Plant Antimicrobial Agents and Their Effects on Plant and Human Pathogens
Abstract
:1. Introduction
2. Phytoanticipins versus Phytoalexins
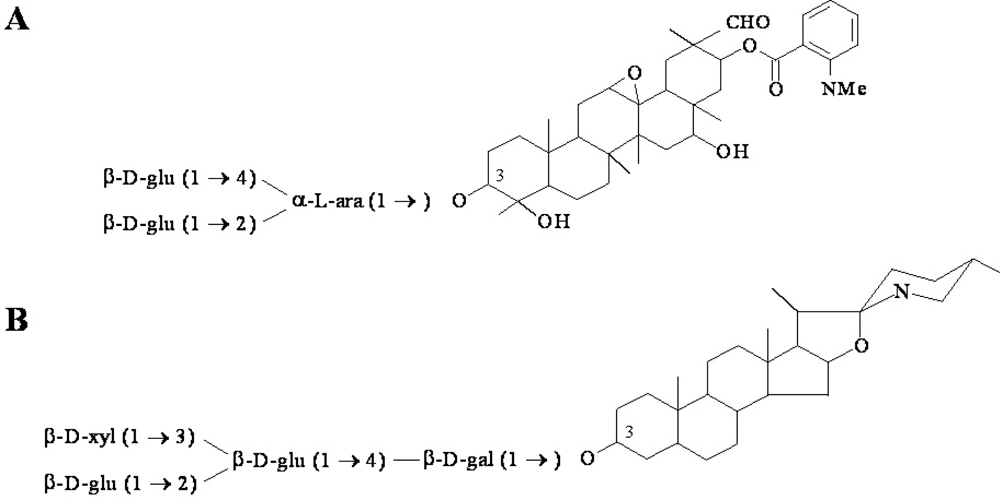
2.1. Phytoanticipins involved in defence responses
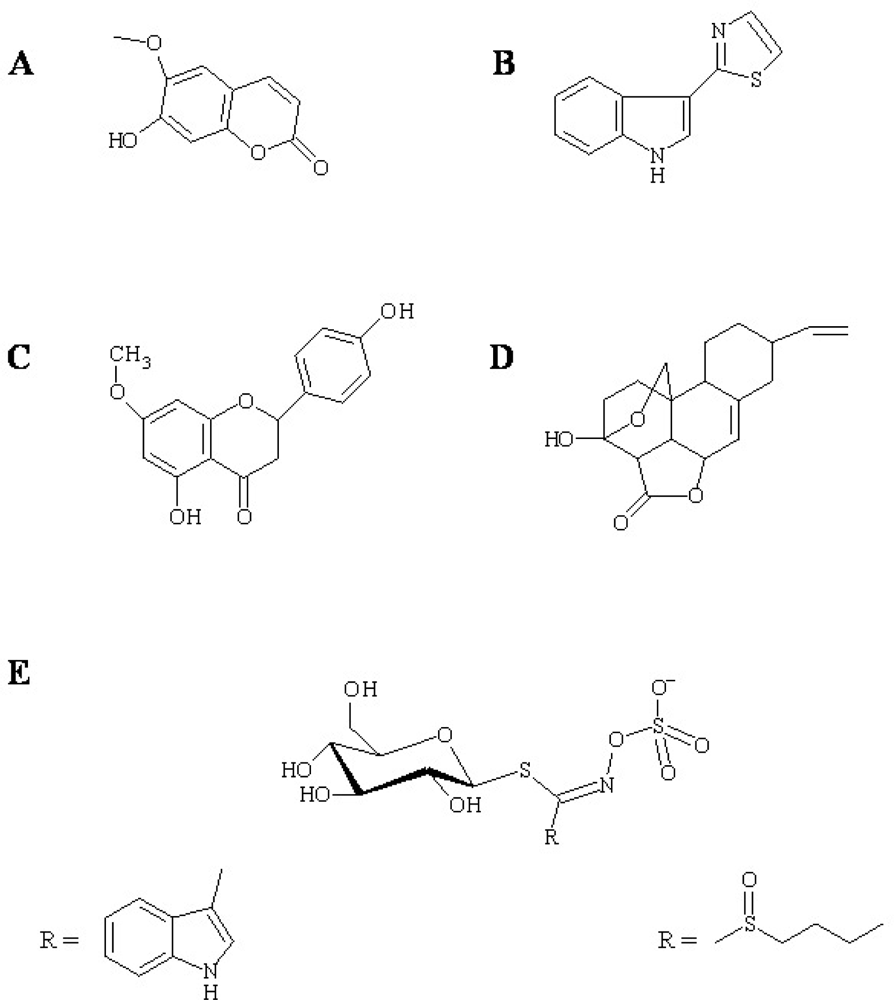
2.2. Phytoalexins: Some biological examples
3. Use of Phytoanticipins and Phytoalexins as Antibacterial Agents in Human Medicine
4. Biotechnological Applications of Phytoanticipins and Phytoalexins in Phytoprotection
5. Conclusions
Acknowledgments
References
- Bais, HP; Weir, TL; Perry, LG; Gilroy, S; Vivanco, JM. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol 2006, 57, 233–266. [Google Scholar]
- Grayer, RJ; Kokubun, T. Plant-fungal interactions: The search for phytoalexins and other antifungal compounds from higher plants. Phytochemistry 2001, 56, 253–263. [Google Scholar]
- Jones, JD; Dangl, JL. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar]
- Lindsay, WP; Lamb, CJ; Dixon, RA. Microbial recognition and activation of plant defense systems. Trends Microbiol 1993, 1, 181–187. [Google Scholar]
- Bednarek, P; Osbourn, A. Plant-microbe interactions: Chemical diversity in plant defense. Science 2009, 324, 746–748. [Google Scholar]
- Müller, KO; Börger, H. Experimentelle Untersuchungen über die Phytophtora-Resistenz, Kartoffel [In German]. Arb. Biol. Reichsanstalt. Landw. Forstw. Berlin 1940, 23, 189–231. [Google Scholar]
- VanEtten, HD; Mansfield, JW; Bailey, JA; Farmer, EE. Two classes of plant antibiotics: Phytoalexins versus phytoanticipins. Plant Cell 1994, 6, 1191–1192. [Google Scholar]
- Osbourn, AE. Preformed antimicrobial compounds and plant defense against fungal attack. Plant Cell 1996, 8, 1821–1831. [Google Scholar]
- Osbourn, AE. Saponins in cereals. Phytochemistry 2003, 62, 1–4. [Google Scholar]
- Morrissey, JP; Osbourn, AE. Fungal resistance to plant antibiotics as a mechanism of pathogenesis. Microbiol. Mol. Biol. Rev 1999, 63, 708–724. [Google Scholar]
- Osbourn, AE; Clarke, BR; Lunness, P; Scott, PR; Daniels, MJ. An oat species lacking avenacin is susceptible to infection by Gaeumannomyces graminis var. tritici. Physiol. Mol. Plant Pathol 1994, 45, 457–467. [Google Scholar]
- Bowyer, P; Clarke, BR; Lunness, P; Daniels, MJ; Osbourn, A. Host range of a plant pathogenic fungus determined by a saponin detoxifying enzyme. Science 1995, 267, 371–374. [Google Scholar]
- Papadopoulou, K; Melton, RE; Legget, M; Daniels, MJ; Osbourn, AE. Compromised disease resistance in saponin deficient plants. Proc. Natl. Acad. Sci. USA 1999, 96, 12923–12928. [Google Scholar]
- Mylona, P; Owatworakit, A; Papadopoulou, K; Jenner, H; Qin, B; Findlay, K; Hill, L; Qi, X; Bakht, S; Melton, R; Osbourn, A. Sad3 and sad4 are required for saponin biosynthesis and root development in oat. Plant Cell 2008, 20, 201–212. [Google Scholar]
- Martín-Hernandez, AM; Dufresne, M; Hugouvieux, V; Melton, R; Osbourn, A. Effects of targeted replacement of the tomatinase gene on the interaction of Septoria lycopersici with tomato plants. Mol. Plant Microbe Interact 2000, 13, 1301–1311. [Google Scholar]
- Bouarab, K; Melton, R; Peart, J; Baulcombe, D; Osbourn, A. Nature 2002, 418, 889–892.
- Maor, R; Shirasu, K. The arms race continues: Battle strategies between plants and fungal pathogens. Curr. Opin. Microbiol 2005, 8, 399–404. [Google Scholar]
- Valle, T; Lopez, JL; Hernandez, JM; Corchete, P. Antifungal activity of scopoletin and its differential accumulation in Ulmus pumila and Ulmus campestris cell suspension cultures infected with Ophiostoma ulmi spores. Plant Sci 1997, 125, 97–101. [Google Scholar]
- Chong, J; Baltz, R; Schmitt, C; Beffa, R; Fritig, B; Saindrenan, P. Downregulation of a pathogen-responsive tobacco UDP-Glc:phenylpropanoid glucosyltransferase reduces scopoletin glucoside accumulation, enhances oxidative stress, and weakens virus resistance. Plant Cell 2002, 14, 1093–1107. [Google Scholar]
- Matros, M; Mock, HP. Ectopic expression of a UDP-glucose:phenylpropanoid glucosyl-transferase leads to increased resistance of transgenic tobacco plants against infection with Potato Virus Y. Plant Cell Physiol 2004, 45, 1185–1193. [Google Scholar]
- Costet, L; Fritig, B; Kauffmann, S. Scopoletin expression in elicitor-treated and tobacco mosaic virus-infected tobacco plants. Physiologia Plantarum 2002, 115, 228–235. [Google Scholar]
- Chong, J; Baltz, R; Fritig, B; Saindrenan, P. An early salicylic acid-, pathogen- and elicitor-inducible tobacco glucosyltransferase: Role in compartmentalization of phenolics and H2O2 metabolism. FEBS Lett 1999, 458, 204–208. [Google Scholar]
- Rakwal, R; Agrawal, GK; Yonekura, M; Kodama, O. Naringenin 7-O-methyltransferase involved in the biosynthesis of the flavanone phytoalexin sakuranetin from rice (Oryza sativa L.). Plant Science 2000, 155, 213–221. [Google Scholar]
- Cartwright, DW; Langcake, P; Pryce, RJ; Leworthy, DP; Ride, JP. Isolation and characterization of two phytoalexins from rice as momilactones A and B. Phytochemistry 1981, 20, 535–537. [Google Scholar]
- Dillon, VM; Overton, J; Grayer, RJ; Harborne, JB. Difference in phytoalexins response among rice cultivars of different resistance to blast. Phytochemistry 1997, 44, 599–603. [Google Scholar]
- Rodrigues, FA; McNally, DJ; Datnoff, LE; Jones, JB; Labbé, C; Benhamou, N; Menzies, JG; Belanger, RR. Silicon enhances the accumulation of diterpenoid phytoalexins in rice: A potential mechanism for blast resistance. Phytopathology 2004, 94, 177–183. [Google Scholar]
- Pedras, MS. The chemical ecology of crucifers and their fungal pathogens: Boosting plant defenses and inhibiting pathogen invasion. Chem. Rec 2008, 8, 109–115. [Google Scholar]
- Pedras, MSC; Zheng, QA; Sarma-Mamillapalle, VK. The phytoalexins from Brassicaceae: Structure, biological activity, synthesis and biosynthesis. Nat. Prod. Commun 2007, 2, 319–330. [Google Scholar]
- Bendarek, P; Pislewska-Bednarek, M; Svatos, A; Schneider, B; Doubsky, J; Mansurova, M; Humphry, M; Consonni, C; Panstruga, R; Sanchez-Vallet, A; Molina, A; Schulze-Lefert, P. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defenses. Science 2009, 323, 101–106. [Google Scholar]
- Clay, NK; Adio, AM; Denoux, C; Jander, G; Ausubel, FM. Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 2009, 323, 95–101. [Google Scholar]
- Glawischnig, E. Camalexin. Phytochemistry 2007, 68, 401–406. [Google Scholar]
- Schuhegger, R; Rauhut, T; Glawischnig, E. Regulatory variability of camalexin biosynthesis. J. Plant Physiol 2007, 164, 636–644. [Google Scholar]
- Kliebenstein, DJ; Rowe, HC; Denby, KJ. Secondary metabolites influence Arabidopsis/Botrytis interactions: Variation in host production and pathogen sensitivity. Plant J 2005, 44, 25–36. [Google Scholar]
- Schuhegger, R; Nafisi, M; Mansourova, M; Petersen, BL; Olsen, CE; Svatos, A; Halkier, BA; Glawischnig, E. CYP71B15 (PAD3) catalyzes the final step in camalexin biosynthesis. Plant Physiol 2006, 141, 1248–1254. [Google Scholar]
- Rogers, EE; Glazebrook, J; Ausubel, FM. Mode of action of the Arabidopsis thaliana phytoalexins camalexin and its role in Arabidopsis-pathogen interactions. Mol. Plant Microb. Interact 1996, 9, 748–757. [Google Scholar]
- Thomma, BP; Nelissen, I; Eggermont, K; Broekaert, WF. Deficiency in phytoalexin production causes enhanced susceptibility of Arabidopsis thaliana to the fungus Alternaria brassicola. Plant J 1999, 19, 163–171. [Google Scholar]
- Chassot, C; Buchala, A; Schoonbeek, HJ; Métraux, JP; Lamotte, O. Wounding of Arabidopsis leaves causes a powerful but transient protection against Botrytis infection. Plant J 2008, 55, 555–567. [Google Scholar]
- Stefanato, FL; Abou-Mansour, E; Buchala, A; Kretschmer, M; Mosbach, A; Bochet, CG; Métraux, JP; Schoonbeek, HJ. The ABC transporter BcatrB from Botrytis cinerea exports camalexin and is a virulence factor on Arabidopsis thaliana. Plant J 2009, 58, 499–510. [Google Scholar]
- Pedras, MSC; Khan, AQ. Biotransformation of the phytoalexins camalexin by the phytopathogen Rhizoctonia solani. Phytochemistry 2000, 53, 59–69. [Google Scholar]
- Pedras, MSC; Ahiahonu, PW. Probing the phytopathogenic stem rot fungus with phytoalexins and analogues: Unprecedented glucosylation of camalexin and 6-methoxycamalexin. Bioorg. Med. Chem 2002, 10, 3307–3312. [Google Scholar]
- Bais, HP; Prithiviraj, B; Jha, AK; Ausubel, FM; Vivanco, JM. Mediation of pathogen resistance by exudation of antimicrobial from roots. Nature 2005, 434, 217–221. [Google Scholar]
- Shah, PM. The need for new therapeutic agents: What is in the pipeline. Clin Microbiol Infect 2005, 11(Suppl. 3), 36–42. [Google Scholar]
- Talbot, GH; Bradley, J; Edwards, JE, Jr; Gilbert, D; Scheld, M; Bartlett, JG. Bad bugs need drugs: An update on the development pipeline from the antimicrobial availability task force of the infectious diseases society of america. Clin. Infect. Dis 2006, 42, 657–668. [Google Scholar]
- Cowan, MM. Plant products as antimicrobial agents. Clin. Microbiol. Rev 1999, 12, 564–582. [Google Scholar]
- Pauli, GF; Case, RJ; Inui, T; Wang, Y; Cho, S; Fischer, HH; Franzblau, SG. New perspectives on natural products in TB drug research. Life Sci 2005, 78, 485–494. [Google Scholar]
- Taguri, T; Tanaka, T; Kouno, I. Antibacterial spectrum of plant polyphenols and extracts depending upon hydrogyphenyl structure. Biol. Pharm. Bull 2006, 29, 2226–2235. [Google Scholar]
- Kovacs, A; Vasas, A; Hohmann, J. Natural phenanthrenes and their biological activity. Phytochem 2008, 69, 1084–1110. [Google Scholar]
- Cushnie, TTP; Lamb, AJ. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar]
- Inou, Y; Shiraishi, A; Hada, T; Hirose, K; Hamashima, H; Shimada, J. The antibacterial effects of terpene alcohols on Staphylococcus aureus and their mode of action. FEMS Microbiol. Lett 2004, 237, 325–331. [Google Scholar]
- Kalemba, D; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Medic. Chem 2003, 10, 813–829. [Google Scholar]
- Rios, JL; Recio, MC. Medicinal plants and antimicrobial activity. J. Ethnopharm 2005, 100, 80–84. [Google Scholar]
- Jaki, BU; Franzblau, SG; Chadwick, LR; Lankin, DC; Zhang, F; Wang, Y; Pauli, GF. Purity-activity relationships of natural products: The case of anti-TB active ursolic acid. J. Nat. Prod 2008, 71, 1742–1748. [Google Scholar]
- Li, X-Z; Nikaido, H. Efflux-mediated drug resistance in bacteria. Drugs 2004, 64, 159–204. [Google Scholar]
- Pagès, J-M; Masi, M; Barbe, J. Inhibitors of efflux pumps in gram-negative bacteria. Trends Mol. Med 2005, 11, 382–389. [Google Scholar]
- Poole, K. Efflux-mediated antimicrobial resistance. J. Antimicrob. Chemother 2005, 56, 20–51. [Google Scholar]
- Lewis, K. In search of natural substrates and inhibitors of MDR pumps. J. Mol. Microbiol. Biotechnol 2001, 3, 247–254. [Google Scholar]
- Roccaro, AS; Blanco, AR; Giuliano, F; Rusciano, D; Enea, V. Epigallocatechin-gallate enhances the activity of tetracycline in Staphylococci by inhibiting its efflux from bacterial cells. Antimicrob. Agents Chemother 2004, 48, 1968–1973. [Google Scholar]
- Zhao, W-H; Hu, Z-Q; Okubo, S; Hara, Y; Shimamura, T. Mechanism of synergy between epigallocatechin gallate and β-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother 2001, 45, 1737–1742. [Google Scholar]
- Fujita, M; Shiota, S; Kuroda, T; Hatano, T; Yoshida, T; Mizushima, T; Tsuchiya, T. Remarkable synergies between baicalein and tetracycline, and baicalein and β-lactams against methicillin-resistant Staphylococcus aureus. Microbiol. Immunol 2005, 49, 391–396. [Google Scholar]
- Stermitz, FR; Lorenz, P; Tawara, JN; Zenewicz, LA; Lewis, K. Synergy in a medicinal plant: Antimicrobial action of bergerine potentiated by 5’-methoxyhydnocarpin, a multidrug pump inhibitor. Proc. Natl. Acad. Sci. USA 2000, 97, 1433–1437. [Google Scholar]
- Michalet, S; Cartier, G; David, B; Mariotte, A-M; Dijoux-franca, M-G; Kaatz, GW; Stavri, M; Gibbons, S. N-Caffeoylphenalkylamide derivatives as bacterial efflux pump inhibitors. Bioorg. Med. Chem. Lett 2007, 17, 1755–1758. [Google Scholar]
- Gibbons, S. Phytochemicals for bacterial resistance-strengths, weaknesses and opportunities. Planta Med 2008, 74, 594–602. [Google Scholar]
- Stavri, M; Piddock, LJV; Gibbons, S. Bacterial efflux pump inhibitors from natural sources. J. Antimicrob. Chemother 2007, 59, 1247–1260. [Google Scholar]
- Tegos, G; Stermitz, FR; Lomovskaya, O; Lewis, K. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob. Agents Chemother 2002, 46, 3133–3141. [Google Scholar]
- Brehm-Stecher, BF; Johnson, EA. Sensitization of Staphylococcus aureus and Escherichia coli to antibiotics by the sesquiterpenoids nerolidol, farnesol, bisabolol, and apritone. Antimicrob. Agents Chemother 2003, 47, 3357–3360. [Google Scholar]
- Kuroda, M; Nagasaki, S; Ohta, T. Sesquiterpene farnesol inhibits recycling of the C55 lipid carrier of the murein monomer precursor contributing to increased susceptibility to β-lactams in methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother 2007, 59, 425–432. [Google Scholar]
- Bjarnsholt, T; Givskov, M. Quorum-sensing blockade as a strategy for enhancing host defences against bacterial pathogens. Phil. Trans. R. Soc. B 2007, 362, 1213–1222. [Google Scholar]
- Fuqua, C; Parsek, MR; Greenberg, EP. Regulation of gene expression by cell-to-cell communication: Acyl-homoserine lactone quorum sensing. Ann. Rev. Genet 2001, 35, 439–468. [Google Scholar]
- Novick, RP; Geisinger, E. Quorum sensing in Staphylococci. Annu. Rev. Genet 2008, 42, 541–564. [Google Scholar]
- Rasmussen, TB; Bjarnsholt, T; Skindersoe, ME; Hentzer, M; Kristoffersen, P; Köte, M; Nielsen, J; Eberl, L; Bivskov, M. Screening for quorum-sensing inhibitors (QSI) by use of a novel genetic system, the QSI selector. J. Bacteriol 2005, 187, 1799–1814. [Google Scholar]
- Bjarnsholt, T; Jensen, PO; Rasmussen, TB; Christophersen, L; Calum, H; Hentzer, M; Hougen, H-P; Rygaard, J; Moser, C; Eberl, L; Hoiby, N; Givskov, M. Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiol 2005, 151, 3873–3880. [Google Scholar]
- Collier, DN; Anderson, L; McKnight, SL; Noah, TL; Knowles, M; Boucher, R; Schwab, U; Gilligan, P; Pesci, EC. A bacterial cell to cell signal in the lungs of cystic fibrosis patients. FEMS Microbiol. Lett 2002, 215, 41–46. [Google Scholar]
- Erickson, DL; Endersby, R; Kirkham, A; Stuber, K; Vollman, DD; Rabin, HR; Mitchell, I; Storey, DG. Pseudomonas aeruginosa quorum-sensing systems may control virulence factor expression in the lungs of patients with cystic fibrosis. Infect. Immun 2002, 70, 1783–1790. [Google Scholar]
- Smith, RS; Harris, SG; Phipps, R; Iglewski, B. The Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl) homoserine lactone contributes to virulence and induces inflammation in vivo. J Bacteriol 2002, 184, 1132–1139. [Google Scholar]
- Telford, G; Wheeler, D; Williams, P; Tomkins, PT; Appleby, P; Sewell, H; Bycroft, BW; Pritchard, DI. The Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-L-homoserine lactone has immunomodulatory activity. Infect. Immun 1998, 66, 36–42. [Google Scholar]
- Yu, L; Xiang, H; Fan, J; Wang, D; Yang, F; Guo, N; Jin, Q; Deng, X. Global transcriptional response of Staphylococcus aureus to rhein, a natural plant product. J. Biotechnol 2008, 135, 304–308. [Google Scholar]
- Wang, D; Yu, L; Xiang, H; Fan, J; He, L; Guo, N; Feng, H; Deng, X. Global transcriptional profiles of Staphylococcus aureus treated with berberine chloride. FEMS Microbiol. Lett 2008, 279, 217–225. [Google Scholar]
- Puupponen-Pimiä, R; Nohynek, L; Alakomi, H-L; Oksman-Caldentey, K-M. Bioactive berry compounds–Novel tools against human pathogens. Appl. Microbiol. Biotechnol 2005, 67, 8–18. [Google Scholar]
- Gattuso, M; Malouin, F; Rempel, H; Diarra, MS. Transcriptomic analysis of Escherichia coli exposed to cranberry extracts. Joint Meeting of 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) and 46th Annual Meeting of the Infectious Diseases Society of America, Washington, DC, USA, October 25–28, 2008; pp. C1–1944.
- Germon, P; Ray, M-C; Vianney, A; Lazzaroni, JC. Energy-dependent conformational change in the TolA protein of Escherichia coli involves its N-terminal domain, TolQ, and TolR. J. Bacteriol 2001, 183, 4110–4114. [Google Scholar]
- Pagès, J-M; James, CE; Winterhalter, M. The porin and the permeating antibiotic: A selective diffusion barrier in gram-negative bacteria. Nat. Rev 2008, 6, 893–903. [Google Scholar]
- Tramonti, A; de Canio, M; Delany, I; Scarlato, V; de Biase, D. Mechanisms of transcription activation exerted by GadX and GadW at the gadA and gadBC gene promoters of the glutamate-based acid resistance system in Echerichia coli. J. Bacteriol 2006, 188, 8118–8127. [Google Scholar]
- Massé, E; Gottesman, S. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc. Natl. Acad. Sci. USA 2002, 99, 4620–4625. [Google Scholar]
- Bouarab, K; El Oirdi, M; Gattuso, M; Moisan, H; Malouin, F. Plant stress response agents affect Staphylococcus aureus virulence genes. ICAAC, Chicago, IL, USA, September 17–20, 2007. Abstract C1–1483.1..
- Proctor, RA; von Eiff, C; Kahl, BC; Becker, K; McNamara, P; Herrmann, M; Peters, G. Small colony variants: A pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol 2006, 4, 295–305. [Google Scholar]
- Moisan, H; Brouillette, E; Jacob, CL; Begin, PL; Michaud, S; Malouin, F. The transcription of virulence factors in Staphylococcus aureus small colony variants isolated from cystic fibrosis patients is influenced by SigB. J. Bacteriol 2006, 188, 64–76. [Google Scholar]
- Mitchell, G; Lamontagne, CA; Brouillette, É; Grondin, G; Talbot, BG; Grandbois, M; Malouin, F. Staphylococcus aureus SigB activity promotes a strong fibronectin-bacterium interaction which may sustain host tissue colonization by small-colony variants isolated from cystic fibrosis patients. Mol. Microbiol 2008, 70, 1540–1555. [Google Scholar]
- Mitchell, G; Gattuso, M; Bouarab, K; Malouin, F. Tomatidine affects virulence regulators of prototypical Staphylococcus aureus and small colony variants of cystic fibrosis patients. ICAAC, San Francisco, CA, USA, September 12–15, 2009. Abstract C1–1341..
- Hain, R; Reif, HJ; Krause, E; Langebartels, R; Kindl, H; Vernau, B; Weise, W; Schmatzer, E; Schreier, PH. Disease resistance results from foreign phytoalexins expression in a novel plant. Nature 1993, 361, 153–156. [Google Scholar]
- VanEtten, H; Temporini, E; Wasmann, C. Phytoalexin (and phytoanticipin) tolerance as a virulence trait: Why is it not required by all pathogens? Physiol. Mol. Plant Pathol 2001, 59, 83–93. [Google Scholar]
- Pedras, MSC; Minic, Z; Jha, M. Brassisin oxidase, a fungal detoxifying enzyme to overcome a plant defense-Purification, characterization and inhibition. FEBS J 2008, 275, 3691–3705. [Google Scholar]
- Pedras, MSC; Minic, Z; Sarma-Mamillapalle, VK. Synthetic inhibitors of the fungal detoxifying enzyme brassinin oxidase based on the phytoalexins camalexin scaffold. J. Agric. Food Chem 2009, 57, 2429–2435. [Google Scholar]

© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
González-Lamothe, R.; Mitchell, G.; Gattuso, M.; Diarra, M.S.; Malouin, F.; Bouarab, K. Plant Antimicrobial Agents and Their Effects on Plant and Human Pathogens. Int. J. Mol. Sci. 2009, 10, 3400-3419. https://doi.org/10.3390/ijms10083400
González-Lamothe R, Mitchell G, Gattuso M, Diarra MS, Malouin F, Bouarab K. Plant Antimicrobial Agents and Their Effects on Plant and Human Pathogens. International Journal of Molecular Sciences. 2009; 10(8):3400-3419. https://doi.org/10.3390/ijms10083400
Chicago/Turabian StyleGonzález-Lamothe, Rocío, Gabriel Mitchell, Mariza Gattuso, Moussa S. Diarra, François Malouin, and Kamal Bouarab. 2009. "Plant Antimicrobial Agents and Their Effects on Plant and Human Pathogens" International Journal of Molecular Sciences 10, no. 8: 3400-3419. https://doi.org/10.3390/ijms10083400
APA StyleGonzález-Lamothe, R., Mitchell, G., Gattuso, M., Diarra, M. S., Malouin, F., & Bouarab, K. (2009). Plant Antimicrobial Agents and Their Effects on Plant and Human Pathogens. International Journal of Molecular Sciences, 10(8), 3400-3419. https://doi.org/10.3390/ijms10083400




