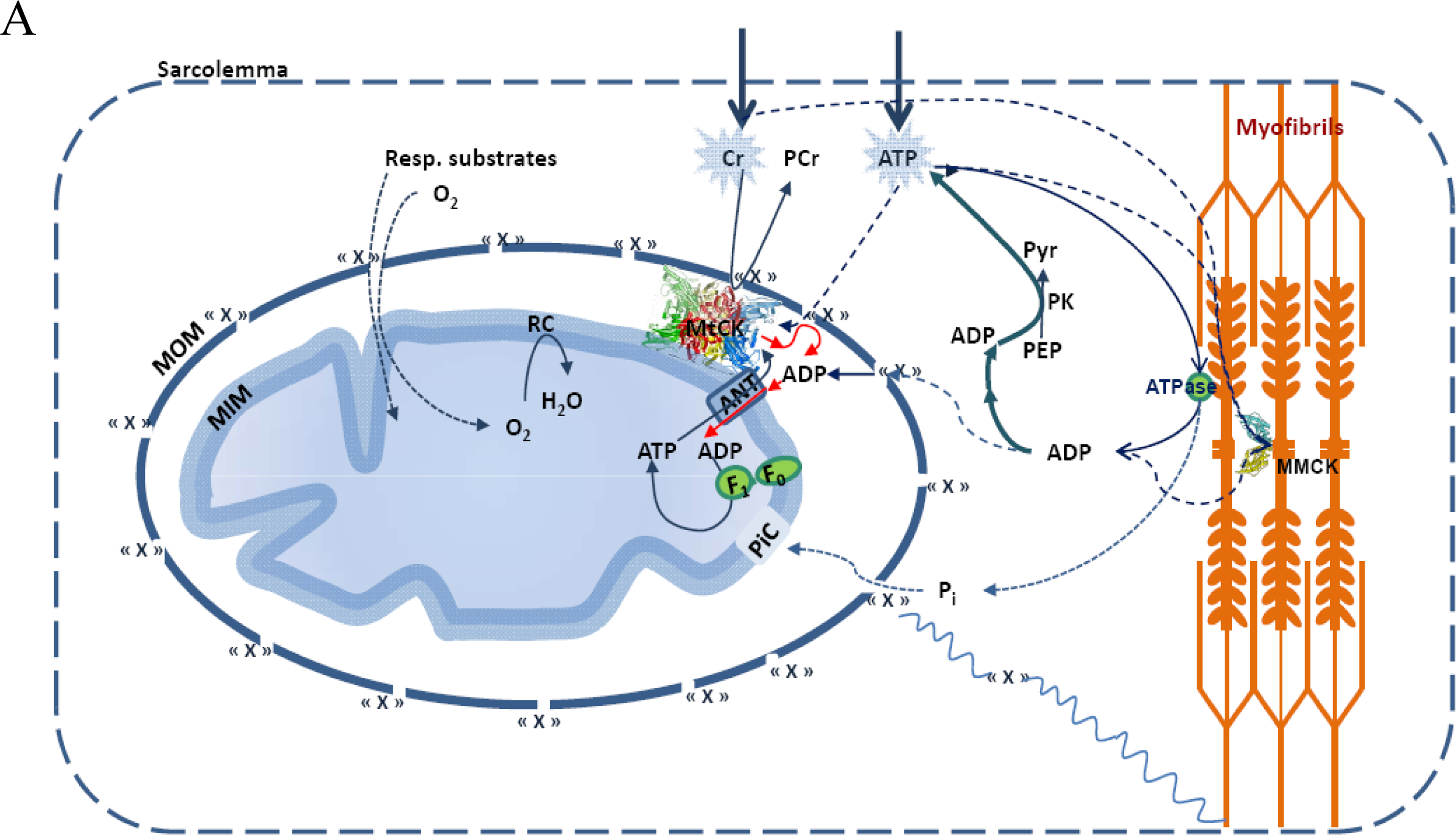
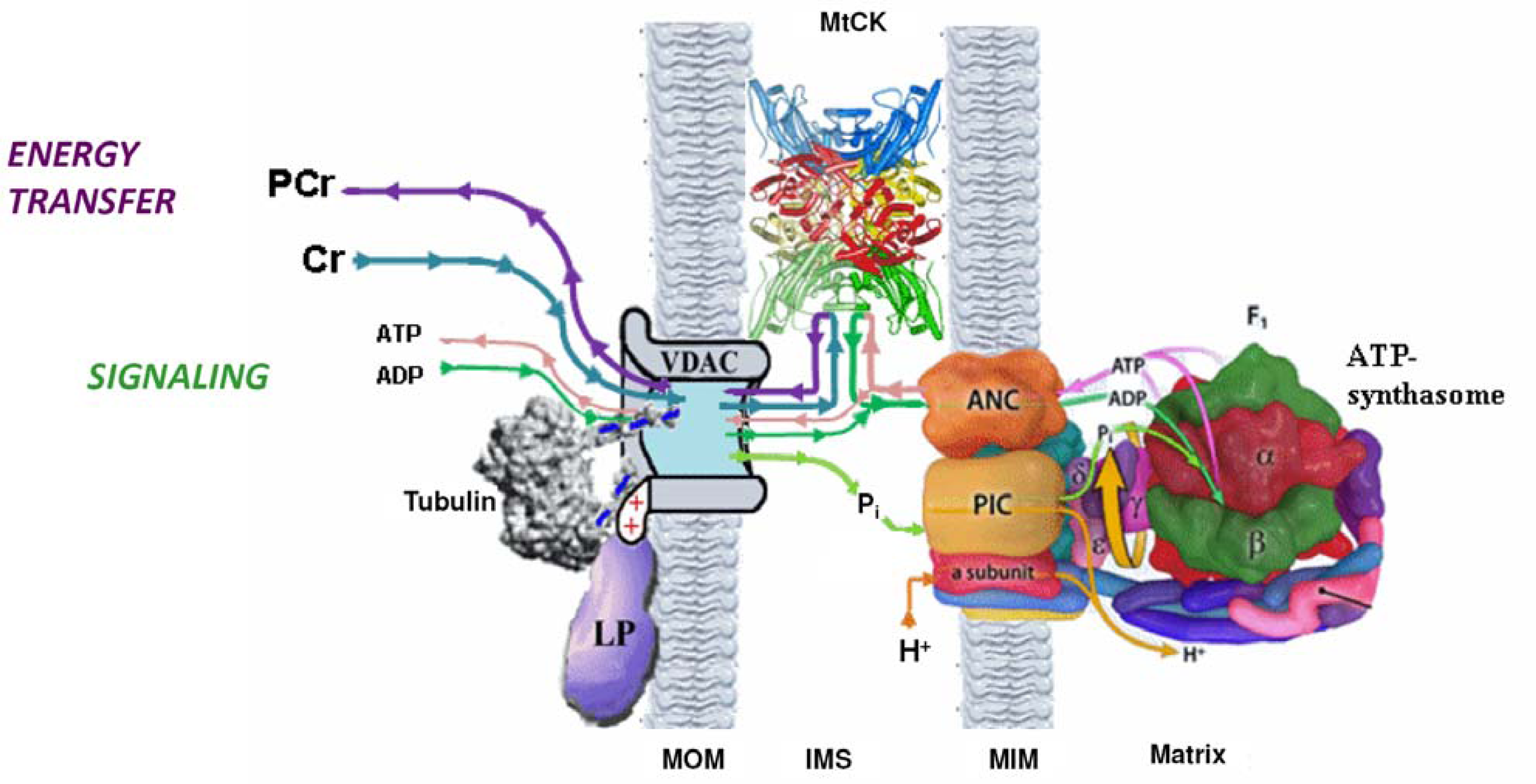
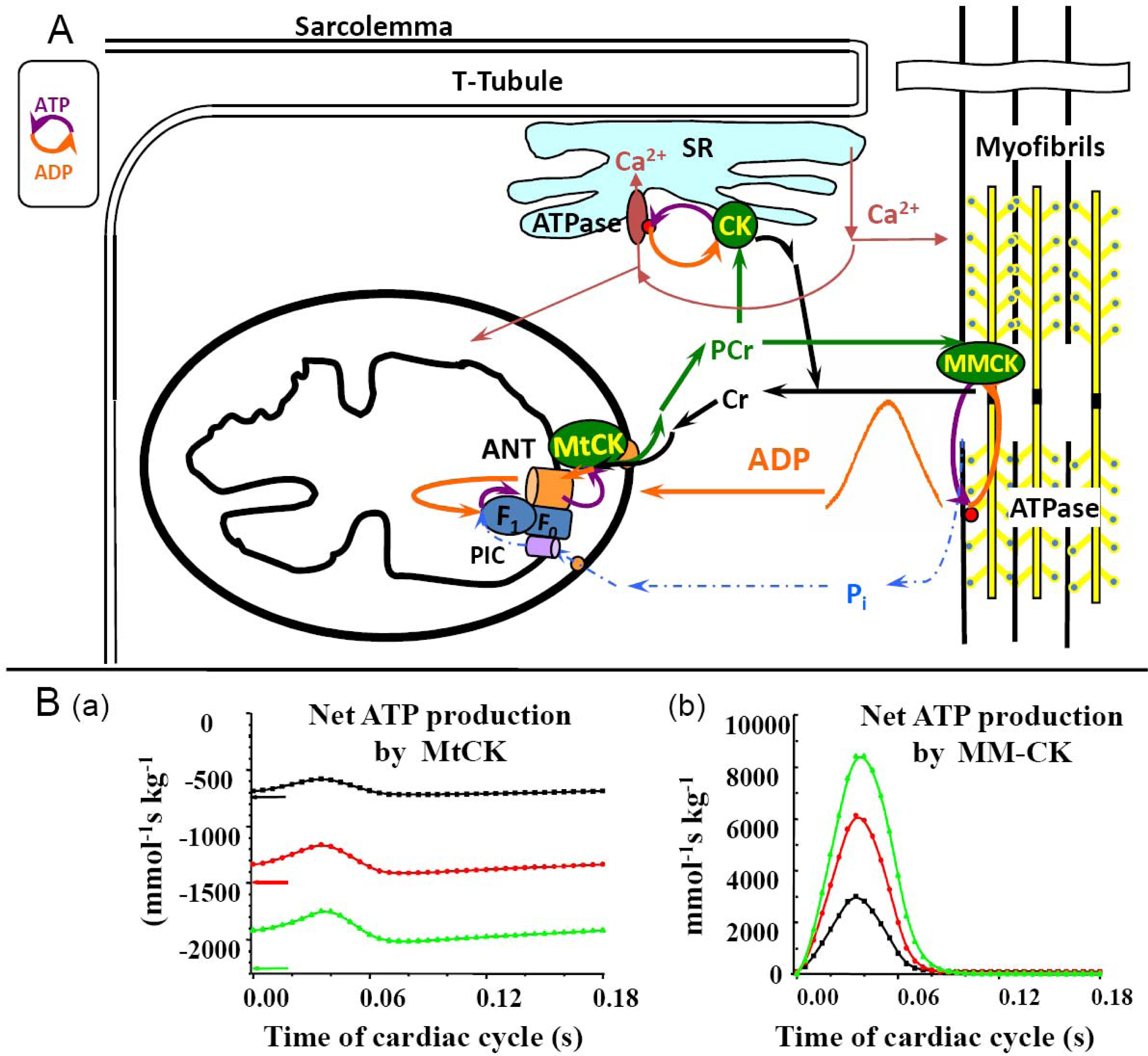
5. Restriction of ADP and ATP Diffusion at the Level of Mitochondrial Outer Membrane–The Mitochondrial Interactosome
6. Role of Cytoplasmic Endogenous ADP in Regulation of Respiration
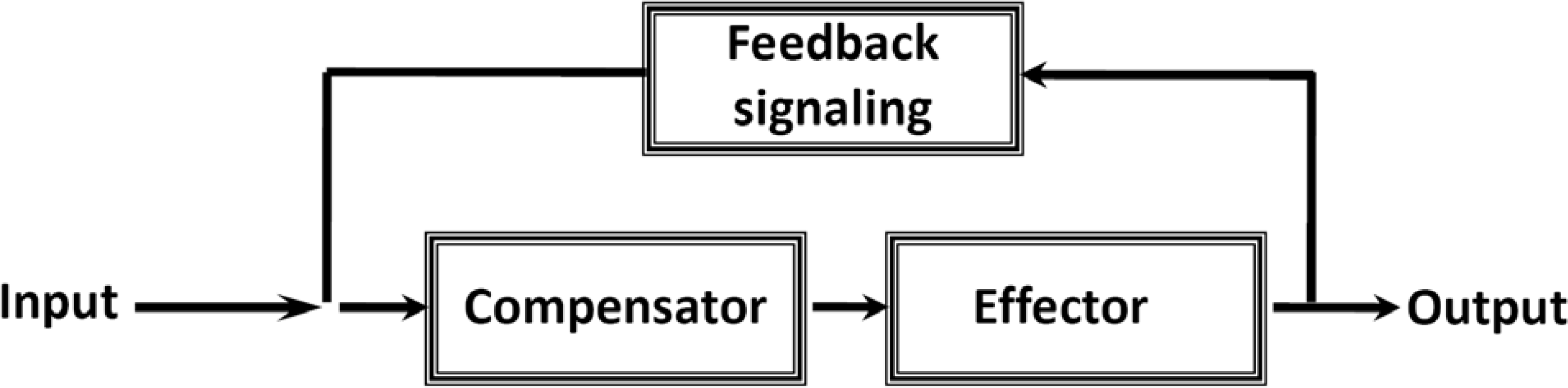
7. Cybernetic Mechanisms of Feedback Regulation
Why the Restriction of Diffusion of ADP across the Outer Mitochondrial Membrane Is Needed in Mitochondrial Interactosome?
8. Four General Characteristics of Cardiac Energy Metabolism to Be Explained by Modeling
9. Feedback Regulation of Respiration: Application of Cybernetic Principles
10. Conclusions
Acknowledgments
References and Notes
- Noble, D. Modeling the heart--from genes to cells to the whole organ. Science 2002, 295, 1678–1682. [Google Scholar]
- Alberghina, L; Westerhoff, HV. Systems Biology: Definitions and Perspectives (Topics in Current Genetics); Springer Verlag: Berlin, Heidelberg, Germany, 2005. [Google Scholar]
- Noble, D. The Music of Life: Biology Beyond the Genome; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Saks, VA; Monge, C; Guzun, R. Philosophical basis and some historical aspects of systems biology: From hegel to noble – applications for bioenergetic research. Int. J. Mol. Sci 2009, 10, 1161–1192. [Google Scholar]
- Westerhoff, HV; Kolodkin, A; Conradie, R; Wilkinson, SJ; Bruggeman, FJ; Krab, K; van Schuppen, JH; Hardin, H; Bakker, BM; Mone, MJ; Rybakova, KN; Eijken, M; van Leeuwen, HJ; Snoep, JL. Systems biology towards life in silico: Mathematics of the control of living cells. J. Math. Biol 2009, 58, 7–34. [Google Scholar]
- Saks, VA; Beraud, N; Wallimann, T. Metabolic compartmentation – a system level property of muscle cells: Real problems of diffusion in living cells. Int. J. Mol. Sci 2008, 9, 751–767. [Google Scholar]
- Hunter, P; Nielsen, P. A strategy for integrative computational physiology. Physiology (Bethesda) 2005, 20, 316–325. [Google Scholar]
- Saks, VA; Dzeja, P; Schlattner, U; Vendelin, M; Terzic, A; Wallimann, T. Cardiac system bioenergetics: Metabolic basis of the Frank-Starling law. J. Physiol 2006, 571, 253–273. [Google Scholar]
- Saks, VA; Favier, R; Guzun, R; Schlattner, U; Wallimann, T. Molecular system bioenergetics: Regulation of substrate supply in response to heart energy demands. J. Physiol 2006, 577, 769–777. [Google Scholar]
- Saks, VA; Monge, C; Anmann, T; Dzeja, P. Integrated and organized cellular energetic systems: Theories of cell energetics, compartmentation and metabolic channeling. In Molecular System Bioenergetics Energy for Life; Saks, VA, Ed.; Wiley-VCH: Weinheim, Germany, 2007; pp. 59–110. [Google Scholar]
- Saks, VA; Monge, C; Guzun, R; Dzeja, P; Wallimann, T. Integrated and organized cellular energetic systems. In Wiley Encyclopedia of Chemical Biology; John Wiley & Sons: Hoboken, NJ, USA, 2009; Volume 2, pp. 366–393. [Google Scholar]
- Dzeja, PP; Terzic, A. Phosphotransfer networks and cellular energetics. J. Exp. Biol 2003, 206, 2039–2047. [Google Scholar]
- Dzeja, PP; Terzic, A. Mitochondrial-nucleus energetic communication: Role of phosphotransfer networks in processing cellular information. In Handbook of Neurochemistry & Molecular Neurobiology: Brain Energetics; Gibson, G, Dienel, G, Eds.; Springer Science: New York, NY, USA, 2005; pp. 614–666. [Google Scholar]
- Dzeja, P; Chung, S; Terzic, A. Integration of adenylate kinase and glycolytic and clycogenolytic circuits in cellular energetics. In Molecular System Bioenergetics Energy for life; Saks, VA, Ed.; Wiley –VCH: Weinheim, Germany, 2007; pp. 195–264. [Google Scholar]
- Monge, C; Beraud, N; Kuznetsov, AV; Rostovtseva, T; Sackett, D; Schlattner, U; Vendelin, M; Saks, VA. Regulation of respiration in brain mitochondria and synaptosomes: Restrictions of ADP diffusion in situ, roles of tubulin, and mitochondrial creatine kinase. Mol. Cell Biochem 2008, 318, 147–165. [Google Scholar]
- Saks, VA; Kaambre, T; Guzun, R; Anmann, T; Sikk, P; Schlattner, U; Wallimann, T; Aliev, M; Vendelin, M. The creatine kinase phosphotransfer network: Thermodynamic and kinetic considerations, the impact of the mitochondrial outer membrane and modelling approaches. Subcell Biochem 2007, 46, 27–65. [Google Scholar]
- Wallimann, T; Tokarska-Schlattner, M; Neumann, D; Epand, RF; Andres, RH; Widmer, HR; Hornemann, T; Saks, VA; Agarkova, I; Schlattner, U. The phosphocreatine circuit: Molecular and cellular physiology of creatine kinases, sensitivity to free radicals, and enhancement by creatine supplementation. In Molecular System Bioenergetics Energy for Life; Saks, VA, Ed.; Wiley-VCH: Weinheim, Germany, 2007; pp. 195–264. [Google Scholar]
- Wallimann, T; Wyss, M; Brdiczka, D; Nicolay, K; Eppenberger, HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: The ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem. J 1992, 281, 21–40. [Google Scholar]
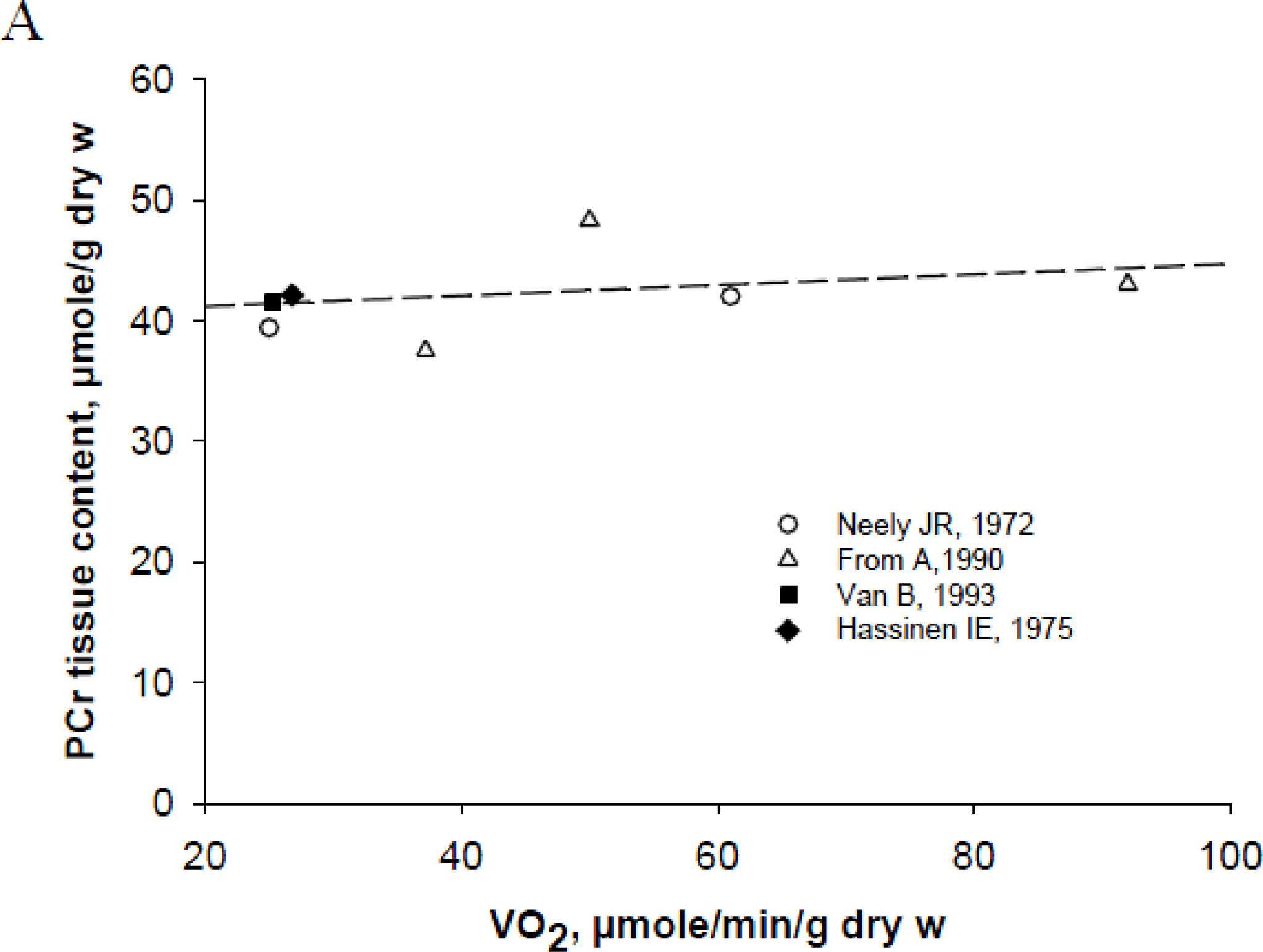
- Neely, JR; Denton, RM; England, PJ; Randle, PJ. The effects of increased heart work on the tricarboxylate cycle and its interactions with glycolysis in the perfused rat heart. Biochem. J 1972, 128, 147–159. [Google Scholar]
- Balaban, RS; Kantor, HL; Katz, LA; Briggs, RW. Relation between work and phosphate metabolite in the in vivo paced mammalian heart. Science 1986, 232, 1121–1123. [Google Scholar]
- Wiener, N. Cybernetics or Control and Communication in the Animal and the Machine, 2nd ed; The MIT Press: Cambridge, Massachussets, UK, 1965. [Google Scholar]
- Saks, VA. Molecular System Bioenergetics Energy for Life; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Balaban, RS. Cardiac energy metabolism homeostasis: Role of cytosolic calcium. J. Mol. Cell Cardiol 2002, 34, 1259–1271. [Google Scholar]
- Beard, DA. Modeling of oxygen transport and cellular energetics explains observations on in vivo cardiac energy metabolism. PLoS Comput. Biol 2006, 2, e107. [Google Scholar]
- van Beek, JH. Multiscale and modular analysis of cardiac energy metabolism: Repairing the broken interfaces of isolated system components. Ann. NY Acad. Sci 2008, 1123, 155–168. [Google Scholar]
- Balaban, RS. Domestication of the cardiac mitochondrion for energy conversion. J. Mol. Cell Cardiol 2009, 46, 832–841. [Google Scholar]
- Beard, DA; Kushmerick, MJ. Strong inference for systems biology. PLoS Comput. Biol 2009, 5, e1000459. [Google Scholar]
- Griffiths, EJ; Rutter, GA. Mitochondrial calcium as a key regulator of mitochondrial ATP production in mammalian cells. Biochim. Biophys. Acta 2009, 1787, 1324–1333. [Google Scholar]
- Guzun, R; Timohhina, N; Tepp, K; Monge, C; Kaambre, T; Sikk, P; Kuznetsov, AV; Pison, C; Saks, VA. Regulation of respiration controlled by mitochondrial creatine kinase in permeabilized cardiac cells in situ. Importance of system level properties. Biochim. Biophys. Acta 2009, 1787, 1089–1105. [Google Scholar]
- Ingwall, JS. Energy metabolism in heart failure and remodelling. Cardiovasc. Res 2009, 81, 412–419. [Google Scholar]
- Liu, T; O’Rourke, B. Regulation of mitochondrial Ca2+ and its effects on energetics and redox balance in normal and failing heart. J. Bioenerg. Biomembr 2009, 41, 127–132. [Google Scholar]
- Timohhina, N; Guzun, R; Tepp, K; Monge, C; Varikmaa, M; Vija, H; Sikk, P; Kaambre, T; Sackett, D; Saks, VA. Direct measurement of energy fluxes from mitochondria into cytoplasm in permeabilized cardiac cells in situ: Some evidence for Mitochondrial Interactosome. J. Bioenerg. Biomembr 2009, 41, 259–275. [Google Scholar]
- Barros, LF; Martinez, C. An enquiry into metabolite domains. Biophys. J 2007, 92, 3878–3884. [Google Scholar]
- Wu, F; Zhang, EY; Zhang, J; Bache, RJ; Beard, DA. Phosphate metabolite concentrations and ATP hydrolysis potential in normal and ischaemic hearts. J. Physiol 2008, 586, 4193–4208. [Google Scholar]
- Wu, F; Beard, DA. Roles of the creatine kinase system and myoglobin in maintaining energetic state in the working heart. BMC Syst Biol 2009, 3, 22. [Google Scholar]
- Dzeja, PP; Bast, P; Pucar, D; Wieringa, B; Terzic, A. Defective metabolic signaling in adenylate kinase AK1 gene knock-out hearts compromises post-ischemic coronary reflow. J. Biol. Chem 2007, 282, 31366–31372. [Google Scholar]
- Saks, VA; Anmann, T; Guzun, R; Kaambre, T; Sikk, P; Schlattner, U; Wallimann, T; Aliev, M; Vendelin, M. The creatine kinase phosphotransfer network: Thermodynamic and kinetic considerations, the impact of the mitochondrial outer membrane and modelling approaches. In Creatine and Creatine Kinase in Health and Disease; Wyss, M, Salomons, G, Eds.; Springer: Dordrecht, The Netherland, 2007; pp. 27–66. [Google Scholar]
- Chance, B; Im, J; Nioka, S; Kushmerick, M. Skeletal muscle energetics with PNMR: Personal views and historic perspectives. NMR Biomed 2006, 19, 904–926. [Google Scholar]
- Howald, H; Hoppeler, H; Claassen, H; Mathieu, O; Straub, R. Influences of endurance training on the ultrastructural composition of the different muscle fiber types in humans. Pflugers Arch 1985, 403, 369–376. [Google Scholar]
- Ponsot, E; Dufour, SP; Zoll, J; Doutrelau, S; N’Guessan, B; Geny, B; Hoppeler, H; Lampert, E; Mettauer, B; Ventura-Clapier, R; Richard, R. Exercise training in normobaric hypoxia in endurance runners. II. Improvement of mitochondrial properties in skeletal muscle. J. Appl. Physiol 2006, 100, 1249–1257. [Google Scholar]
- Kiens, B; Essen-Gustavsson, B; Christensen, NJ; Saltin, B. Skeletal muscle substrate utilization during submaximal exercise in man: Effect of endurance training. J. Physiol 1993, 469, 459–478. [Google Scholar]
- Zoll, J; Koulmann, N; Bahi, L; Ventura-Clapier, R; Bigard, AX. Quantitative and qualitative adaptation of skeletal muscle mitochondria to increased physical activity. J. Cell Physiol 2003, 194, 186–193. [Google Scholar]
- Williamson, JR; Ford, C; Illingworth, J; Safer, B. Coordination of citric acid cycle activity with electron transport flux. Circ. Res 1976, 38, S139–S151. [Google Scholar]
- From, AH; Zimmer, SD; Michurski, SP; Mohanakrishnan, P; Ulstad, VK; Thoma, WJ; Ugurbil, K. Regulation of the oxidative phosphorylation rate in the intact cell. Biochemistry 1990, 29, 3731–3743. [Google Scholar]
- Kushmerick, MJ; Moerland, TS; Wiseman, RW. Mammalian skeletal muscle fibers distinguished by contents of phosphocreatine, ATP, and Pi. Proc. Natl. Acad. Sci. USA 1992, 89, 7521–7525. [Google Scholar]
- Meyer, RA; Sweeney, HL; Kushmerick, MJ. A simple analysis of the “phosphocreatine shuttle”. Am. J. Physiol 1984, 246, C365–C377. [Google Scholar]
- McFarland, EW; Kushmerick, MJ; Moerland, TS. Activity of creatine kinase in a contracting mammalian muscle of uniform fiber type. Biophys. J 1994, 67, 1912–1924. [Google Scholar]
- Jeneson, JA; Wiseman, RW; Westerhoff, HV; Kushmerick, MJ. The signal transduction function for oxidative phosphorylation is at least second order in ADP. J. Biol. Chem 1996, 271, 27995–27998. [Google Scholar]
- Jeneson, JA; Westerhoff, HV; Kushmerick, MJ. A metabolic control analysis of kinetic controls in ATP free energy metabolism in contracting skeletal muscle. Am. J. Physiol. Cell Physiol 2000, 279, C813–C832. [Google Scholar]
- Kushmerick, MJ; Conley, KE. Energetics of muscle contraction: The whole is less than the sum of its parts. Biochem. Soc. Trans 2002, 30, 227–231. [Google Scholar]
- Beard, DA. A biophysical model of the mitochondrial respiratory system and oxidative phosphorylation. PLoS Comput. Biol 2005, 1, e36. [Google Scholar]
- Korzeniewski, B. Regulation of oxidative phosphorylation through parallel activation. Biophys. Chem 2007, 129, 93–110. [Google Scholar]
- van Beek, JH. Adenine nucleotide-creatine-phosphate module in myocardial metabolic system explains fast phase of dynamic regulation of oxidative phosphorylation. Am. J. Physiol. Cell Physiol 2007, 293, C815–C829. [Google Scholar]
- Wu, F; Zhang, J; Beard, DA. Experimentally observed phenomena on cardiac energetics in heart failure emerge from simulations of cardiac metabolism. Proc. Natl. Acad. Sci. USA 2009, 106, 7143–7148. [Google Scholar]
- Ingwall, JS. ATP and the Heart; Kluwer Academic Publishers: Dordrecht, The Netherland, 2002; pp. 1–244. [Google Scholar]
- Frank, O. Zur dynamik des herzmuskels. Z. Biologie 1885, 32, 370–447. [Google Scholar]
- Starling, EH; Visscher, MB. The regulation of the energy output of the heart. J. Physiol 1927, 62, 243–261. [Google Scholar]
- Hassinen, IE; Hiltunen, K. Respiratory control in isolated perfused rat heart. Role of the equilibrium relations between the mitochondrial electron carriers and the adenylate system. Biochim. Biophys. Acta 1975, 408, 319–330. [Google Scholar]
- Dedkova, EN; Blatter, LA. Mitochondrial Ca2+ and the heart. Cell Calcium 2008, 44, 77–91. [Google Scholar]
- Korzeniewski, B; Deschodt-Arsac, V; Calmettes, G; Franconi, JM; Diolez, P. Physiological heart activation by adrenaline involves parallel activation of ATP usage and supply. Biochem. J 2008, 413, 343–347. [Google Scholar]
- Hom, J; Sheu, SS. Morphological dynamics of mitochondria – A special emphasis on cardiac muscle cells. J. Mol. Cell Cardiol 2009, 46, 811–820. [Google Scholar]
- Territo, PR; French, SA; Balaban, RS. Simulation of cardiac work transitions, in vitro: Effects of simultaneous Ca2+ and ATPase additions on isolated porcine heart mitochondria. Cell Calcium 2001, 30, 19–27. [Google Scholar]
- Brandes, R; Bers, DM. Simultaneous measurements of mitochondrial NADH and Ca2+ during increased work in intact rat heart trabeculae. Biophys. J 2002, 83, 587–604. [Google Scholar]
- Brookes, PS; Yoon, Y; Robotham, JL; Anders, MW; Sheu, SS; Calcium, ATP; ROS. A mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol 2004, 287, C817–C833. [Google Scholar]
- Baniene, R; Nauciene, Z; Maslauskaite, S; Baliutyte, G; Mildaziene, V. Contribution of ATP synthase to stimulation of respiration by Ca2+ in heart mitochondria. Syst. Biol. (Stevenage) 2006, 153, 350–353. [Google Scholar]
- Grueter, CE; Abiria, SA; Dzhura, I; Wu, Y; Ham, AJ; Mohler, PJ; Anderson, ME; Colbran, RJ. L-type Ca2+ channel facilitation mediated by phosphorylation of the beta subunit by CaMKII. Mol. Cell 2006, 23, 641–650. [Google Scholar]
- Nicholls, DG; Ferguson, SJ. Bioenergetics 3, 3rd ed; Academic Press: New York, NY, USA, 2002. [Google Scholar]
- Mizuno, J; Araki, J; Mohri, S; Minami, H; Doi, Y; Fujinaka, W; Miyaji, K; Kiyooka, T; Oshima, Y; Iribe, G; Hirakawa, M; Suga, H. Frank-Starling mechanism retains recirculation fraction of myocardial Ca2+ in the beating heart. Jpn. J. Physiol 2001, 51, 733–743. [Google Scholar]
- Shimizu, J; Todaka, K; Burkhoff, D. Load dependence of ventricular performance explained by model of calcium-myofilament interactions. Am. J. Physiol. Heart Circ. Physiol 2002, 282, H1081–H1091. [Google Scholar]
- Kobayashi, T; Solaro, RJ. Calcium, thin filaments, and the integrative biology of cardiac contractility. Annu. Rev. Physiol 2005, 67, 39–67. [Google Scholar]
- Katz, LA; Swain, JA; Portman, MA; Balaban, RS. Relation between phosphate metabolites and oxygen consumption of heart in vivo. Am. J. Physiol 1989, 256, H265–H274. [Google Scholar]
- McCormack, JG; Halestrap, AP; Denton, RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol. Rev 1990, 70, 391–425. [Google Scholar]
- Jouaville, LS; Pinton, P; Bastianutto, C; Rutter, GA; Rizzuto, R. Regulation of mitochondrial ATP synthesis by calcium: Evidence for a long-term metabolic priming. Proc. Natl. Acad. Sci. USA 1999, 96, 13807–13812. [Google Scholar]
- Territo, PR; French, SA; Dunleavy, MC; Evans, FJ; Balaban, RS. Calcium activation of heart mitochondrial oxidative phosphorylation: Rapid kinetics of mVO2, NADH, AND light scattering. J. Biol. Chem 2001, 276, 2586–2599. [Google Scholar]
- Bers, DM. Cardiac excitation-contraction coupling. Nature 2002, 415, 198–205. [Google Scholar]
- Brini, M. Ca2+ signalling in mitochondria: Mechanism and role in physiology and pathology. Cell Calcium 2003, 34, 399–405. [Google Scholar]
- Bianchi, K; Rimessi, A; Prandini, A; Szabadkai, G; Rizzuto, R. Calcium and mitochondria: Mechanisms and functions of a troubled relationship. Biochim. Biophys. Acta 2004, 1742, 119–131. [Google Scholar]
- Gunter, TE; Yule, DI; Gunter, KK; Eliseev, RA; Salter, JD. Calcium and mitochondria. FEBS Lett 2004, 567, 96–102. [Google Scholar]
- Opie, L. The Heart Physiology, from Cell to Circulation; Lippincott-Raven Publishers: Philadelphia, PA, USA, 1998. [Google Scholar]
- Jeneson, JA; Schmitz, JP; van den Broek, NM; van Riel, NA; Hilbers, P; Nicolay, K; Prompers, JJ. Magnitude and control of mitochondrial adp sensitivity. Am. J. Physiol. Endocrinol. Metab 2009, 297, E774–E784. [Google Scholar]
- Klingenberg, M. The state of ADP or ATP fixed to the mitochondria by bongkrekate. Eur. J. Biochem 1976, 65, 601–605. [Google Scholar]
- Carroll, AK; Clevenger, WR; Szabo, T; Ackermann, LE; Pei, Y; Ghosh, SS; Glasco, S; Nazarbaghi, R; Davis, RE; Anderson, CM. Ectopic expression of the human adenine nucleotide translocase, isoform 3 (ANT-3). Characterization of ligand binding properties. Mitochondrion 2005, 5, 1–13. [Google Scholar]
- Klingenberg, M. The ADP and ATP transport in mitochondria and its carrier. Biochim. Biophys. Acta 2008, 1778, 1978–2021. [Google Scholar]
- Metelkin, E; Goryanin, I; Demin, O. Mathematical modeling of mitochondrial adenine nucleotide translocase. Biophys. J 2006, 90, 423–432. [Google Scholar]
- Schiaffino, S; Sandri, M; Murgia, M. Activity-dependent signaling pathways controlling muscle diversity and plasticity. Physiology (Bethesda) 2007, 22, 269–278. [Google Scholar]
- Forbes, SC; Paganini, AT; Slade, JM; Towse, TF; Meyer, RA. Phosphocreatine recovery kinetics following low- and high-intensity exercise in human triceps surae and rat posterior hindlimb muscles. Am. J. Physiol. Regul. Integr. Comp. Physiol 2009, 296, R161–R170. [Google Scholar]
- Saks, VA; Veksler, VI; Kuznetsov, AV; Kay, L; Sikk, P; Tiivel, T; Tranqui, L; Olivares, J; Winkler, K; Wiedemann, F; Kunz, WS. Permeabilized cell and skinned fiber techniques in studies of mitochondrial function in vivo. Mol. Cell Biochem 1998, 184, 81–100. [Google Scholar]
- Kuznetsov, AV; Veksler, V; Gellerich, FN; Saks, VA; Margreiter, R; Kunz, WS. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat. Protoc 2008, 3, 965–976. [Google Scholar]
- Schlattner, U; Gehring, F; Vernoux, N; Tokarska-Schlattner, M; Neumann, D; Marcillat, O; Vial, C; Wallimann, T. C-terminal lysines determine phospholipid interaction of sarcomeric mitochondrial creatine kinase. J. Biol. Chem 2004, 279, 24334–24342. [Google Scholar]
- Schlattner, U; Tokarska-Schlattner, M; Wallimann, T. Mitochondrial creatine kinase in human health and disease. Biochim. Biophys. Acta 2006, 1762, 164–180. [Google Scholar]
- Walsh, B; Tonkonogi, M; Sahlin, K. Effect of endurance training on oxidative and antioxidative function in human permeabilized muscle fibres. Pflugers Arch 2001, 442, 420–425. [Google Scholar]
- Cherpec, R. Molecular system bioenergetics of muscle cells: Mechanisms of regulation of respiration in vivo – importance of system level properties. . Ph.D. Thesis. University Grenoble, Grenoble, France, 2009. [Google Scholar]
- Saks, VA; Kaambre, T; Sikk, P; Eimre, M; Orlova, E; Paju, K; Piirsoo, A; Appaix, F; Kay, L; Regitz-Zagrosek, V; Fleck, E; Seppet, E. Intracellular energetic units in red muscle cells. Biochem. J 2001, 356, 643–657. [Google Scholar]
- Saks, VA; Khuchua, ZA; Vasilyeva, EV; Belikova, O; Kuznetsov, AV. Metabolic compartmentation and substrate channelling in muscle cells. Role of coupled creatine kinases in in vivo regulation of cellular respiration – a synthesis. Mol Cell Biochem 1994, 133–134, 155–192. [Google Scholar]
- Dzeja, PP; Zeleznikar, RJ; Goldberg, ND. Suppression of creatine kinase-catalyzed phosphotransfer results in increased phosphoryl transfer by adenylate kinase in intact skeletal muscle. J. Biol. Chem 1996, 271, 12847–12851. [Google Scholar]
- Dzeja, PP; Vitkevicius, KT; Redfield, MM; Burnett, JC; Terzic, A. Adenylate kinase-catalyzed phosphotransfer in the myocardium: Increased contribution in heart failure. Circ. Res 1999, 84, 1137–1143. [Google Scholar]
- Abraham, MR; Selivanov, VA; Hodgson, DM; Pucar, D; Zingman, LV; Wieringa, B; Dzeja, PP; Alekseev, AE; Terzic, A. Coupling of cell energetics with membrane metabolic sensing. Integrative signaling through creatine kinase phosphotransfer disrupted by M-CK gene knock-out. J. Biol. Chem 2002, 277, 24427–24434. [Google Scholar]
- Saks, VA; Kuznetsov, AV; Vendelin, M; Guerrero, K; Kay, L; Seppet, EK. Functional coupling as a basic mechanism of feedback regulation of cardiac energy metabolism. Mol Cell Biochem 2004, 256–257, 185–199. [Google Scholar]
- Schlattner, U; Wallimann, T. Metabolite channeling: Creatine kinase microcompartments. In In Encyclopedia of Biological Chemistry; Lennarz, WJ, Lane, MD, Eds.; Academic Press: New York, NY, USA, 2004; pp. 646–651. [Google Scholar]
- Selivanov, VA; Alekseev, AE; Hodgson, DM; Dzeja, PP; Terzic, A. Nucleotide-gated KATP channels integrated with creatine and adenylate kinases: Amplification, tuning and sensing of energetic signals in the compartmentalized cellular environment. Mol Cell Biochem 2004, 256–257, 243–256. [Google Scholar]
- Vendelin, M; Lemba, M; Saks, VA. Analysis of functional coupling: Mitochondrial creatine kinase and adenine nucleotide translocase. Biophys. J 2004, 87, 696–713. [Google Scholar]
- Vendelin, M; Eimre, M; Seppet, E; Peet, N; Andrienko, T; Lemba, M; Engelbrecht, J; Seppet, EK; Saks, VA. Intracellular diffusion of adenosine phosphates is locally restricted in cardiac muscle. Mol Cell Biochem 2004, 256–257, 229–241. [Google Scholar]
- Selivanov, VA; Krause, S; Roca, J; Cascante, M. Modeling of spatial metabolite distributions in the cardiac sarcomere. Biophys. J 2007, 92, 3492–3500. [Google Scholar]
- Mitchell, P. Compartmentation and communication in living systems. Ligand conduction: A general catalytic principle in chemical, osmotic and chemiosmotic reaction systems. Eur. J. Biochem 1979, 95, 1–20. [Google Scholar]
- Engelhardt, WA. Life and Science. Annu. Rev. Biochem 1982, 51, 1–19. [Google Scholar]
- Belitzer, VA; Tsybakova, ET. About mechanism of phosphorylation, respiratory coupling. Biokhimiya 1939, 4, 516–534. [Google Scholar]
- Lundsgaard, E. Untersuhungen uber Muskelkontractionen ohne Milchsaurebildung. Biochem. Z 1930, 217, 162–177. [Google Scholar]
- Mommaerts, WF. Energetics of muscular contraction. Physiol. Rev 1969, 49, 427–508. [Google Scholar]
- Hill, AV. A challenge to biochemists. Biochim. Biophys. Acta 1950, 4, 4–11. [Google Scholar]
- Infante, AA; Davies, RE. The effect of 2,4-dinitrofluorobenzene on the activity of striated muscle. J. Biol. Chem 1965, 240, 3996–4001. [Google Scholar]
- Veech, RL; Lawson, JW; Cornell, NW; Krebs, HA. Cytosolic phosphorylation potential. J. Biol. Chem 1979, 254, 6538–6547. [Google Scholar]
- Jacobs, H; Heldt, HW; Klingenberg, M. High activity of creatine kinase in mitochondria from muscle and brain and evidence for a separate mitochondrial isoenzyme of creatine kinase. Biochem. Biophys. Res. Commun 1964, 16, 516–521. [Google Scholar]
- Bessman, SP; Fonyo, A. The possible role of the mitochondrial bound creatine kinase in regulation of mitochondrial respiration. Biochem. Biophys. Res. Commun 1966, 22, 597–602. [Google Scholar]
- Jacobus, WE; Lehninger, AL. Creatine kinase of rat heart mitochondria. Coupling of creatine phosphorylation to electron transport. J. Biol. Chem 1973, 248, 4803–4810. [Google Scholar]
- Bessman, SP; Carpenter, CL. The creatine-creatine phosphate energy shuttle. Annu. Rev. Biochem 1985, 54, 831–862. [Google Scholar]
- Schlattner, U; Forstner, M; Eder, M; Stachowiak, O; Fritz-Wolf, K; Wallimann, T. Functional aspects of the X-ray structure of mitochondrial creatine kinase: A molecular physiology approach. Mol. Cell Biochem 1998, 184, 125–140. [Google Scholar]
- Schlattner, U; Dolder, M; Wallimann, T; Tokarska-Schlattner, M. Mitochondrial creatine kinase and mitochondrial outer membrane porin show a direct interaction that is modulated by calcium. J. Biol. Chem 2001, 276, 48027–48030. [Google Scholar]
- Schlattner, U; Tokarska-Schlattner, M; Wallimann, T. Molecular structure and function of mitochondrial creatine kinases. In Creatine Kinase; Vial, C, Ed.; Nova Science Publishers: New York, NY, USA, 2006; pp. 123–170. [Google Scholar]
- Wyss, M; Smeitink, J; Wevers, RA; Wallimann, T. Mitochondrial creatine kinase: A key enzyme of aerobic energy metabolism. Biochim. Biophys. Acta 1992, 1102, 119–166. [Google Scholar]
- Dolder, M; Walzel, B; Speer, O; Schlattner, U; Wallimann, T. Inhibition of the mitochondrial permeability transition by creatine kinase substrates. Requirement for microcompartmentation. J. Biol. Chem 2003, 278, 17760–17766. [Google Scholar]
- Saks, VA; Aliev, MK. Is there the creatine kinase equilibrium in working heart cells? Biochem. Biophys. Res. Commun 1996, 227, 360–367. [Google Scholar]
- Saks, VA; Chernousova, GB; Voronkov, II; Smirnov, VN; Chazov, EI. Study of energy transport mechanism in myocardial cells. Circ. Res 1974, 35, S138–S149. [Google Scholar]
- Saks, VA; Chernousova, GB; Gukovsky, DE; Smirnov, VN; Chazov, EI. Studies of energy transport in heart cells. Mitochondrial isoenzyme of creatine phosphokinase: Kinetic properties and regulatory action of Mg2+ ions. Eur. J. Biochem 1975, 57, 273–290. [Google Scholar]
- Gellerich, F; Saks, VA. Control of heart mitochondrial oxygen consumption by creatine kinase: The importance of enzyme localization. Biochem. Biophys. Res. Commun 1982, 105, 1473–1481. [Google Scholar]
- Jacobus, WE; Saks, VA. Creatine kinase of heart mitochondria: Changes in its kinetic properties induced by coupling to oxidative phosphorylation. Arch. Biochem. Biophys 1982, 219, 167–178. [Google Scholar]
- Barbour, RL; Ribaudo, J; Chan, SH. Effect of creatine kinase activity on mitochondrial ADP/ATP transport. Evidence for a functional interaction. J. Biol. Chem 1984, 259, 8246–8251. [Google Scholar]
- Kuznetsov, AV; Khuchua, ZA; Vassil’eva, EV; Medved’eva, NV; Saks, VA. Heart mitochondrial creatine kinase revisited: The outer mitochondrial membrane is not important for coupling of phosphocreatine production to oxidative phosphorylation. Arch. Biochem. Biophys 1989, 268, 176–190. [Google Scholar]
- Saks, VA; Kuznetsov, AV; Kupriyanov, VV; Miceli, MV; Jacobus, WE. Creatine kinase of rat heart mitochondria. The demonstration of functional coupling to oxidative phosphorylation in an inner membrane-matrix preparation. J. Biol. Chem 1985, 260, 7757–7764. [Google Scholar]
- Joubert, F; Mateo, P; Gillet, B; Beloeil, JC; Mazet, JL; Hoerter, JA. CK flux or direct ATP transfer: Versatility of energy transfer pathways evidenced by NMR in the perfused heart. Mol Cell Biochem 2004, 256–257, 43–58. [Google Scholar]
- Ventura-Clapier, R; Kuznetsov, A; Veksler, V; Boehm, E; Anflous, K. Functional coupling of creatine kinases in muscles: Species and tissue specificity. Mol. Cell Biochem 1998, 184, 231–247. [Google Scholar]
- Segel, I. Enzyme Kinetics; Wiley Interscience Publishers: New York, NY, USA, 1975; pp. 1–957. [Google Scholar]
- Qian, H. Phosphorylation energy hypothesis: Open chemical systems and their biological functions. Annu. Rev. Phys. Chem 2007, 58, 113–142. [Google Scholar]
- Qian, H. Cooperativity and specificity in enzyme kinetics: A single-molecule time-based perspective. Biophys. J 2008, 95, 10–17. [Google Scholar]
- De La Fuente, IM; Martinez, L; Perez-Samartin, AL; Ormaetxea, L; Amezaga, C; Vera-Lopez, A. Global self-organization of the cellular metabolic structure. PLoS ONE 2008, 3, e3100. [Google Scholar]
- Aon, MA; Gomez-Casati, DF; Iglesias, AA; Cortassa, S. Ultrasensitivity in (supra)molecularly organized and crowded environments. Cell Biol. Int 2001, 25, 1091–1099. [Google Scholar]
- Aon, MA; O’Rourke, B; Cortassa, S. The fractal architecture of cytoplasmic organization: Scaling, kinetics and emergence in metabolic networks. Mol Cell Biochem 2004, 256–257, 169–184. [Google Scholar]
- Cortassa, S; O’Rourke, B; Winslow, RL; Aon, MA. Control and regulation of mitochondrial energetics in an integrated model of cardiomyocyte function. Biophys. J 2009, 96, 2466–2478. [Google Scholar]
- Saks, VA; Belikova, YO; Kuznetsov, AV. In vivo regulation of mitochondrial respiration in cardiomyocytes: Specific restrictions for intracellular diffusion of ADP. Biochim. Biophys. Acta 1991, 1074, 302–311. [Google Scholar]
- Vendelin, M; Beraud, N; Guerrero, K; Andrienko, T; Kuznetsov, AV; Olivares, J; Kay, L; Saks, VA. Mitochondrial regular arrangement in muscle cells: A “crystal-like” pattern. Am. J. Physiol. Cell Physiol 2005, 288, C757–C767. [Google Scholar]
- Beraud, N; Pelloux, S; Usson, Y; Kuznetsov, AV; Ronot, X; Tourneur, Y; Saks, VA. Mitochondrial dynamics in heart cells: Very low amplitude high frequency fluctuations in adult cardiomyocytes and flow motion in non beating Hl-1 cells. J. Bioenerg. Biomembr 2009, 41, 195–214. [Google Scholar]
- Pelloux, S; Robillard, J; Ferrera, R; Bilbaut, A; Ojeda, C; Saks, VA; Ovize, M; Tourneur, Y. Non-beating HL-1 cells for confocal microscopy: Application to mitochondrial functions during cardiac preconditioning. Prog. Biophys. Mol. Biol 2006, 90, 270–298. [Google Scholar]
- Anmann, T; Guzun, R; Beraud, N; Pelloux, S; Kuznetsov, AV; Kogerman, L; Kaambre, T; Sikk, P; Paju, K; Peet, N; Seppet, E; Ojeda, C; Tourneur, Y; Saks, VA. Different kinetics of the regulation of respiration in permeabilized cardiomyocytes and in HL-1 cardiac cells. Importance of cell structure/organization for respiration regulation. Biochim. Biophys. Acta 2006, 1757, 1597–1606. [Google Scholar]
- Kuznetsov, AV; Tiivel, T; Sikk, P; Kaambre, T; Kay, L; Daneshrad, Z; Rossi, A; Kadaja, L; Peet, N; Seppet, E; Saks, VA. Striking differences between the kinetics of regulation of respiration by ADP in slow-twitch and fast-twitch muscles in vivo. Eur. J. Biochem 1996, 241, 909–915. [Google Scholar]
- Appaix, F; Kuznetsov, AV; Usson, Y; Kay, L; Andrienko, T; Olivares, J; Kaambre, T; Sikk, P; Margreiter, R; Saks, VA. Possible role of cytoskeleton in intracellular arrangement and regulation of mitochondria. Exp. Physiol 2003, 88, 175–190. [Google Scholar]
- Brdizka, D. Mitochondrial VDAC and its complexes. In Molecular System Bioenergetics Energy for Life; Saks, VA, Ed.; Wiley-VCH: Weinheim, Germany, 2007; pp. 165–194. [Google Scholar]
- Rostovtseva, TK; Bezrukov, SM. VDAC regulation: Role of cytosolic proteins and mitochondrial lipids. J. Bioenerg. Biomembr 2008, 40, 163–170. [Google Scholar]
- Rostovtseva, TK; Sheldon, KL; Hassanzadeh, E; Monge, C; Saks, VA; Bezrukov, SM; Sackett, DL. Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc. Natl. Acad. Sci. USA 2008, 105, 18746–18751. [Google Scholar]
- Monge, C; Grichine, A; Rostovtseva, T; Sackett, P; Saks, VA. Compartmentation of ATP in cardiomyocytes and mitochondria. Kinetic studies and direct measurements. Biophys. J 2009, 96, 241a. [Google Scholar]
- Pedersen, PL; Ko, YH; Hong, S. ATP synthases in the year 2000: Evolving views about the structures of these remarkable enzyme complexes. J. Bioenerg. Biomembr 2000, 32, 325–332. [Google Scholar]
- Ko, YH; Delannoy, M; Hullihen, J; Chiu, W; Pedersen, PL. Mitochondrial ATP synthasome. Cristae-enriched membranes and a multiwell detergent screening assay yield dispersed single complexes containing the ATP synthase and carriers for Pi and ADP/ATP. J. Biol. Chem 2003, 278, 12305–12309. [Google Scholar]
- Chen, C; Ko, Y; Delannoy, M; Ludtke, SJ; Chiu, W; Pedersen, PL. Mitochondrial ATP synthasome: Three-dimensional structure by electron microscopy of the ATP synthase in complex formation with carriers for Pi and ADP/ATP. J. Biol. Chem 2004, 279, 31761–31768. [Google Scholar]
- Pedersen, PL. Transport ATPases into the year 2008: A brief overview related to types, structures, functions and roles in health and disease. J. Bioenerg. Biomembr 2007, 39, 349–355. [Google Scholar]
- Pedersen, PL. Warburg, me and Hexokinase 2: Multiple discoveries of key molecular events underlying one of cancers’ most common phenotypes, the “Warburg Effect”, i.e., elevated glycolysis in the presence of oxygen. J. Bioenerg. Biomembr 2007, 39, 211–222. [Google Scholar]
- Smith, SA; Montain, SJ; Zientara, GP; Fielding, RA. Use of phosphocreatine kinetics to determine the influence of creatine on muscle mitochondrial respiration: An in vivo 31P-MRS study of oral creatine ingestion. J. Appl. Physiol 2004, 96, 2288–2292. [Google Scholar]
- Lenaz, G; Genova, ML. Kinetics of integrated electron transfer in the mitochondrial respiratory chain: Random collisions vs. solid state electron channeling. Am. J. Physiol. Cell Physiol 2007, 292, C1221–C1239. [Google Scholar]
- Vonck, J; Schafer, E. Supramolecular organization of protein complexes in the mitochondrial inner membrane. Biochim. Biophys. Acta 2009, 1793, 117–124. [Google Scholar]
- Zeleznikar, RJ; Dzeja, PP; Goldberg, ND. Adenylate kinase-catalyzed phosphoryl transfer couples ATP utilization with its generation by glycolysis in intact muscle. J. Biol. Chem 1995, 270, 7311–7319. [Google Scholar]
- Vendelin, M; Kongas, O; Saks, VA. Regulation of mitochondrial respiration in heart cells analyzed by reaction-diffusion model of energy transfer. Am. J. Physiol. Cell Physiol 2000, 278, C747–C764. [Google Scholar]
- Aliev, MK; Saks, VA. Compartmentalized energy transfer in cardiomyocytes: Use of mathematical modeling for analysis of in vivo regulation of respiration. Biophys. J 1997, 73, 428–445. [Google Scholar]
- Dos Santos, P; Aliev, MK; Diolez, P; Duclos, F; Besse, P; Bonoron-Adele, S; Sikk, P; Canioni, P; Saks, VA. Metabolic control of contractile performance in isolated perfused rat heart. Analysis of experimental data by reaction: Diffusion mathematical model. J. Mol. Cell Cardiol 2000, 32, 1703–1734. [Google Scholar]
- Forbes, SC; Raymer, GH; Kowalchuk, JM; Thompson, RT; Marsh, GD. Effects of recovery time on phosphocreatine kinetics during repeated bouts of heavy-intensity exercise. Eur. J. Appl. Physiol 2008, 103, 665–675. [Google Scholar]
- Forbes, SC; Slade, JM; Francis, RM; Meyer, RA. Comparison of oxidative capacity among leg muscles in humans using gated (31)P 2-D chemical shift imaging. NMR Biomed 2009, 22, 1063–1071. [Google Scholar]
- Larsen, RG; Callahan, DM; Foulis, SA; Kent-Braun, JA. In vivo oxidative capacity varies with muscle and training status in young adults. J. Appl. Physiol 2009, 107, 873–879. [Google Scholar]
- Spindler, M; Illing, B; Horn, M; de Groot, M; Ertl, G; Neubauer, S. Temporal fluctuations of myocardia high-energy phosphate metabolite with the cardiac cycle. Basic Res. Cardiol 2001, 96, 553–556. [Google Scholar]
- Honda, H; Tanaka, K; Akita, N; Haneda, T. Cyclical changes in high-energy phosphates during the cardiac cycle by pacing-Gated 31P nuclear magnetic resonance. Circ. J 2002, 66, 80–86. [Google Scholar]
- Gudbjarnason, S; Mathes, P; Ravens, KG. Functional compartmentation of ATP and creatine phosphate in heart muscle. J. Mol. Cell Cardiol 1970, 1, 325–339. [Google Scholar]
- Kammermeier, H; Roeb, E; Jungling, E; Meyer, B. Regulation of systolic force and control of free energy of ATP-hydrolysis in hypoxic hearts. J. Mol. Cell Cardiol 1990, 22, 707–713. [Google Scholar]
- Griese, M; Perlitz, V; Jungling, E; Kammermeier, H. Myocardial performance and free energy of ATP-hydrolysis in isolated rat hearts during graded hypoxia, reoxygenation and high Ke+-perfusion. J. Mol. Cell Cardiol 1988, 20, 1189–1201. [Google Scholar]
- Weiss, RG; Gerstenblith, G; Bottomley, PA. ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc. Natl. Acad. Sci. USA 2005, 102, 808–813. [Google Scholar]
- Weiss, JN; Yang, L; Qu, Z. Systems biology approaches to metabolic and cardiovascular disorders: Network perspectives of cardiovascular metabolism. J. Lipid Res 2006, 47, 2355–2366. [Google Scholar]
- Ingwall, JS. On the hypothesis that the failing heart is energy starved: Lessons learned from the metabolism of ATP and creatine. Curr. Hypertens Rep 2006, 8, 457–464. [Google Scholar]
- Ingwall, JS; Weiss, RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ. Res 2004, 95, 135–145. [Google Scholar]
- Ventura-Clapier, R; Garnier, A; Veksler, V. Energy metabolism in heart failure. J. Physiol 2004, 555, 1–13. [Google Scholar]
- Neubauer, S. The failing heart--an engine out of fuel. N. Engl. J. Med 2007, 356, 1140–1151. [Google Scholar]
- Ventura-Clapier, R. Exercise training, energy metabolism, and heart failure. Appl. Physiol. Nutr. Metab 2009, 34, 336–339. [Google Scholar]
- Ventura-Clapier, R; De Sousa, E; Veksler, V. Metabolic myopathy in heart failure. News Physiol. Sci 2002, 17, 191–196. [Google Scholar]
- Saks, VA; Kongas, O; Vendelin, M; Kay, L. Role of the creatine/phosphocreatine system in the regulation of mitochondrial respiration. Acta Physiol. Scand 2000, 168, 635–641. [Google Scholar]
- Bose, S; French, S; Evans, FJ; Joubert, F; Balaban, RS. Metabolic network control of oxidative phosphorylation: Multiple roles of inorganic phosphate. J. Biol. Chem 2003, 278, 39155–39165. [Google Scholar]
- Scheibye-Knudsen, M; Quistorff, B. Regulation of mitochondrial respiration by inorganic phosphate; comparing permeabilized muscle fibers and isolated mitochondria prepared from type-1 and type-2 rat skeletal muscle. Eur. J. Appl. Physiol 2009, 105, 279–287. [Google Scholar]
- Bernard, C. Introduction À L’étude De La Médicine Expérimentale; Champs-Flammarion: Paris, France, 1984. [Google Scholar]
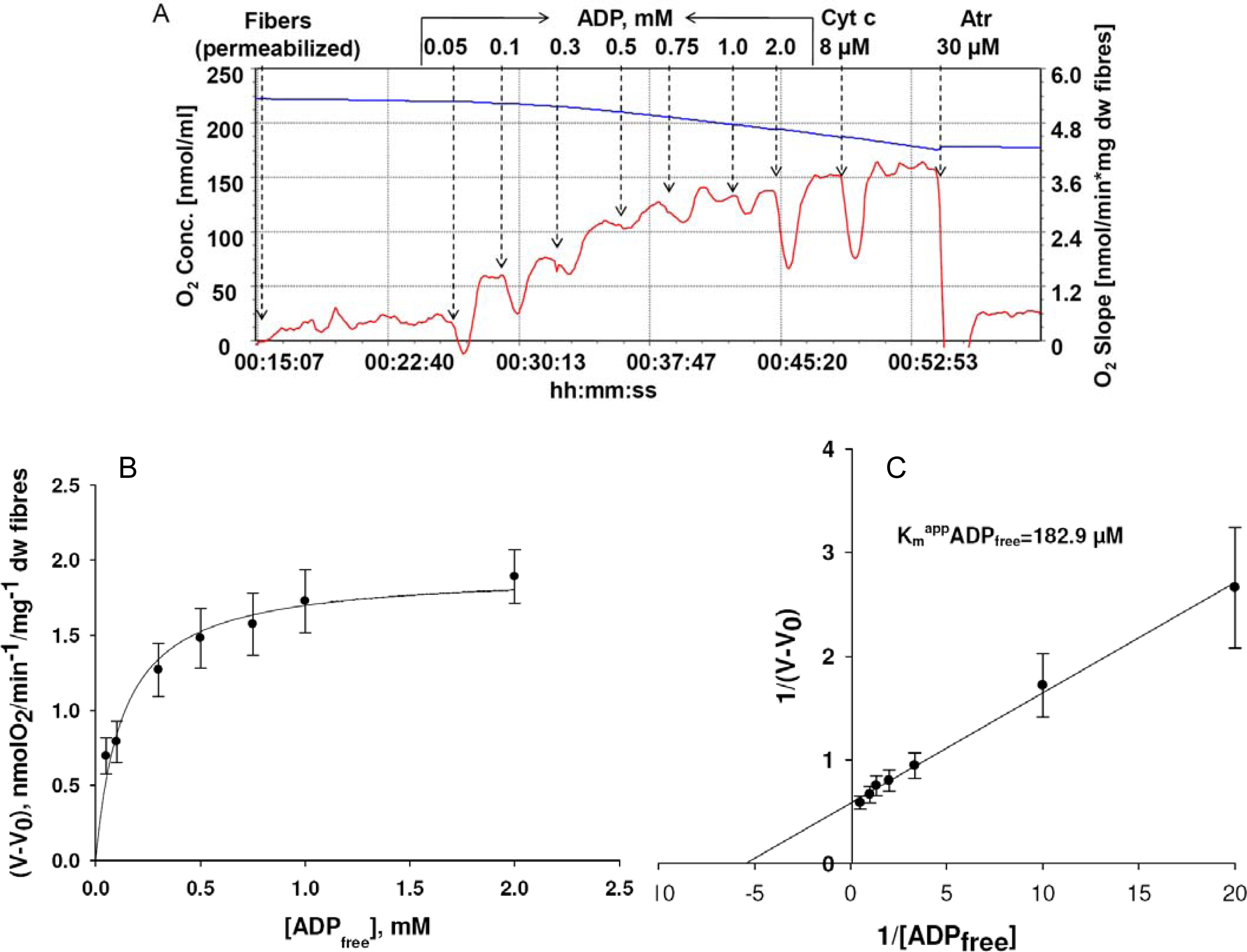

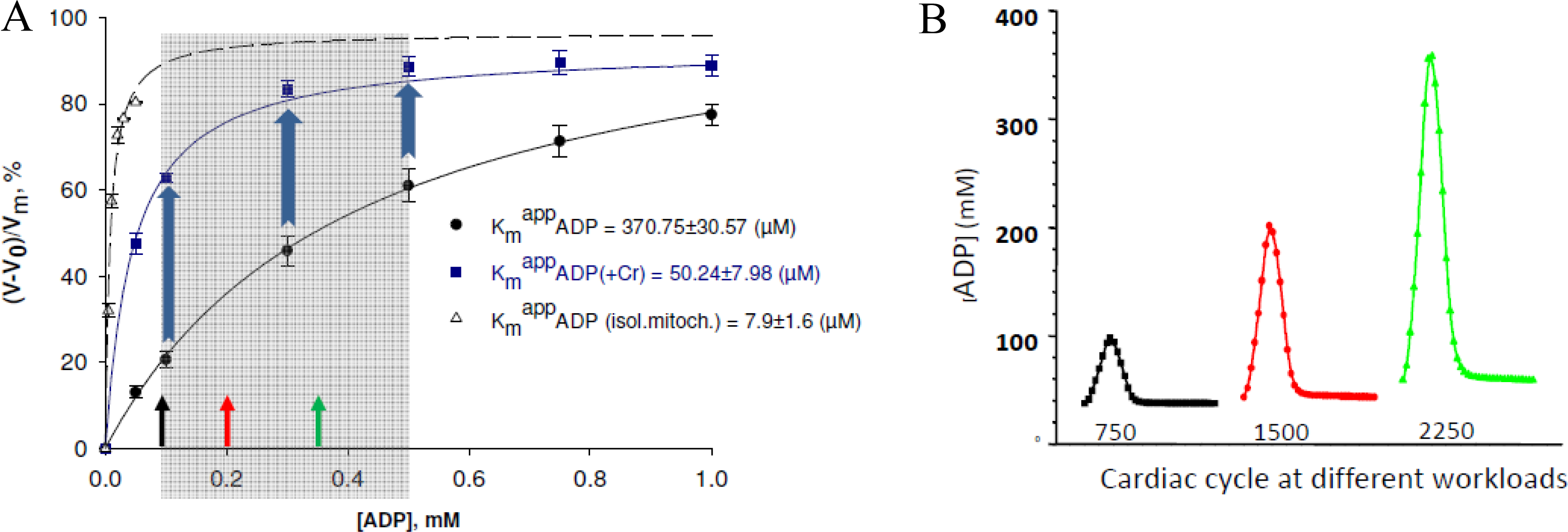
 ) – in the presence of both trapping system for free ADP and 20 mM creatine. Reproduced from [32] with permission.
) – in the presence of both trapping system for free ADP and 20 mM creatine. Reproduced from [32] with permission.
 ) – in the presence of both trapping system for free ADP and 20 mM creatine. Reproduced from [32] with permission.
) – in the presence of both trapping system for free ADP and 20 mM creatine. Reproduced from [32] with permission.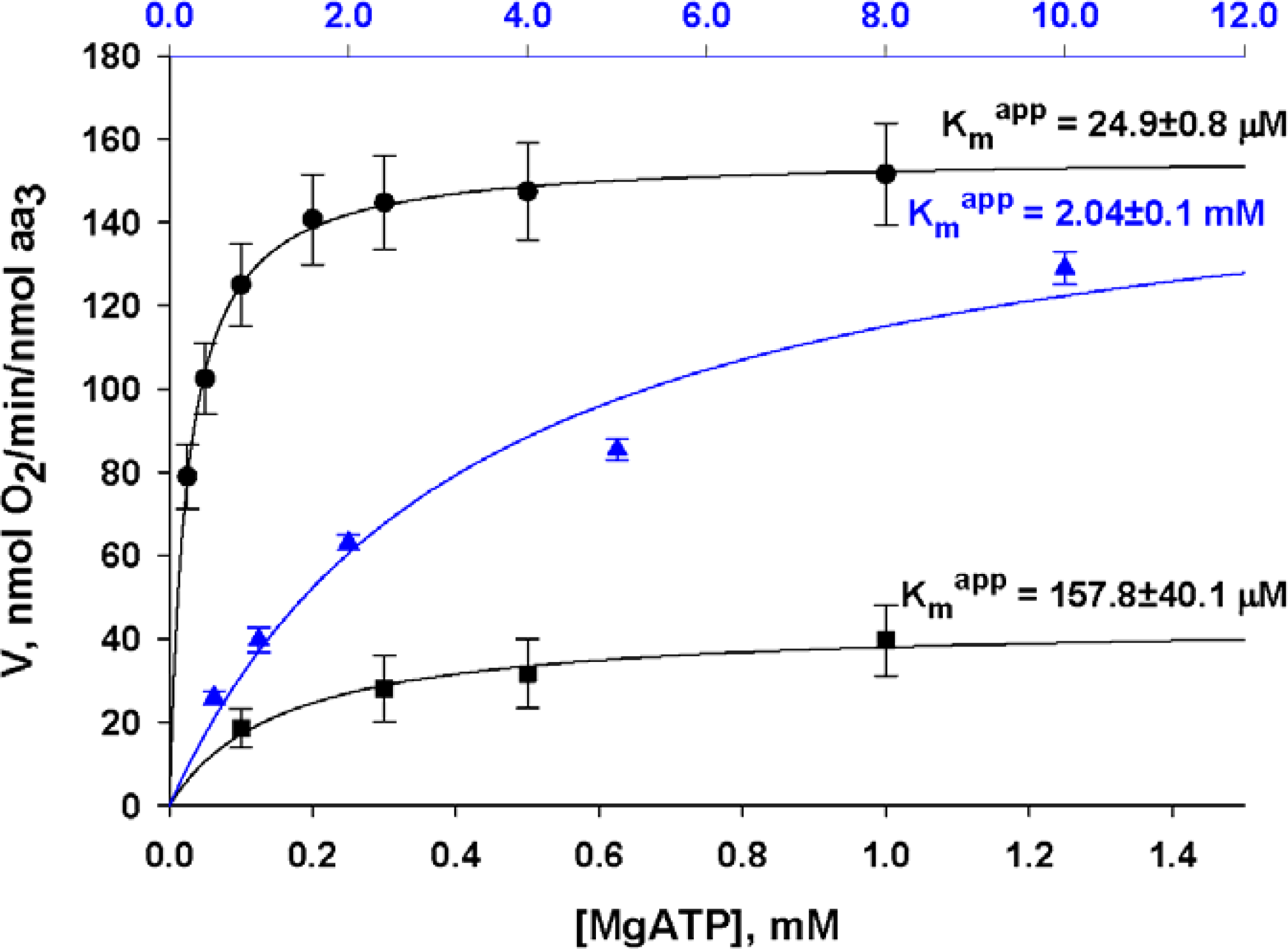


| Parameter | Mitochondria in vitro | Mitochondria in situ (permeabilized cardiomyocytes) |
|---|---|---|
| V0, nmolO2·min−1·mg prot−1 | 26.37 ± 7.93 | 7.53 ± 1.61 |
| V3 (2 mM ADP), nmolO2·min−1·mg prot−1 | 187.94 ± 40.68 | 84.45 ± 13.85 |
| [Cyt aa3], nmol·mg prot−1 | 1.00 ± 0.04 | 0.46 ± 0.09 |
| V3 (2 mM ADP), nmolO2·min−1·nmol cyt aa3−1 | 187.94 ± 40.68 | 178.23 ± 33.96 |
| VCr,ATP, nmolO2·min−1·nmol cyt aa3−1 | 197.90 ± 31.86 | 162.63 ± 26.87 |
| Kia (MgATP), mM | Ka (MgATP), mM | Kib (Cr), mM | Kb (Cr), mM | Kip (PCr), mM | ||
|---|---|---|---|---|---|---|
| Isolated Mitoch. | −OxPhosph | 0.92 ± 0.09 | 0.15 ± 0.023 | 30 ± 4.5 | 5.2 ± 0.3 | |
| +OxPhosph | 0.44 ± 0.08 | 0.016 ± 0.01 | 28 ± 7 | 5 ± 1.2 | 0.84 ± 0.22 | |
| Mitoch. in situ (PEP-PK) | 1.94 ± 0.86 | 2.04 ± 0.14 | 2.12 ± 0.21 | 2.17 ± 0.40 | 0.89 ± 0.17 | |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Guzun, R.; Saks, V. Application of the Principles of Systems Biology and Wiener's Cybernetics for Analysis of Regulation of Energy Fluxes in Muscle Cells in Vivo. Int. J. Mol. Sci. 2010, 11, 982-1019. https://doi.org/10.3390/ijms11030982
Guzun R, Saks V. Application of the Principles of Systems Biology and Wiener's Cybernetics for Analysis of Regulation of Energy Fluxes in Muscle Cells in Vivo. International Journal of Molecular Sciences. 2010; 11(3):982-1019. https://doi.org/10.3390/ijms11030982
Chicago/Turabian StyleGuzun, Rita, and Valdur Saks. 2010. "Application of the Principles of Systems Biology and Wiener's Cybernetics for Analysis of Regulation of Energy Fluxes in Muscle Cells in Vivo" International Journal of Molecular Sciences 11, no. 3: 982-1019. https://doi.org/10.3390/ijms11030982
APA StyleGuzun, R., & Saks, V. (2010). Application of the Principles of Systems Biology and Wiener's Cybernetics for Analysis of Regulation of Energy Fluxes in Muscle Cells in Vivo. International Journal of Molecular Sciences, 11(3), 982-1019. https://doi.org/10.3390/ijms11030982




