Purification, Crystallization and Preliminary X-ray Crystallographic Studies of RAIDD Death-Domain (DD)
Abstract
:1. Introduction
2. Results and Discussion
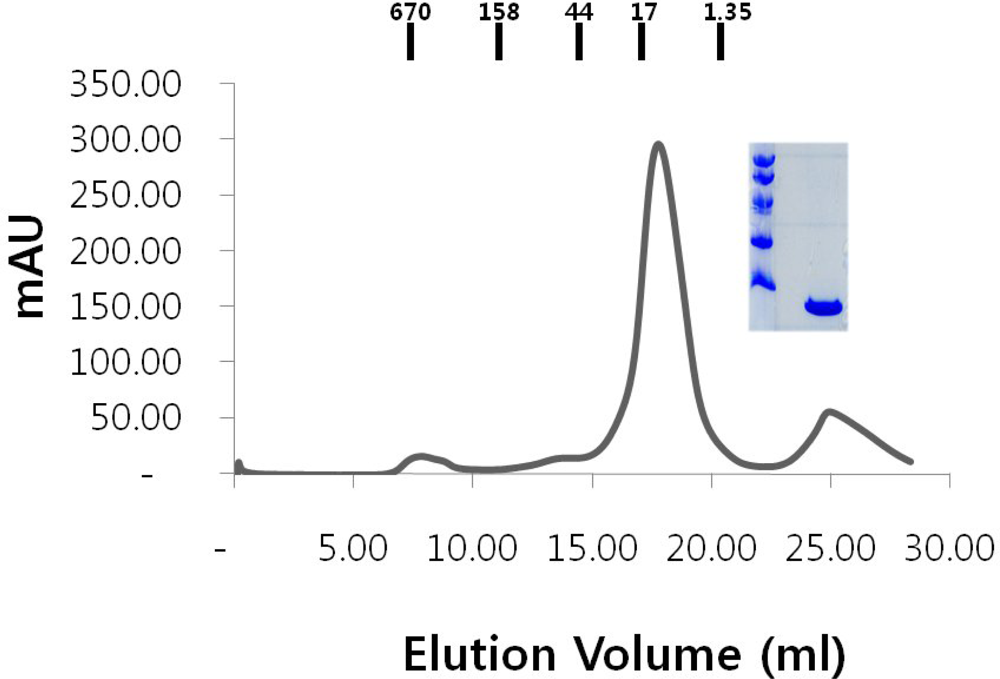
2.1. Over-expression and purification of RAIDD DD
2.2. Crystallization of RAIDD DD
2.3. Preliminary X-ray diffraction studies
2.4. Functional test of RAIDD DD
3. Experimental Section
3.1. Expression and purification
3.2. Crystallization
3.3. Data collection and analysis
3.4. Gel filtration chromatography
3.5. Ultracentrifugation
4. Conclusions
Acknowledgments
References and Notes
- Reed, JC; Doctor, KS; Godzik, A. The domains of apoptosis: A genomics perspective. Sci STKE 2004. [Google Scholar]
- Park, HH; Lo, Y; Lin, S; Wang, L; Yang, JK; Wu, H. The death domain superfamily in intracellular signaling of apoptosis and inflammation. Annu. Rev. Immunol 2007, 25, 561–586. [Google Scholar]
- Wajant, H. The Fas signaling pathway: More than a paradigm. Science 2002, 296, 1635–1636. [Google Scholar]
- Zou, H; Henzel, WJ; Liu, X; Lutschg, A; Wang, X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 1997, 90, 405–413. [Google Scholar]
- Tinel, A; Tschopp, J. The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress. Science 2004, 304, 843–846. [Google Scholar]
- Salvesen, GS. Caspases and apoptosis. Essays Biochem 2002, 38, 9–19. [Google Scholar]
- Janssens, S; Tinel, A; Lippens, S; Tschopp, J. PIDD mediates NF-kappaB activation in response to DNA damage. Cell 2005, 123, 1079–1092. [Google Scholar]
- Lin, Y; Ma, W; Benchimol, S. Pidd, a new death-domain-containing protein, is induced by p53 and promotes apoptosis. Nat. Genet 2000, 26, 122–127. [Google Scholar]
- Lassus, P; Opitz-Araya, X; Lazebnik, Y. Requirement for caspase-2 in stress-induced apoptosis before mitochondrial permeabilization. Science 2002, 297, 1352–1354. [Google Scholar]
- Duan, H; Dixit, VM. RAIDD is a new ‘death’ adaptor molecule. Nature 1997, 385, 86–89. [Google Scholar]
- Read, SH; Baliga, BC; Ekert, PG; Vaux, DL; Kumar, SJ. A novel Apaf-1-independent putative caspase-2 activation complex. Cell Biol 2002, 159, 739–745. [Google Scholar]
- Xiao, T; Towb, P; Wasserman, SA; Sprang, SR. Three-dimensional structure of a complex between the death domains of pelle and tube. Cell 1999, 99, 545–555. [Google Scholar]
- Huang, H; Eberstadt, M; Olejniczak, ET; Meadows, RP; Fesik, S. NMR structure and mutagenesis of the Fas (APO-1/CD95) death domain. Nature 1996, 384, 638–641. [Google Scholar]
- Jeong, EJ; Bang, S; Lee, TH; Park, YI; Sim, WS; Kim, KS. The solution structure of FADD death domain. Structural basis of death domain interactions of Fas and FADD. J. Biol. Chem 1999, 274, 16337–16342. [Google Scholar]
- Terwilliger, TC; Berendzen, J. Bayesian correlated MAD phasing. Acta Cryst 1997, D53, 571–579. [Google Scholar]
- Otwinoski, Z; Minor, W. Processing of x-ray diffraction data collected in oscillation mode. Methods Enzymol 1997, 276, 307–326. [Google Scholar]
- Brünger, AT; Adams, PD; Clore, GM; DeLano, WL; Gros, P; Grosse-Kunsleve, RW; Jiang, JS; Kuszewski, J; Nilges, M; Pannu, NS; Read, RJ; Rice, LM; Simonson, T; Warren, GL. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Cryst 1998, D54, 905–921. [Google Scholar]
- Park, HH; Emmanuelle, L; Stefan, R; Solange, C; Thomas, W; Jurg, T; Wu, H. Death domain assembly mechanism revealed by crystal structure of the oligomeric PIDDosome core complex. Cell 2007, 128, 533–546. [Google Scholar]
- Park, HH; Wu, H. Crystal structure of RAIDD death domain implicates potential mechanism of PIDDosome assembly. J. Mol. Biol 2006, 357, 358–364. [Google Scholar]


| Data collection | Se-Met | Native |
|---|---|---|
| Space group | P3121 | P3121 |
| Cell dimensions | ||
| a, b, c | 56.3Å, 56.3Å, 64.9Å | 56.1Å, 56.1Å, 64.9Å |
| Resolution | 50–2.0Å | 50–2.0Å |
| †Rsym | 6.2% (27.4%) | 5.5% (17.9%) |
| †I/σ(I) | 17.2 (2.8) | 14.5 (2.3) |
| †Completeness | 100.0% (100.0%) | 99.7% (99.8%) |
| †Redundancy | 11.0 (10.6) | 8.9 (8.9) |
| Refinement | |
|---|---|
| Resolution | 50–2.0Å |
| No. reflections used (completeness) | 8063 (96.9%) |
| Rwork/Rfree | 23.1%/24.1% |
| No. atoms | |
| Protein | 704 |
| Water and other small molecule | 55 |
| Average B-factors | |
| Protein | 32.0 Å2 |
| Water and other small molecule | 40.4 Å2 |
| R.m.s deviations | |
| Bond lengths | 0.005Å |
| Bond angles | 1.0° |
| Ramachandran Plot | |
| Most favored regions | 91.3% |
| Additional allowed regions | 8.7% |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jang, T.-h.; Park, H.H. Purification, Crystallization and Preliminary X-ray Crystallographic Studies of RAIDD Death-Domain (DD). Int. J. Mol. Sci. 2009, 10, 2501-2509. https://doi.org/10.3390/ijms10062501
Jang T-h, Park HH. Purification, Crystallization and Preliminary X-ray Crystallographic Studies of RAIDD Death-Domain (DD). International Journal of Molecular Sciences. 2009; 10(6):2501-2509. https://doi.org/10.3390/ijms10062501
Chicago/Turabian StyleJang, Tae-ho, and Hyun Ho Park. 2009. "Purification, Crystallization and Preliminary X-ray Crystallographic Studies of RAIDD Death-Domain (DD)" International Journal of Molecular Sciences 10, no. 6: 2501-2509. https://doi.org/10.3390/ijms10062501
APA StyleJang, T.-h., & Park, H. H. (2009). Purification, Crystallization and Preliminary X-ray Crystallographic Studies of RAIDD Death-Domain (DD). International Journal of Molecular Sciences, 10(6), 2501-2509. https://doi.org/10.3390/ijms10062501




