Neuroprotective Properties of Mildronate, a Small Molecule, in a Rat Model of Parkinson’s Disease
Abstract
:1. Introduction
2. Results
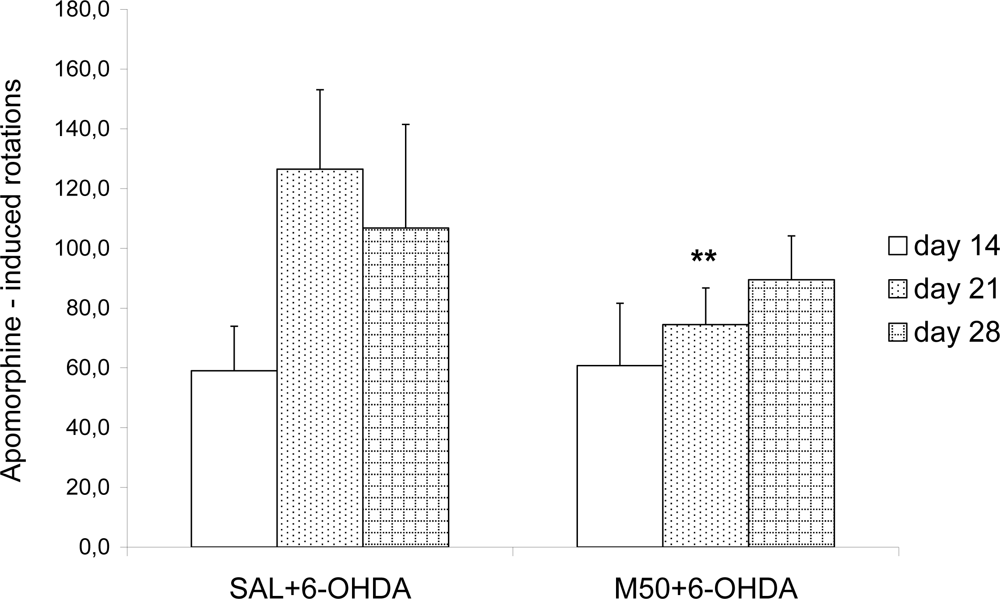
2.1. Effects on Apomorphine-Induced Rotations
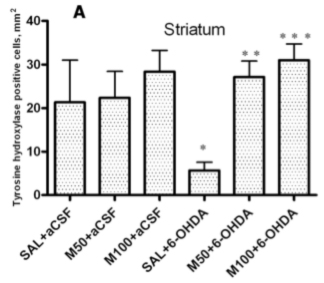
2.2. Tyrosine Hydroxylase (TH) Expression in Striatum and Substantia Nigra
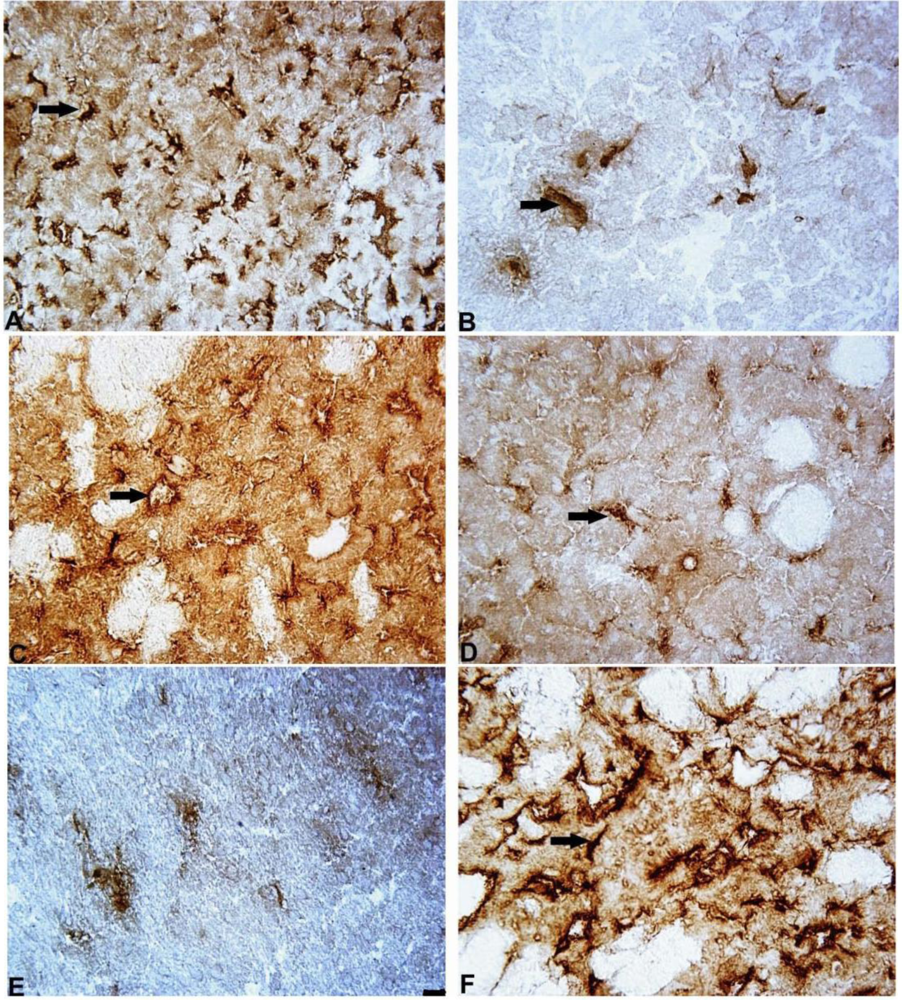
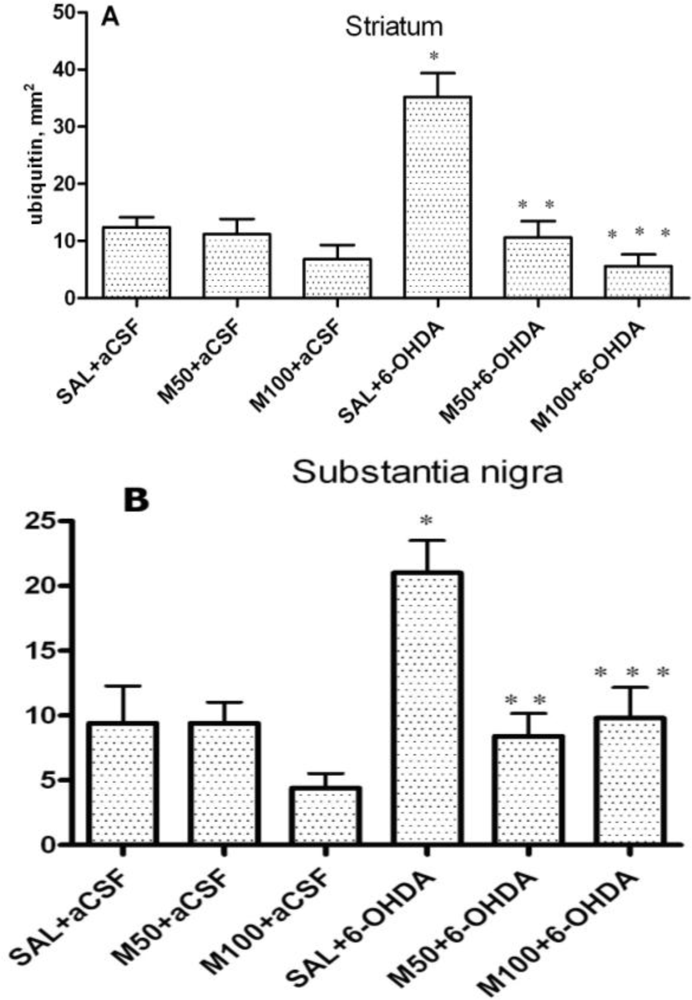
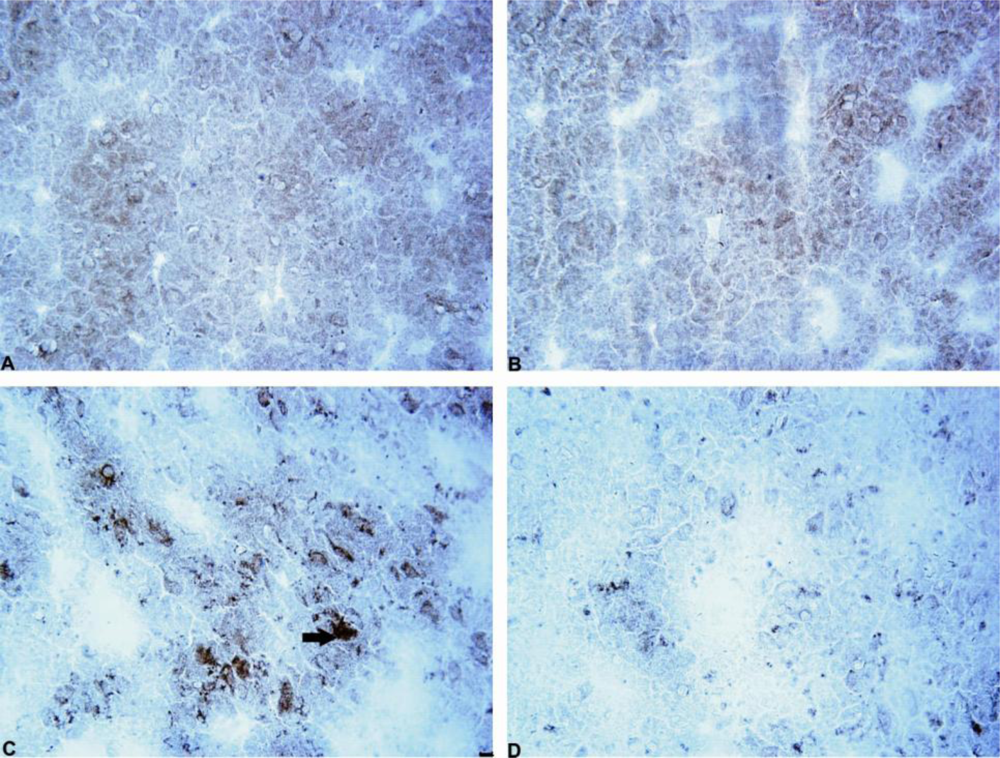
2.3. The Number of Cells with Intracellular Ubiquitin-Positive Inclusions in Striatum and Substantia Nigra
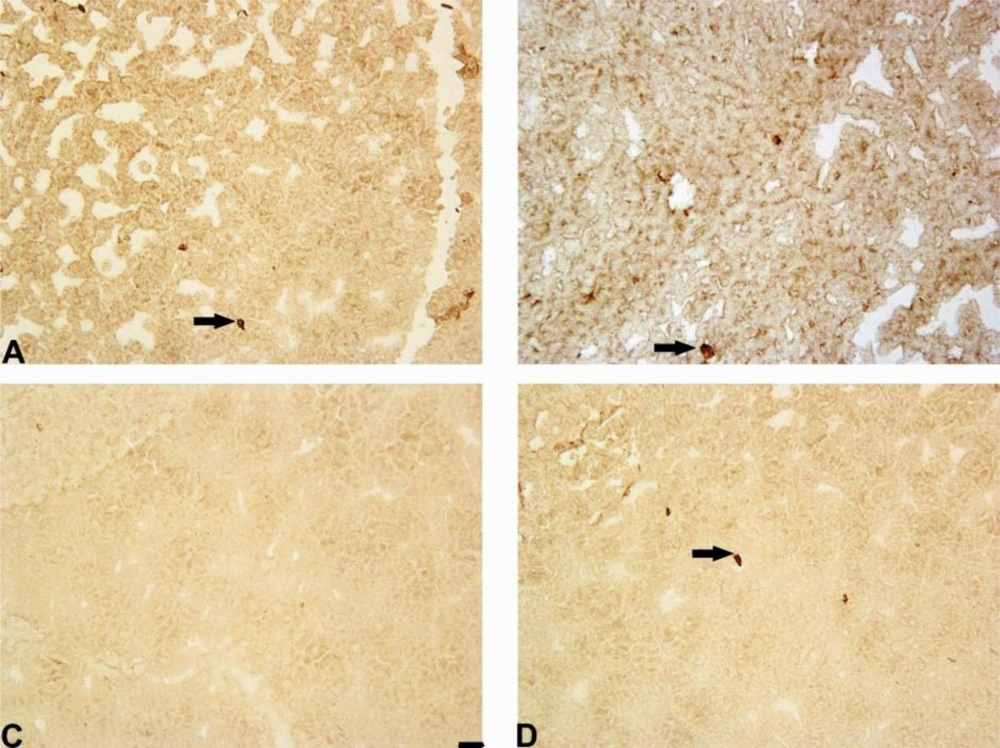
2.4. Notch-3 Expression in Striatum and Substantia Nigra
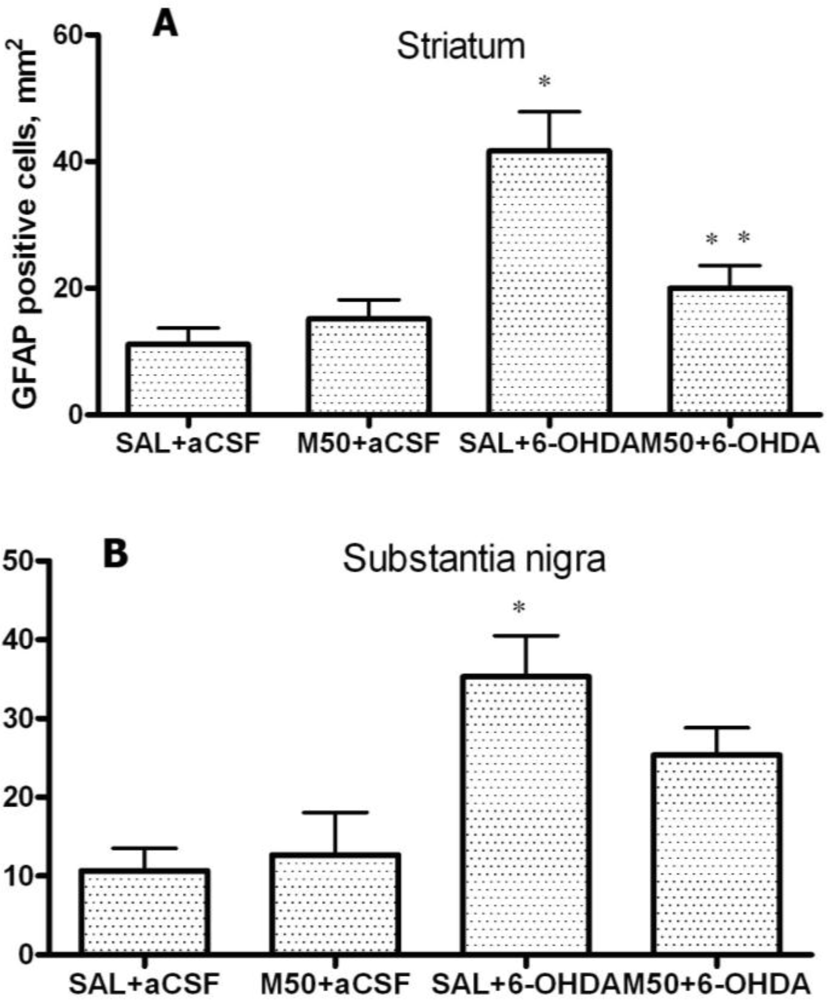
2.5. GFAP Expression in Striatum and Substantia Nigra
2.6. IBA-1-Expression in Striatum and Substantia Nigra
2.7. iNOS Expression in Striatum and Substantia Nigra
3. Discussion
4. Experimental Section
4.1. Materials and Methods
4.2. Experimental Design
- Group 1: Saline (i.p.) for two weeks followed by aCSF (intrastriatal)(corpus striatum).
- Group 2: Mildronate, 50 mg/kg i.p. for two weeks followed by aCSF (intrastriatal).
- Group 3: Mildronate, 100 mg/kg i.p. for two weeks followed by aCSF (intrastriatal).
- Group 4: Saline i.p. for two weeks followed by 6-OHDA (intrastriatal).
- Group 5: Mildronate, 50 mg/kg i.p. for two weeks followed by 6-OHDA (intrastriatal).
- Group 6: Mildronate, 100 mg/kg i.p. for two weeks followed by 6-OHDA (intrastriatal).
4.3. Surgical Procedures
4.4. Rotational Behavior
4.5. Brain Tissue Processing
4.6. Immunohistochemical Examination of the Brain Tissue
4.7. Imaging and Quantitation of Fibers and Cells
4.8. Statistics
5. Conclusions
Acknowledgments
References
- Moore, DJ; West, AB; Dawson, VL; Dawson, TM. Molecular pathophysiology of Parkinson’s disease. Annu. Rev. Neurosci 2005, 28, 57–87. [Google Scholar]
- Olanow, CW; Tatton, WG. Etiology and pathogenesis of Parkinson’s disease. Annu. Rev. Neurosci 1999, 22, 123–144. [Google Scholar]
- Ahlskog, JE. Beating a dead horse: Dopamine and Parkinson disease. Neurology 2007, 69, 1701–1711. [Google Scholar]
- Schapira, AH. Mitochondrial involvement in Parkinson’s disease, Huntington’s disease, hereditary spastic paraplegia and Friedreich’s ataxia. Biochim. Biophys. Acta 1999, 1410, 159–170. [Google Scholar]
- Mandemakers, W; Morais, VA; De Strooper, B. A cell biological perspective on mitochondrial dysfunction in Parkinson disease and other neurodegenerative diseases. J. Cell Sci 2007, 120, 1707–1716. [Google Scholar]
- Abeliovich, A. Parkinson’s disease: Mitochondrial damage control. Nature 2010, 463, 744–745. [Google Scholar]
- Vila, M; Przedborski, S. Targeting programmed cell death in neurodegenerative diseases. Nat. Rev. Neurosci 2003, 4, 365–375. [Google Scholar]
- Dawson, TM; Dawson, VL. Molecular pathways of neurodegeneration in Parkinson’s disease. Science 2003, 302, 819–822. [Google Scholar]
- Hightower, LE. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell 1991, 66, 191–197. [Google Scholar]
- Meriin, AB; Sherman, MY. Role of molecular chaperones in neurodegenerative disorders. Int. J. Hyperthermia 2005, 21, 403–419. [Google Scholar]
- Dedmon, MM; Christodoulou, J; Wilson, MR; Dobson, CM. Heat shock protein 70 inhibits alpha-synuclein fibril formation via preferential binding to prefibrillar species. J. Biol. Chem 2005, 280, 14733–14740. [Google Scholar]
- Conn, PM; Janovick, JA. Drug development and the cellular quality control system. Trends Pharmacol. Sci 2009, 30, 228–233. [Google Scholar]
- Caldas, T; Demont-Caulet, N; Ghazi, A; Richarme, G. Thermoprotection by glycine betaine and choline. Microbiology 1999, 145, 2543–2548. [Google Scholar]
- Diamant, S; Rosenthal, D; Azem, A; Eliahu, N; Ben-Zvi, AP; Goloubinoff, P. Dicarboxylic amino acids and glycine-betaine regulate chaperone-mediated protein-disaggregation under stress. Mol. Microbiol 2003, 49, 401–410. [Google Scholar]
- Li, J; Zhu, M; Rajamani, S; Uversky, VN; Fink, AL. Rifampicin inhibits alpha-synuclein fibrillation and disaggregates fibrils. Chem. Biol 2004, 11, 1513–1521. [Google Scholar]
- Zhu, M; Rajamani, S; Kaylor, J; Han, S; Zhou, F; Fink, AL. The flavonoid baicalein inhibits fibrillation of alpha-synuclein and disaggregates existing fibrils. J. Biol. Chem 2004, 279, 26846–26857. [Google Scholar]
- Rigat, B; Mahuran, D. Diltiazem, a L-type Ca2+ channel blocker, also acts as a pharmacological chaperone in Gaucher patient cells. Mol. Genet. Metab 2009, 96, 225–232. [Google Scholar]
- Giasson, BI; Lee, VM. Are ubiquitination pathways central to Parkinson’s disease? Cell 2003, 114, 1–8. [Google Scholar]
- Huang, Q; Figueiredo-Pereira, ME. Ubiquitin/proteasome pathway impairment in neurodegeneration: Therapeutic implications. Apoptosis 2010. doi: 10.1007/s10495-010-0466-z.. [Google Scholar]
- Hernandez, F; Diaz-Hernandez, M; Avila, J; Lucas, JJ. Testing the ubiquitin-proteasome hypothesis of neurodegeneration in vivo. Trends Neurosci 2004, 27, 66–69. [Google Scholar]
- Rodriguez-Pallares, J; Parga, JA; Munoz, A; Rey, P; Guerra, MJ; Labandeira-Garcia, JL. Mechanism of 6-hydroxydopamine neurotoxicity: The role of NADPH oxidase and microglial activation in 6-hydroxydopamine-induced degeneration of dopaminergic neurons. J. Neurochem 2007, 103, 145–156. [Google Scholar]
- Liu, B; Gao, HM; Hong, JS. Parkinson’s disease and exposure to infectious agents and pesticides and the occurrence of brain injuries: Role of neuroinflammation. Environ. Health Perspect 2003, 111, 1065–1073. [Google Scholar]
- Marinova-Mutafchieva, L; Sadeghian, M; Broom, L; Davis, JB; Medhurst, AD; Dexter, DT. Relationship between microglial activation and dopaminergic neuronal loss in the substantia nigra: A time course study in a 6-hydroxydopamine model of Parkinson’s disease. J. Neurochem 2009, 110, 966–975. [Google Scholar]
- Pinto, L; Gotz, M. Radial glial cell heterogeneity--the source of diverse progeny in the CNS. Prog. Neurobiol 2007, 83, 2–23. [Google Scholar]
- Allen, NJ; Barres, BA. Neuroscience: Glia-more than just brain glue. Nature 2009, 457, 675–677. [Google Scholar]
- Neumann, J; Gunzer, M; Gutzeit, HO; Ullrich, O; Reymann, KG; Dinkel, K. Microglia provide neuroprotection after ischemia. FASEB J 2006, 20, 714–716. [Google Scholar]
- Di Virgilio, F; Ceruti, S; Bramanti, P; Abbracchio, MP. Purinergic signalling in inflammation of the central nervous system. Trends Neurosci 2009, 32, 79–87. [Google Scholar]
- Krieglstein, K. Factors promoting survival of mesencephalic dopaminergic neurons. Cell Tissue Res 2004, 318, 73–80. [Google Scholar]
- Voutilainen, MH; Back, S; Porsti, E; Toppinen, L; Lindgren, L; Lindholm, P; Peranen, J; Saarma, M; Tuominen, RK. Mesencephalic astrocyte-derived neurotrophic factor is neurorestorative in rat model of Parkinson’s disease. J. Neurosci 2009, 29, 9651–9659. [Google Scholar]
- Deierborg, T; Soulet, D; Roybon, L; Hall, V; Brundin, P. Emerging restorative treatments for Parkinson’s disease. Prog. Neurobiol 2008, 85, 407–432. [Google Scholar]
- Pluchino, S; Zanotti, L; Deleidi, M; Martino, G. Neural stem cells and their use as therapeutic tool in neurological disorders. Brain Res. Brain Rev 2005, 48, 211–219. [Google Scholar]
- Aponso, PM; Faull, RL; Connor, B. Increased progenitor cell proliferation and astrogenesis in the partial progressive 6-hydroxydopamine model of Parkinson’s disease. Neuroscience 2008, 151, 1142–1153. [Google Scholar]
- Hoglinger, GU; Rizk, P; Muriel, MP; Duyckaerts, C; Oertel, WH; Caille, I; Hirsch, EC. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat. Neurosci 2004, 7, 726–735. [Google Scholar]
- Su, B; Wang, X; Zheng, L; Perry, G; Smith, MA; Zhu, X. Abnormal mitochondrial dynamics and neurodegenerative diseases. Biochim. Biophys. Acta 2010, 1802, 135–142. [Google Scholar]
- Arora, PS; Ansari, AZ. Chemical biology: A Notch above other inhibitors. Nature 2009, 462, 171–173. [Google Scholar]
- Vilšķērsts, R; Zvejniece, L; Strogova, I; Alijeva, J; Muceniece, R; Dambrova, M; Liepiņš, E. Mildronate decreases l-carnitine concentration, but does not influence glucose uptake and related gene expression in mice brain. Scientific papers, University of Latvia: Riga, Latvia, 2009; Volume 750, pp. 106–116. (in Latvian). [Google Scholar]
- Pupure, J; Isajevs, S; Skapare, E; Rumaks, J; Svirskis, S; Svirina, D; Kalvinsh, I; Klusa, V. Neuroprotective properties of mildronate, a mitochondria-targeted small molecule. Neurosci. Lett 2010, 470, 100–105. [Google Scholar]
- Pupure, J; Fernandes, MA; Santos, MS; Moreno, AJ; Kalvinsh, I; Klusa, V; Oliveira, CR. Mitochondria as the target for mildronate’s protective effects in azidothymidine (AZT)-induced toxicity of isolated rat liver mitochondria. Cell Biochem. Funct 2008, 26, 620–631. [Google Scholar]
- Schapira, AH. Neurobiology and treatment of Parkinson’s disease. Trends Pharmacol. Sci 2009, 30, 41–47. [Google Scholar]
- Hamberger, A; Blomstrand, C; Lehninger, AL. Comparative studies on mitochondria isolated from neuron-enriched and glia-enriched fractions of rabbit and beef brain. J. Cell Biol 1970, 45, 221–234. [Google Scholar]
- Glinka, Y; Tipton, KF; Youdim, MB. Nature of inhibition of mitochondrial respiratory complex I by 6-Hydroxydopamine. J. Neurochem 1996, 66, 2004–2010. [Google Scholar]
- Jellinger, K; Linert, L; Kienzl, E; Herlinger, E; Youdim, MB. Chemical evidence for 6-hydroxydopamine to be an endogenous toxic factor in the pathogenesis of Parkinson’s disease. J. Neural Transm. Suppl 1995, 46, 297–314. [Google Scholar]
- Pierson, J; Svenningsson, P; Caprioli, RM; Andren, PE. Increased levels of ubiquitin in the 6-OHDA-lesioned striatum of rats. J. Proteome Res 2005, 4, 223–226. [Google Scholar]
- Mazzio, EA; Reams, RR; Soliman, KF. The role of oxidative stress, impaired glycolysis and mitochondrial respiratory redox failure in the cytotoxic effects of 6-hydroxydopamine in vitro. Brain Res 2004, 1004, 29–44. [Google Scholar]
- Irvin, DK; Zurcher, SD; Nguyen, T; Weinmaster, G; Kornblum, HI. Expression patterns of Notch1, Notch2, and Notch3 suggest multiple functional roles for the Notch-DSL signaling system during brain development. J. Comp. Neurol 2001, 436, 167–181. [Google Scholar]
- Campos, AH; Wang, W; Pollman, MJ; Gibbons, GH. Determinants of Notch-3 receptor expression and signaling in vascular smooth muscle cells: Implications in cell-cycle regulation. Circ. Res 2002, 91, 999–1006. [Google Scholar]
- Yano, R; Yokoyama, H; Kuroiwa, H; Kato, H; Araki, T. A novel anti-Parkinsonian agent, zonisamide, attenuates MPTP-induced neurotoxicity in mice. J. Mol. Neurosci 2009, 39, 211–219. [Google Scholar]
- Buffo, A; Rite, I; Tripathi, P; Lepier, A; Colak, D; Horn, AP; Mori, T; Gotz, M. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc. Natl. Acad. Sci. USA 2008, 105, 3581–3586. [Google Scholar]
- Sofroniew, MV; Vinters, HV. Astrocytes: Biology and pathology. Acta Neuropathol 2010, 119, 7–35. [Google Scholar]
- Batchelor, PE; Liberatore, GT; Wong, JY; Porritt, MJ; Frerichs, F; Donnan, GA; Howells, DW. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J. Neurosci 1999, 19, 1708–1716. [Google Scholar]
- Silver, DJ; Steindler, DA. Common astrocytic programs during brain development, injury and cancer. Trends Neurosci 2009, 32, 303–311. [Google Scholar]
- Depino, AM; Earl, C; Kaczmarczyk, E; Ferrari, C; Besedovsky, H; del Rey, A; Pitossi, FJ; Oertel, WH. Microglial activation with atypical proinflammatory cytokine expression in a rat model of Parkinson’s disease. Eur. J. Neurosci 2003, 18, 2731–2742. [Google Scholar]
- Singh, S; Das, T; Ravindran, A; Chaturvedi, RK; Shukla, Y; Agarwal, AK; Dikshit, M. Involvement of nitric oxide in neurodegeneration: A study on the experimental models of Parkinson’s disease. Redox. Rep 2005, 10, 103–109. [Google Scholar]





© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Klusa, V.Z.; Isajevs, S.; Svirina, D.; Pupure, J.; Beitnere, U.; Rumaks, J.; Svirskis, S.; Jansone, B.; Dzirkale, Z.; Muceniece, R.; et al. Neuroprotective Properties of Mildronate, a Small Molecule, in a Rat Model of Parkinson’s Disease. Int. J. Mol. Sci. 2010, 11, 4465-4487. https://doi.org/10.3390/ijms11114465
Klusa VZ, Isajevs S, Svirina D, Pupure J, Beitnere U, Rumaks J, Svirskis S, Jansone B, Dzirkale Z, Muceniece R, et al. Neuroprotective Properties of Mildronate, a Small Molecule, in a Rat Model of Parkinson’s Disease. International Journal of Molecular Sciences. 2010; 11(11):4465-4487. https://doi.org/10.3390/ijms11114465
Chicago/Turabian StyleKlusa, Vija Z., Sergejs Isajevs, Darja Svirina, Jolanta Pupure, Ulrika Beitnere, Juris Rumaks, Simons Svirskis, Baiba Jansone, Zane Dzirkale, Ruta Muceniece, and et al. 2010. "Neuroprotective Properties of Mildronate, a Small Molecule, in a Rat Model of Parkinson’s Disease" International Journal of Molecular Sciences 11, no. 11: 4465-4487. https://doi.org/10.3390/ijms11114465
APA StyleKlusa, V. Z., Isajevs, S., Svirina, D., Pupure, J., Beitnere, U., Rumaks, J., Svirskis, S., Jansone, B., Dzirkale, Z., Muceniece, R., Kalvinsh, I., & Vinters, H. V. (2010). Neuroprotective Properties of Mildronate, a Small Molecule, in a Rat Model of Parkinson’s Disease. International Journal of Molecular Sciences, 11(11), 4465-4487. https://doi.org/10.3390/ijms11114465