Liquid Surface-Enhanced Raman Spectroscopy (SERS) Sensor-Based Au-Ag Colloidal Nanoparticles for Easy and Rapid Detection of Deltamethrin Pesticide in Brewed Tea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation and Synthesis of Au-Ag Nanoparticles
2.3. Detection of Deltamethrin Residue in Tea Using Liquid SERS
3. Results
3.1. The Characterization of Au-Ag Colloidal Nanoparticles
3.2. The Detection of Deltamethrin in a Brewed Tea Using Liquid SERS
3.3. The Limit of Detection (LOD) of Deltamethrin Pesticide in Tea Using Au0Ag100 Liquid SERS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khaliqi, M.; Gurning, H.R.H.; Novanda, R.R.; Simamora, O.N. Competitiveness Indonesia tea in international market. In IOP Conference Series: Earth and Environmental Science, Proceedings of the International Conference on Agriculture, Environment and Food Security (AEFS) 2019, Medan, Indonesia, 10 October 2019; IOP Publishing: Bristol, UK, 2020; Volume 454, p. 012039. [Google Scholar]
- Hendriadi, A.; Sulistiyorini, S.; Devilana, M.R. Pesticides residues in Fresh Food of plant origin: Case study in Indonesia. AGRIVITA J. Agric. Sci. 2021, 43, 285–299. [Google Scholar] [CrossRef]
- Rodríguez, R.; Picó, Y.; Font, G.; Mañes, J. Analysis of thiabendazole and procymidone in fruits and vegetables by capillary electrophoresis-electrospray mass spectrometry. J. Chromatogr. A 2002, 949, 359–366. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Pesticide Evaluation Scheme, Vector Ecology and Management; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Chrustek, A.; Hołyńska-Iwan, I.; Dziembowska, I.; Bogusiewicz, J.; Wróblewski, M.; Cwynar, A.; Olszewska-Słonina, D. Current research on the safety of pyrethroids used as insecticides. Medicina 2018, 54, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hugli, T.E.; Moore, S. Determination of the tryptophan content of proteins by ion-exchange chromatography of alkaline hydrolysates. J. Biol. Chem. 1972, 247, 2828–2834. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Zhao, R.; Xu, R. Fast pesticide multiresidue analysis in American ginseng (Panax quinquefolium L.) by gas chromatography with electron capture detection. J. Nat. Med. 2011, 65, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Rong, T.; Yang, R.; Young, J.C.; Zhu, H. Polyphenolic profiles in eight apple cultivars using high-performance liquid chromatography (HPLC). J. Agric. Food Chem. 2003, 51, 6347–6353. [Google Scholar]
- Jaabiri, I.; Belhaj, D.; Turki, N.; Kallel, M.; Ayadi, H.; Ksantini, M.; Bouzid, J.; Gargouri, R. Development and method validation for determination of Deltamethrin residue in olive oil using a reversed-phase high-performance liquid chromatography. OSR J. Appl. Chem 2013, 6, 1–8. [Google Scholar] [CrossRef]
- Kasicka, V. Recent developments in capillary electrophoresis and capillary electrochromatography of peptides. Electrophoresis 2006, 27, 142–175. [Google Scholar] [CrossRef]
- Chang, P.L.; Hsieh, M.M.; Chiu, T.C. Recent advances in the determination of pesticides in environmental samples by capillary electrophoresis. Int. J. Environ. Res. Public Health 2016, 13, 409. [Google Scholar] [CrossRef] [Green Version]
- Yi, L.; Li, H.; Sun, L.; Liu, L.; Zhang, C.; Xi, Z. A highly sensitive fluorescence probe for fast thiol-quantification assay of glutathione reductase. Angew. Chem. 2009, 48, 4034–4037. [Google Scholar] [CrossRef]
- Cserhati, T.; Szogyi, M. Chromatographic determination of pesticides in foods and food products. J. Nutr. Food Sci. 2012, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- Zou, M.; Zhang, X.; Qi, X.; Liu, F. Scattering liquid sensor for quantitative detection of trace melamine in dairy products. In State of the Art in Biosensors: Environmental and Medical Applications; Rinken, T., Ed.; IntechOpen: London, UK, 2013; Available online: https://www.intechopen.com/chapters/43589 (accessed on 6 May 2021).
- Yande, L.; Yuxiang, Z.; Haiyang, W.; Bing, Y. Detection of pesticides on navel orange skin by surface-enhanced Raman spectroscopy coupled with Ag nanostructures. Int. J. Agric. Biol. Eng. 2016, 9, 179. [Google Scholar]
- Durickovic, I. Using Raman spectroscopy for characterization of aqueous media and quantification of species in aqueous solution. In Applications of Molecular Spectroscopy to Current Research in the Chemical and Biological Sciences; Stauffer, M., Ed.; Intech: Rijeka, Croatia, 2016. [Google Scholar]
- Li, Y.S.; Church, J.S. Raman spectroscopy in the analysis of food and pharmaceutical nanomaterials. J. Food Drug Anal. 2014, 22, 29–48. [Google Scholar] [CrossRef] [Green Version]
- Vašková, H. A powerful tool for material identification: Raman spectroscopy. Int. J. Math. Models Methods Appl. Sci. 2011, 5, 1205–1212. [Google Scholar]
- Moreira, L.M.; Junior, L.S.; Santos, F.V.; Lyon, J.P.; Rocha, R.; Zângaro, R.A.; Villaverde, A.B.; Pachec, M.T.T. Raman spectroscopy: A powerful technique for biochemical analysis and diagnosis. J. Spectrosc. 2008, 22, 1–191. [Google Scholar] [CrossRef]
- Cyrankiewicz, M.; Wybranowski, T.; Kruszewski, S. Study of SERS efficiency of metallic colloidal systems. In Journal of Physics: Conference Series, Proceedings of the XIII International Seminar on Physics and Chemistry of Solids, Ustron, Poland, 10–13 June 2007; IOP Publishing: Bristol, UK, 2007; Volume 79, p. 01203. [Google Scholar]
- Zhang, Y.; Mi, X.; Tan, X.; Xiang, R. Recent Progress on Liquid Biopsy Analysis using Surface-Enhanced Raman Spectroscopy. Theranostic 2019, 9, 491–525. [Google Scholar] [CrossRef]
- Fromm, D.P.; Sundaramurthy, A.; Kinkhabwala, A.; Schuck, P.J.; Kino, G.S.; Moerner, W.E. Exploring the chemical enhancement for surface-enhanced Raman scattering with Au bowtie nanoantennas. J. Chem. Phys. 2006, 124, 61101. [Google Scholar] [CrossRef] [Green Version]
- Davies, R.A.; Chong, N.S.; Ooi, B.G. Chemical enhancement of the surface enhanced Raman scattering signals of anilines via their Ortho-substituents. Opt. Photonics J. 2013, 3, 13–23. [Google Scholar] [CrossRef]
- Ding, S.Y.; You, E.M.; Tian, Z.Q.; Moskovits, M. Electromagnetic theories of surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef]
- Wang, J.; Lin, W.; Cao, E.; Xu, X.; Liang, W.; Zhang, X. Surface plasmon resonance sensors on Raman and fluorescence spectroscopy. Sensors 2017, 17, 2719. [Google Scholar] [CrossRef] [Green Version]
- Ling, Y.; Zhuo, Y.; Huang, L.; Mao, D. Using Ag-embedded TiO2 nanotubes array as recyclable SERS substrate. Appl. Surf. Sci. 2016, 388, 169–173. [Google Scholar] [CrossRef]
- Roguska, A.; Kudelski, A.; Pisarek, M.; Opara, M.; Janik-Czachor, M. Surface-enhanced Raman scattering (SERS) activity of Ag, Au and Cu nanoclusters on TiO2-nanotubes/Ti substrate. Appl. Surf. Sci. 2011, 257, 8182–8189. [Google Scholar] [CrossRef]
- Wang, K.; Huang, M.; Chen, J.; Lin, L.; Kong, L.; Liu, X.; Wang, H.; Lin, M. A ‘drop-wipe-test’ SERS method for rapid detection of pesticide residues in fruits. J. Raman Spectrosc. 2018, 49, 493–498. [Google Scholar] [CrossRef]
- Jiang, J.; Zou, S.; Ma, L.; Wang, S.; Liao, J.; Zhang, Z. SERS detection of pesticide residues using transparent adhesive tape and coated silver nanorod. ACS Appl. Mater. Interfaces 2018, 10, 9129–9135. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Du, Y.; Zhang, L.; Yang, Y.; Gao, Y. Improving SERS hot spots for on-site pesticide detection by combining silver nanoparticles with nanowires. J. Mater. Chem. C 2018, 6, 8793–8803. [Google Scholar] [CrossRef]
- Nowicka, A.B.; Czaplicka, M.; Kowalska, A.A.; Szymborski, T.; Kamínska, A. Flexible PET/ITO/Ag SERS platform for label-free detection of pesticides. Biosensors 2019, 9, 111. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.F.; Zou, M.; Qi, X.H.; Liu, F.; Zhu, X.H.; Zhao, B.H. Detection of melamine in liquid milk using surface-enhanced Raman scattering spectroscopy. J. Raman Spectrosc. 2010, 41, 1655–1660. [Google Scholar] [CrossRef]
- Pieczonka, N.P.W.; Aroca, R.F. Inherent complexities of trace detection by surface-enhanced Raman scattering. ChemPhysChem 2005, 6, 2473–2484. [Google Scholar] [CrossRef]
- Alvarez-Puebla, R.A.; Liz-Marzán, L.M. SERS-based diagnosis and biodetection. Small 2010, 6, 604–610. [Google Scholar] [CrossRef]
- Watson, D.A.; Brown, L.O.; Gaskill, D.F.; Naivar, M.; Graves, S.W.; Doorn, S.K.; Nolan, J.P. A flow cytometer for thr measurement of Raman spectra. Cytom. Part A. 2008, 73A, 119–128. [Google Scholar] [CrossRef]
- Piorek, B.D.; Andreou, C.; Moskovits, M.; Meinhart, C.D. Discrete free-surface millifluidics for rapid capture and analysis of airborne molecules using surface-enhanced Raman spectroscopy. Anal. Chem. 2014, 86, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Stamplecoskie, K.G.; Scaiano, J.C.; Tiwari, V.S.; Anis, H. Optimal size of silver nanoparticles for surface-enhanced Raman spectroscopy. J. Phys. Chem. C 2011, 115, 1403–1409. [Google Scholar] [CrossRef]
- He, Y.; Xiao, S.; Dong, T.; Nie, P. Gold nanoparticles for qualitative detection of deltamethrin and carbofuran residues in soil by surface enhanced Raman scattering (SERS). Int. J. Mol. Sci. 2019, 20, 1731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, T.; Lin, L.; He, Y.; Nie, P.; Qu, F.; Xiao, S. Density functional theory analysis of deltamethrin and its application in strawberry by surface-enhanced Raman spectroscopy. Molecules 2018, 23, 1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, A.Q.; Chang, C.M.; Mou, C.Y. Evolution of catalytic activity of Au-Ag bimetallic nanoparticles on mesoporous support for CO oxidation. J. Phys. Chem. B 2005, 109, 18860–18867. [Google Scholar] [CrossRef] [PubMed]
- Valley, N.; Greeneltch, N.; Van Duyne, R.P.; Schatz, G.C. A look at the origin and magnitude of the chemical contribution to the enhancement mechanism of surface-enhanced Raman spectroscopy (SERS): Theory and experiment. J. Phys. Chem. Lett. 2013, 4, 2599–2604. [Google Scholar] [CrossRef]
- Caro, C.; Quaresma, P.; Pereira, E.; Franco, J.; Pernia Leal, M.P.; García-Martín, M.L.; Royo, J.L.; Oliva-Montero, J.M.; Merkling, P.J.; Zaderenko, A.P.; et al. Synthesis and characterization of elongated-shaped silver nanoparticles as a biocompatible anisotropic SERS probe for Intracellular Imaging: Theoretical modeling and experimental verification. Nanomaterials 2019, 9, 256. [Google Scholar] [CrossRef] [Green Version]
- Han, Q.; Zhang, C.; Gao, W.; Han, Z.; Liu, T.; Li, C.; Wang, Z.; He, E.; Zheng, H.H. Ag-Au alloy nanoparticles: Synthesis and in situ monitoring SERS of plasmonic catalysis. Sens. Actuators Part B 2016, 231, 609–614. [Google Scholar] [CrossRef] [Green Version]
- Plieth, W.; Dietz, H.; Anders, A.; Sandmann, G.; Meixner, A.; Weber, M.; Kneppe, H. Electrochemical preparation of silver and gold nanoparticles: Characterization by confocal and surface enhanced Raman microscopy. Surf. Sci. 2005, 597, 119–126. [Google Scholar] [CrossRef]






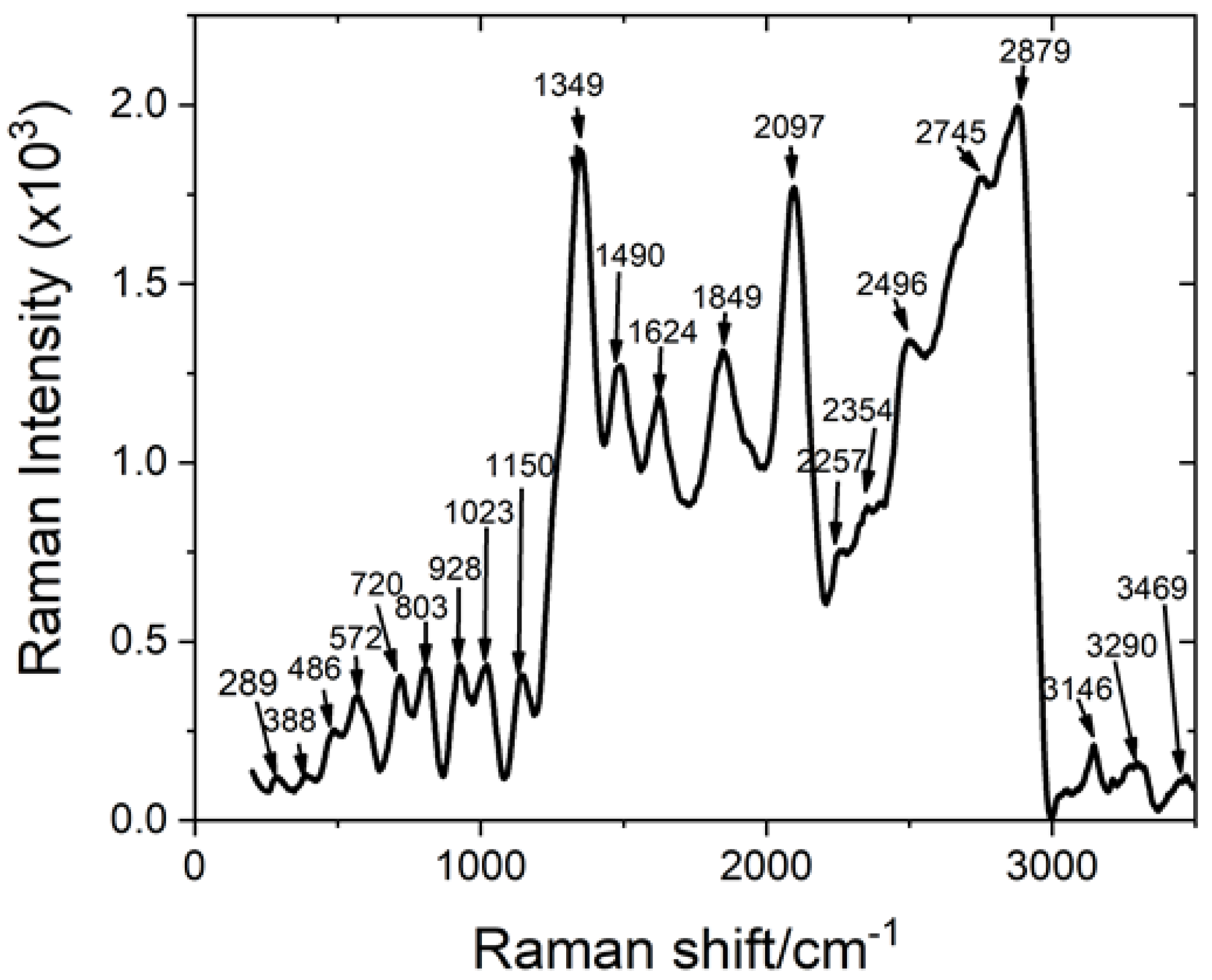

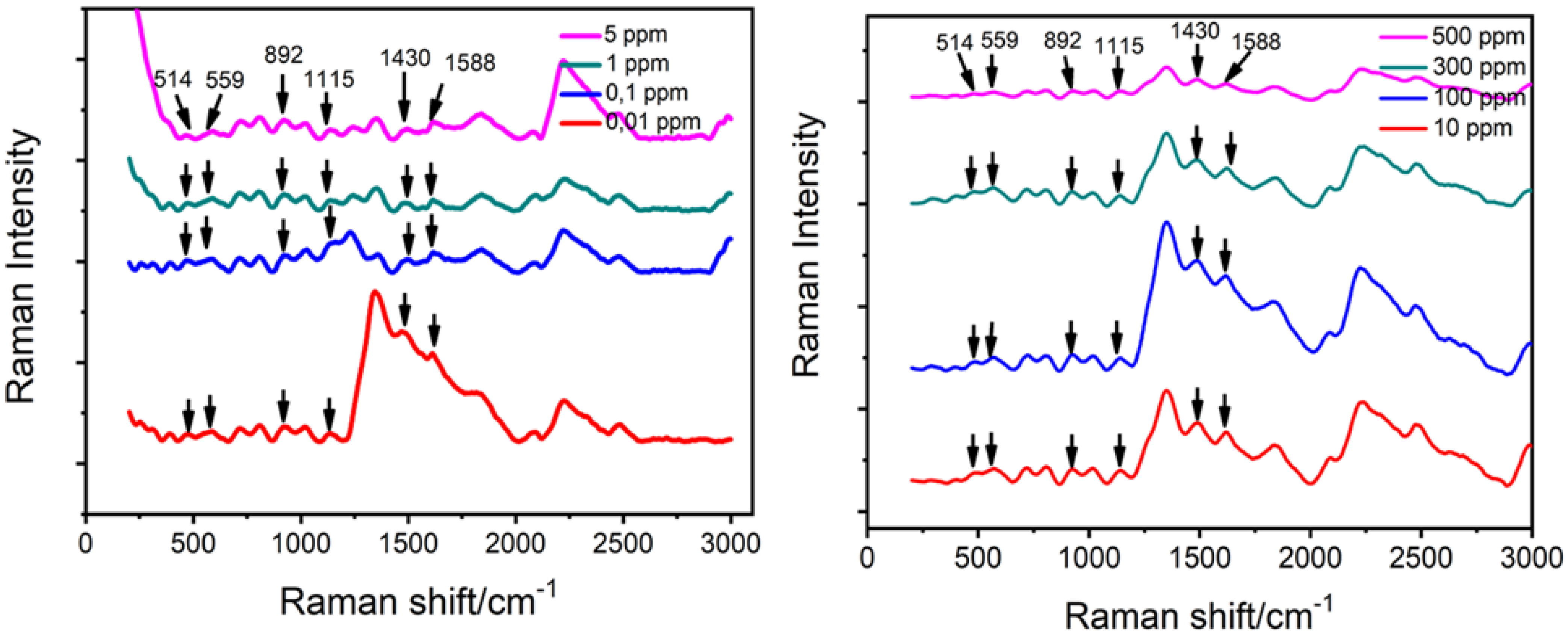

| Raman Shift (cm−1) | Raman Intensity (a.u.) | Peak Assignment [39] | |||
|---|---|---|---|---|---|
| Tea and Pesticide | Au100Ag0 | Au50Ag50 | Au0Ag100 | ||
| 514 | 135.15 | 38.06 | 98.00 | 432.16 | υ (C-Br) ip |
| 559 | 164.33 | 47.40 | 121.22 | 527.63 | υ (C-Br) ip + υ (C-C) |
| 892 | 154.75 | 59.48 | 132.32 | 858.09 | δ (C-H) oop |
| 1115 | 95.15 | 80.30 | 80.30 | 927.50 | δ (C-H) oop |
| 1430 | 94.57 | 24,88 | 290.76 | 1600.19 | δ (C-H) oop |
| 1588 | 75.57 | 5.03 | 229.03 | 1321.27 | υ (C=C) ip |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidayah, A.N.; Triyono, D.; Herbani, Y.; Saleh, R. Liquid Surface-Enhanced Raman Spectroscopy (SERS) Sensor-Based Au-Ag Colloidal Nanoparticles for Easy and Rapid Detection of Deltamethrin Pesticide in Brewed Tea. Crystals 2022, 12, 24. https://doi.org/10.3390/cryst12010024
Hidayah AN, Triyono D, Herbani Y, Saleh R. Liquid Surface-Enhanced Raman Spectroscopy (SERS) Sensor-Based Au-Ag Colloidal Nanoparticles for Easy and Rapid Detection of Deltamethrin Pesticide in Brewed Tea. Crystals. 2022; 12(1):24. https://doi.org/10.3390/cryst12010024
Chicago/Turabian StyleHidayah, Affi Nur, Djoko Triyono, Yuliati Herbani, and Rosari Saleh. 2022. "Liquid Surface-Enhanced Raman Spectroscopy (SERS) Sensor-Based Au-Ag Colloidal Nanoparticles for Easy and Rapid Detection of Deltamethrin Pesticide in Brewed Tea" Crystals 12, no. 1: 24. https://doi.org/10.3390/cryst12010024
APA StyleHidayah, A. N., Triyono, D., Herbani, Y., & Saleh, R. (2022). Liquid Surface-Enhanced Raman Spectroscopy (SERS) Sensor-Based Au-Ag Colloidal Nanoparticles for Easy and Rapid Detection of Deltamethrin Pesticide in Brewed Tea. Crystals, 12(1), 24. https://doi.org/10.3390/cryst12010024






