Isolation and Structure Elucidation of a Flavanone, a Flavanone Glycoside and Vomifoliol from Echiochilon Fruticosum Growing in Tunisia
Abstract
:Introduction
Results and Discussion
Compound 1
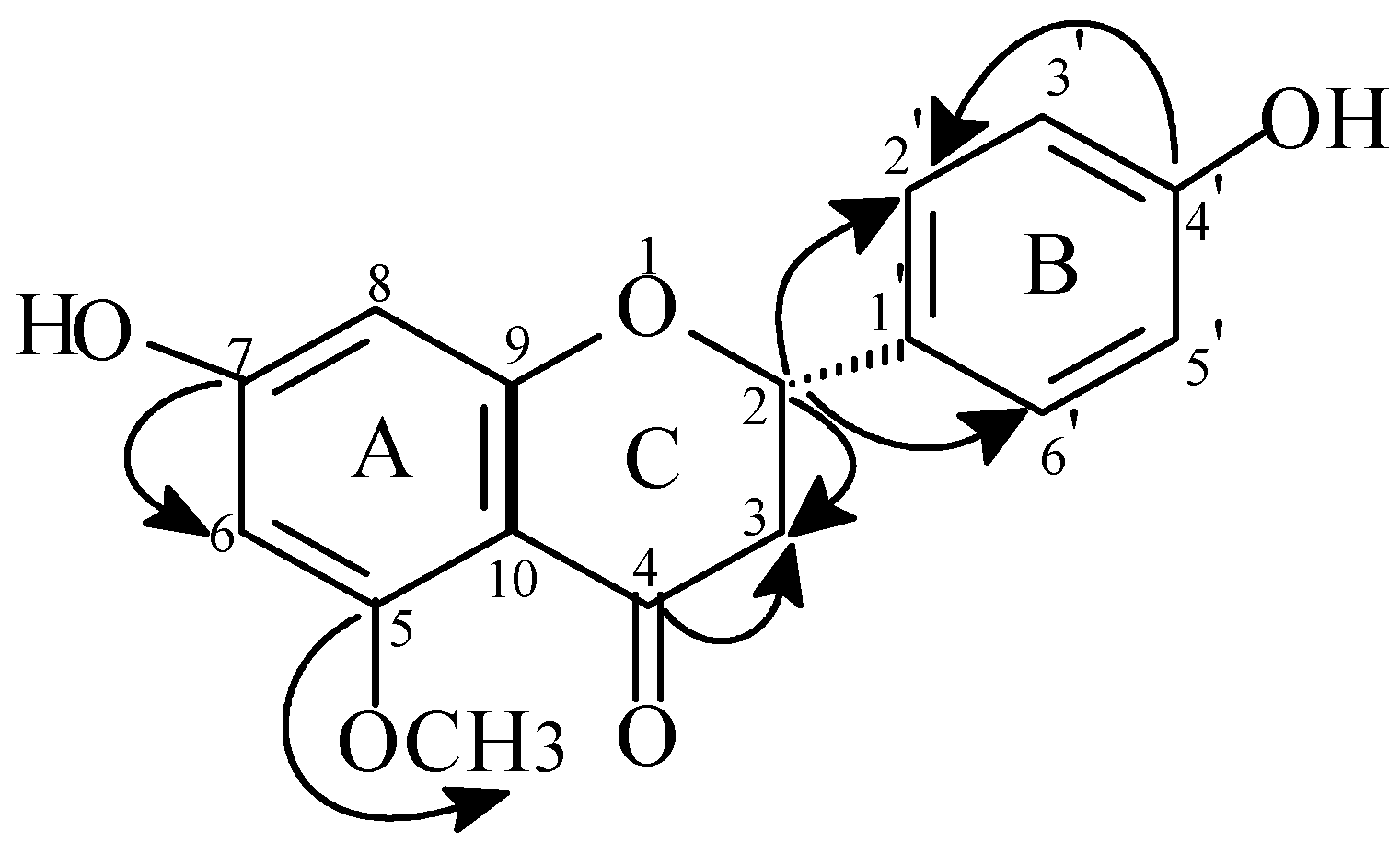
| Compound 1 | Compound 2 | Compound 3 | ||||
| Position | 13C | 1H | 13C | 1H | 13C | 1H |
| 1 | 78.9 | |||||
| 2 | 78.6 | 5.38; dd ; 13.1 ; 2.9 | 79.3 | 5.48; dd ; 2.9 ; 13.1 | 41.0 | |
| 3a | 52.9 | 3.12; dd; 2.9; 17.1 | 43.2 | 2.86; dd; 17.1; 2.9 | 49.6 | 2.25 (a); d; 16.9 |
| 3b | 2.85; dd; 17.1; 13.1 | 3.12; dd; 17.1; 13.1 | 2.45 (b); d; 16.9 | |||
| 4 | 196.4 | 196.5 | 195.5 | |||
| 5 | 168.0 | 162.9 | 127.9 | 5.90; m | ||
| 6 | 93.8 | 6.09; d; 2.17 | 97.0 | 6.21; d; 2.1 | 162.2 | |
| 7 | 162.9 | 165.6 | 129.0 | 5.81; m | ||
| 8 | 96.3 | 6.02; d; 2.3 | 95.8 | 6.23; d; 2.1 | 135.7 | 5.84; m |
| 9 | 164.0 | 162.8 | 68.1 | 4.41; m | ||
| 10 | 102.0 | 105.0 | 23.7 | 1.29; d; 6.4 | ||
| 11 | 24.0 | 1.01; s | ||||
| 12 | 23.0 | 1.08; s | ||||
| 13 | 18.8 | 1.89; s | ||||
| 1’ | 129.9 | 138.3 | ||||
| 2’ | 127.7 | 7.35; d; 8.4 | 126.0 | 7.48; d; 7.5 | ||
| 3’ | 115.4 | 6.90; d; 2.3 | 129.6 | 7.41; m | ||
| 4’ | 156.4 | 128.6 | 7.39; m | |||
| 5’ | 115.4 | 6.90; d; 8.4 | 129.6 | 7.41; m | ||
| 6’ | 127.7 | 5.35; d; 8.4 | 126.0 | 7.48; d; 7.5 | ||
| 1’’ | 101.0 | 4.97; d; 7.1 | ||||
| 2’’ | ||||||
| 3’’ | ||||||
| 4’’ | ||||||
| 5’’ | ||||||
| 6”a | 61.1 | 3.74; dd | ||||
| 6”b | 61.1 | 3.87; dd |
Compound 2

Compound 3
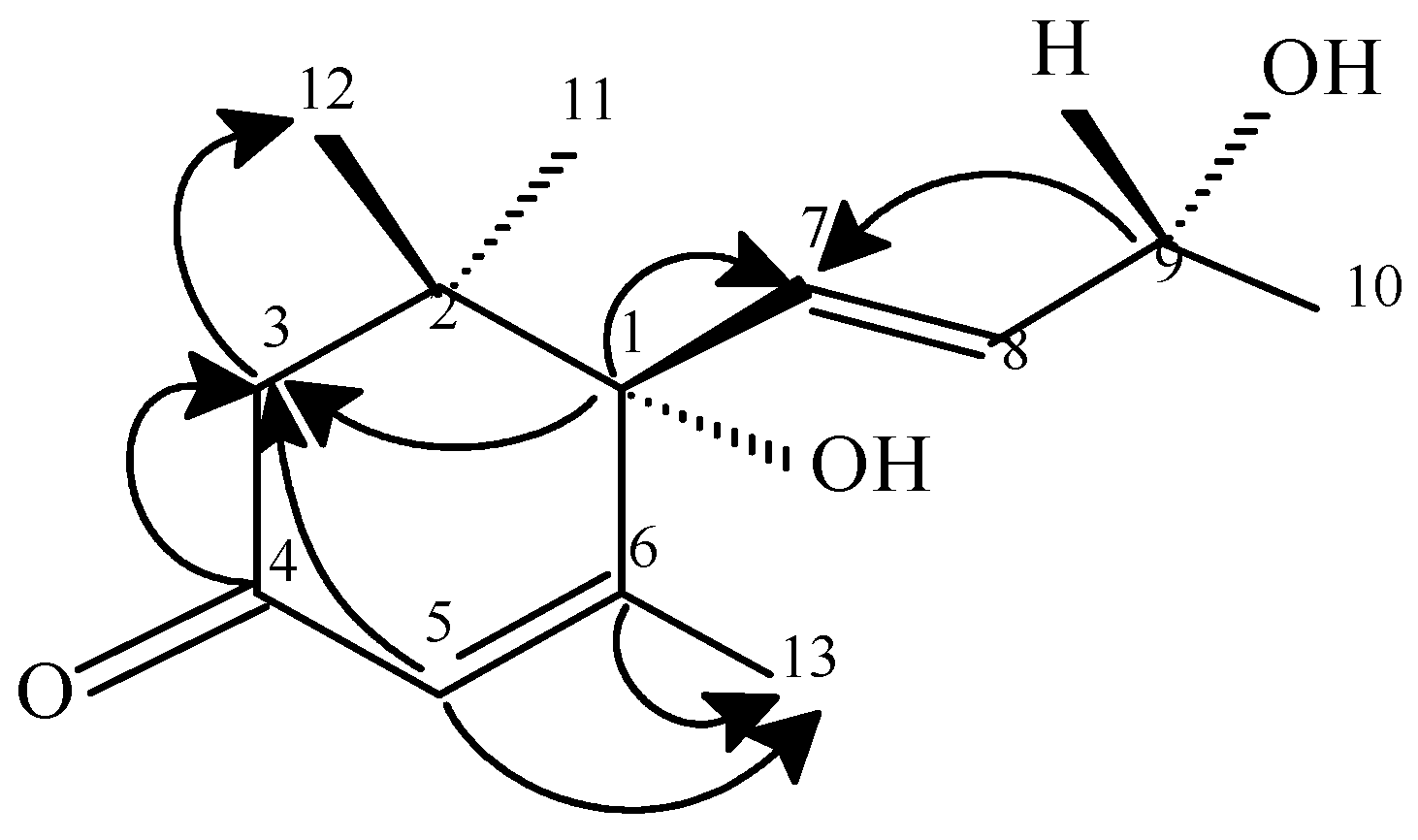
Conclusions
Experimental
General
Plant material
Extraction
Isolation of pure compounds 1-3
Acknowledgements
References
- Hichri, F.; Ben Jannet, H.; Cheriaa, J.; Jegham, S.; Mighri, Z. Antibacterial activities of a new prepared derivatives of oleanolic acid and of other natural triterpenic compounds. C. R. Chimie. 2003, 6, 473–483, and references cited therein. [Google Scholar] [CrossRef]
- Moursi, M. A.; Abdel Gawad, A. A.; Ibrahim, K. M.; Osman, R. Pasture productivity in north west coastal region in Egypt. 1. Effect of location on chemical composition of some forage plants at Sidi-Barrani. Egypt. J. Agron. 1979, 2, 129–139. [Google Scholar]
- El-Ghonemy, A. A. Socio-ecological studies of the natural plant communities along a desert transect 200 km long between Alexandria and Cairo. III. Ecological relation of vegetation on siliceous deposit north of Wadi El-Natrun. Egypt. J. Bot. 1976, 19, 43–62. [Google Scholar]
- El-Din, A. S.; El-Kady, H. F. Nutritive value of the range plants in the western Mediterranean Desert of Egypt. Arab Gulf J. Sci. Res. 2001, 19, 19–27. [Google Scholar]
- Alapetite, G. P. Flore de la Tunisie; Publications Scientifiques Tunisiennes, Imprimerie officielle de la République Tunisienne: Tunis (Tunisia), 1981. [Google Scholar]
- Issaoui, A.; Kallala, A.; Neffati, M.; Akrimi, N. Plantes Naturelles du Sud Tunisien; Ministère de l’environnement et de l’amenagement du territoire: Tunis (Tunisia), 1996; pp. 68–69. [Google Scholar]
- Singh, V.P.; Bineeta, Y.; Pandey, V.B. Flavanone glycosides from Alhagi pseudalhagi. Phytochemistry 1999, 51, 587–590. [Google Scholar] [CrossRef]
- Kang, T. H.; Jeong, S. J.; Ko, W. G.; Kim, Na. Y.; Lee, B. H.; Inagaki, M.; Miyamoto, T.; Higuchi, R.; Chul Kim, Y. Cytotoxic lavandulyl flavanones from Sophora flavescens. J. Nat. Prod. 2000, 63, 680–681. [Google Scholar] [CrossRef] [PubMed]
- Demole, E.; Enggist, P. Novel synthesis of 3,5,5-trimethyl-4-(2-butenylidene)-cyclohex-2-en-1- one, a major constituent of Burley Tobacco flavour. Helv. Chim. Acta 1974, 7, 2087–2091. [Google Scholar] [CrossRef]
- Andersson, R.; Lundgren, R. Monoaryl and cyclohexenone glycosides from needles of Pinus sylverstris. Phytochemistry 1988, 27, 559–562. [Google Scholar] [CrossRef]
- Grayer, R. J. Method. Plant Biochem. 1989, 1, 287–288.
- Sample availability: Samples of compounds 1 (6 mg), 2 (1 mg) and 3 (0.8 mg) are available from the authors.
© 2004 by MDPI (http:www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Hammami, S.; Jannet, H.B.; Bergaoui, A.; Ciavatta, L.; Cimino, G.; Mighri, Z. Isolation and Structure Elucidation of a Flavanone, a Flavanone Glycoside and Vomifoliol from Echiochilon Fruticosum Growing in Tunisia. Molecules 2004, 9, 602-608. https://doi.org/10.3390/90700602
Hammami S, Jannet HB, Bergaoui A, Ciavatta L, Cimino G, Mighri Z. Isolation and Structure Elucidation of a Flavanone, a Flavanone Glycoside and Vomifoliol from Echiochilon Fruticosum Growing in Tunisia. Molecules. 2004; 9(7):602-608. https://doi.org/10.3390/90700602
Chicago/Turabian StyleHammami, S., H. Ben Jannet, A. Bergaoui, L. Ciavatta, G. Cimino, and Z. Mighri. 2004. "Isolation and Structure Elucidation of a Flavanone, a Flavanone Glycoside and Vomifoliol from Echiochilon Fruticosum Growing in Tunisia" Molecules 9, no. 7: 602-608. https://doi.org/10.3390/90700602
APA StyleHammami, S., Jannet, H. B., Bergaoui, A., Ciavatta, L., Cimino, G., & Mighri, Z. (2004). Isolation and Structure Elucidation of a Flavanone, a Flavanone Glycoside and Vomifoliol from Echiochilon Fruticosum Growing in Tunisia. Molecules, 9(7), 602-608. https://doi.org/10.3390/90700602




